Abstract
Programmed cell death 1 (PDCD1, best known as PD-1) is a central negative regulator of effector T cells that is involved in the etiology of chronic inflammatory conditions, viral diseases, and cancer. We have recently sought to improve T-cell functions by means of a novel chimeric co-stimulatory molecule that could divert the negative signals normally transmitted by PD-1 into positive ones. Human T cells transduced to express a fusion protein encompassing the extracellular domain of PD-1 and the intracellular portion of the co-stimulatory molecule CD28, which we named PD-1/28, exhibited an increase in cytokine secretion, the upregulation of activation markers, an improved proliferative potential and superior antineoplastic activity in xenograft models of human melanoma.
Keywords: Adoptive T cell transfer, PD1, CD28, T-cell engineering, immunotherapy
Inhibitory (or negative) co-stimulatory molecules such as programmed cell death 1 (PDCD1, best known as PD-1) and cytotoxic T lymphocyte-associated protein 4 (CTLA4) have been shown to actively modulate T-cell responses upon activation.1 Interestingly, they have also been implicated in the escape of malignant cells from immunosurveillance, as the signal they convey can impair T-cell functions, often leading to exhaustion, decreased secretion of multiple cytokines including interleukin-2 (IL-2), interferon γ (IFNγ) and tumor necrosis factor α (TNFα), dampened proliferation and limited cytotoxic activity.2 PD-1, which is expressed on effector T cells shortly after T-cell receptor (TCR)-dependent activation, can negatively regulate T-cell function by itself. PD-1 binds to 2 different ligands, CD274 (best known as PD-L1 or B7-H1) and PD-1 ligand 2 (PDL2, also known as B7-DC), that can be expressed by professional antigen-presenting cells as well as by tumor cells of distinct histological origin (e.g., breast, kidney, ovarian, pancreatic, bladder, and gastric cancer cells).3 Because of its critical immunosuppressive role, PD-1 has been extensively studied and therapeutic approaches aimed at eliminating its negative impact on T cell-dependent antitumor responses have been devised, mostly based on the blockade of PD-1 signaling with anti-PD-1 or anti-PD-L1 antibodies. These agents can reverse T-cell exhaustion ex vivo and in vivo, hence inducing durable tumor regressions or prolonged disease stabilization in patients with advanced cancers.4 In contrast to PD-1, several co-stimulatory molecules, such as CD28, provide positive signals that are required for the full activation and effector activity of naïve T cells. Upon binding to their cognate ligands, these receptors—which belong to either the B7/CD28 family or the TNFα receptor (TNFR) family—convey TCR-independent intracellular signals that can lead to T-cell expansion as well as to the acquisition of effector functions. Thus, the balance between co-stimulatory and co-inhibitory signals regulate the response, function and expansion of T cells in multiple pathophysiological scenario.
The adoptive transfer of tumor-infiltrating lymphocytes (TILs) or genetically engineered T cells has received increasing attention over the past decade as this approach appears to mediate impressive tumor regressions in some patients bearing advanced neoplasms.5 In addition to receptors that endow T cells with a new specificity (including TCRs and so-called chimeric antigen receptors, CARs), co-stimulatory receptors such as CD28 can be genetically introduced into T cells in order to enhance their effector functions, persistence and antitumor activity.6-8 However, due to the paucity of some activatory ligands (e.g., B7 family members) and the overexpression of inhibitory ligands (such as PD-L1) in the tumor microenvironment, T cells expressing co-stimulatory receptors are expected to function inadequately within neoplastic lesions. To circumvent this issue and generate T cells that are supposed to exhibit robust effector functions in the tumor microenvironment, we designed and optimized a re-targeting molecule that we termed “co-stimulatory converter,” which comprises the extracellular domain of PD-1 fused to the signaling domains of CD28 and/or TNFR superfamily, member 9 (TNFRSF9, best known as 4–1BB).9 The rationale of this approach was to take advantage of the elevated levels of PD-L1 found on malignant cells to stimulate genetically-engineered T cells (Fig. 1). Moreover, to emulate clinical conditions, we designed a tripartite retroviral vector that encodes the α and β chains of a clinically-tested melan A (MLANA)-specific TCR (F4) as well as one of our chimeric receptors, the PD-1/28 molecule. Following transduction, we were able to achieve high levels of expression of both PD-1/28 and F4 TCR in primary human T cells. We then evaluated the function of human T cells co-expressing PD-1/28 and F4 exposed to different melanoma cell lines, and we found that PD-1/28-engineered human T-cells secreted high amounts of various cytokines (including IL-2, IFNγ and TNFα) and expressed increased levels of activation markers including CD25, CD69, and 4–1BB. PD-1/28-expressing T cells also manifested an improved proliferative response as compared with control cells. These observations prompted us to investigate the cytotoxic functions of PD-1/28-expressing T cells in 2 xenograft models of human melanoma. First, we took advantage of a system that we recently adopted for adoptive T-cell transfer studies,8 which that is based on the growth of human tumors on the chick embryo chorioallantoic membrane (CAM). Following the intravenous transfer of PD-1/28-transduced T cells, we observed improved tumor regression as compared with control conditions, and we were able to detect by flow cytometry the accumulation of adoptively transferred T cells within neoplastic lesions. In addition, by using an immunodeficient mouse model, we demonstrate that PD-1/28-transduced T cells are highly efficient at delaying the growth of human melanoma in vivo.
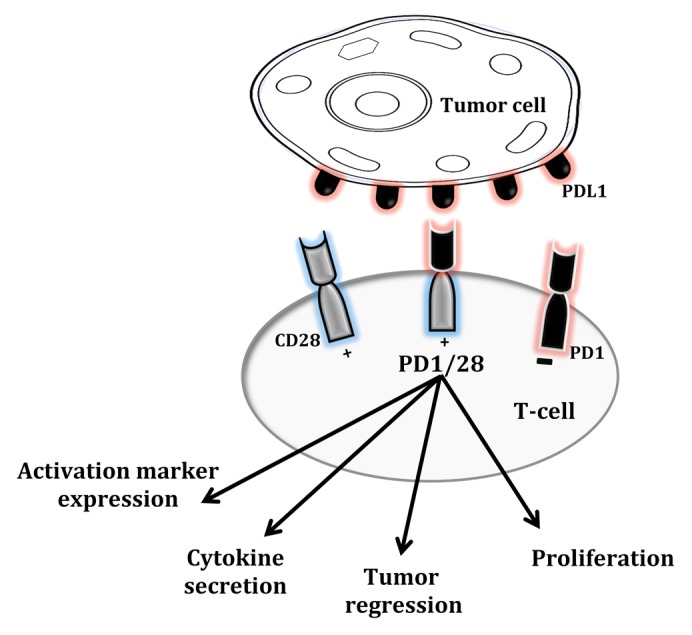
Figure 1. PD-1/28, a chimeric co-stimulatory converter. PD-1/28 is composed of the extracellular domain of the co-inhibitory receptor programmed cell death 1 (PDCD1, best known as PD-1) and the intracellular domain of the co-stimulatory molecule CD28. Upon binding to PD-1 ligands expressed on the surface of cancer cells, PD-1/28 results in increased cytokine secretion, upregulation of T-cell activation markers, improved proliferative potential and superior antitumor activity in xenograft models of human melanoma.
Using other co-inhibitory10 and co-stimulatory molecules for the generation of additional co-stimulatory converters is an attractive perspective. We believe that this type of strategy could be useful in circumstances in T cells undergo exhaustion owing to the PD-1/PD-L1 signaling axis, such as in the course of chronic viral diseases. As malignant cells that escape T-cell responses could be selected in vivo over time based on their high levels of PD-L1, co-stimulatory converters may be useful for reverting this situation, reducing immunosuppression and hence enabling a robust T cell-mediated antitumor response.
In summary, our results suggest that the PD-1/28 co-stimulatory converter improves the antitumor activity of adoptively transferred antigen-specific T cells, resulting to tumor regression. We trust that our findings highlight the importance of manipulating co-stimulatory pathways for the improvement of T cell-based treatments using gene transfer approaches.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Ankri C, Cohen C. Out of the bitter came forth sweet: Activating CD28-dependent co-stimulation via PD-1 ligands. OncoImmunology 2013; 2:e27399; 10.4161/onci.27399
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/27399
References
- 1.Seliger B, Marincola FM, Ferrone S, Abken H. The complex role of B7 molecules in tumor immunology. Trends Mol Med. 2008;14:550–9. doi: 10.1016/j.molmed.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 3.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–12. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–81. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merhavi-Shoham E, Haga-Friedman A, Cohen CJ. Genetically modulating T-cell function to target cancer. Semin Cancer Biol. 2012;22:14–22. doi: 10.1016/j.semcancer.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Topp MS, Riddell SR, Akatsuka Y, Jensen MC, Blattman JN, Greenberg PD. Restoration of CD28 expression in CD28- CD8+ memory effector T cells reconstitutes antigen-induced IL-2 production. J Exp Med. 2003;198:947–55. doi: 10.1084/jem.20021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniel-Meshulam I, Horovitz-Fried M, Cohen CJ. Enhanced antitumor activity mediated by human 4-1BB-engineered T cells. Int J Cancer. 2013;133:2903–13. doi: 10.1002/ijc.28320. [DOI] [PubMed] [Google Scholar]
- 9.Ankri C, Shamalov K, Horovitz-Fried M, Mauer S, Cohen CJ. Human T cells engineered to express a programmed death 1/28 costimulatory retargeting molecule display enhanced antitumor activity. J Immunol. 2013;191:4121–9. doi: 10.4049/jimmunol.1203085. [DOI] [PubMed] [Google Scholar]
- 10.Shin JH, Park HB, Oh YM, Lim DP, Lee JE, Seo HH, Lee SJ, Eom HS, Kim IH, Lee SH, et al. Positive conversion of negative signaling of CTLA4 potentiates antitumor efficacy of adoptive T-cell therapy in murine tumor models. Blood. 2012;119:5678–87. doi: 10.1182/blood-2011-09-380519. [DOI] [PubMed] [Google Scholar]


