Abstract
Tumor-infiltrating lymphocytes (TILs) play an important role in the response of neoplastic lesions to therapy. We have recently shown that a robust tumor infiltration by CD3+ and CD8+ T cells correlates with improved disease outcome upon definitive chemoradiotherapy in patients with head and neck cancer, in a manner that is influenced by tumor compartment and human papilloma virus status. Our data highlight the prognostic and therapeutic relevance of TILs in head and neck cancer.
Keywords: head and neck cancer, HPV, prognostic value, radiotherapy, tumor infiltrating lymphocytes
Head and neck squamous cell carcinoma (HNSCC) constitutes the sixth most common malignancy in developed countries.1 Definitive chemoradiotherapy (CRT) represents the cornerstone in the management of patients with HNSCC. Despite the advent of modern radiation techniques, loco-regional and distant recurrence occur in approximately 20–30% of CRT-treated patients, resulting in significant morbidity and mortality.1 Hence, the identification of biological markers that predict an increased risk of recurrence among HNSCC patients subjected to CRT has profound clinical relevance.
CD3 is a pan-T cell marker and constitutes a protein complex involving four distinct chains that is integral part the T-cell receptor (TCR) signaling machinery. Together with the TCR, the transmembrane co-receptor CD8 binds to peptide-loaded MHC class I molecules expressed on the surface of target cells, resulting in the activation of T-cell cytotoxicity.2,3 We have recently demonstrated that the robust infiltration of HNSCC lesions by CD3+ and CD8+ T cells is a favorable prognostic factor in HNSCC patients following definitive CRT.4 Indeed, these patients exhibited improved overall survival (OS), progression-free survival (PFS) and distant metastasis-free survival (DMFS) in multivariate analyses as compared with patients bearing tumors that were poorly infiltrated by CD3+ and CD8+ T cells, independently of other predictive clinicopathological parameters. The intratumoral abundance of neither CD4+ T cells nor forkhead box P3 (FOXP3)+ regulatory T cells possessed a prognostic value in our study.4
A robust tumor infiltration by CD3+ and CD8+ cytotoxic T cells has been associated with a favorable disease outcome in patients affected by different tumor types including HNSCC as well as colorectal, breast, esophageal, renal, lung, ovarian, and anal carcinomas.5 Hence, our results are in line with previous reports. Notably, in a recent meta-analysis on the prognostic role of TILs in cancer, Gooden et al. failed to detect a significant prognostic value for CD4 or FOXP3 expression with regard to disease progression and patient survival,5 which is similar to our observations.
We quantified the abundance of CD3+ and CD8+ TILs in 3 different tumor compartments (in close proximity of malignant cells, in the tumor stroma and in the periphery).4 Interestingly, the prognostic value of TILs varied according to their intratumoral localization. Thus, patients manifesting high levels of CD3+ T lymphocytes in the proximity of malignant cells had an improved disease outcome whereas robust CD3+ T-cell infiltration of the tumor stroma or periphery failed to have a prognostic value. Elevated levels of CD8+ T cells in the tumor stroma correlated with improved OS, PFS, DMFS and local failure-free survival (LFFS). Finally, a robust infiltration of the tumor periphery by CD8+ T cells was associated with ameliorations in PFS and LFFS, whereas high levels of CD3+ T lymphocytes in the proximity of malignant cells were only associated with improved OS and DMFS. Previous reports have demonstrated differences in the prognostic value of TIL levels that varied according to the tumor compartment,5,6 like in our work.
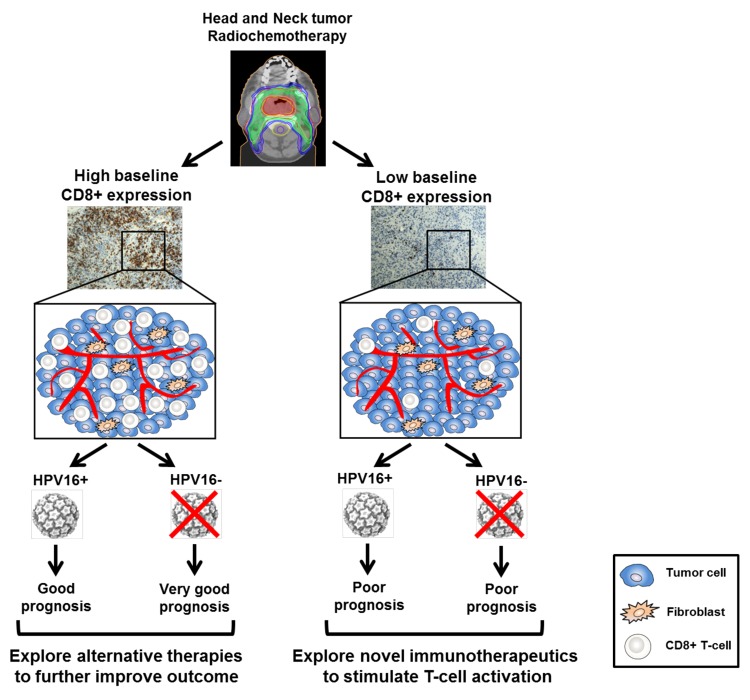
Approximately 20% of HNSCC cases are infected by the human papillomavirus (HPV).7 Cyclin-dependent kinase inhibitor 2A (CDKN2A, also known as p16INK4) is a cellular marker of HPV infection associated with the expression of the E6 and E7 viral oncoproteins. Although some HPV variants, including HPV-16, promote the development of HNSCC, HPV+ patients generally manifest improved responses to chemo- and radiotherapy than their HPV− counterparts, and present distinct clinicopathological features.7 Notably, we did not observe a difference in patient survival according to HPV-16 status in our cohort.4 This discrepancy can be attributed to the high percentage of old patients included in our cohort and to the fact that our study enrolled patients affected by HNSCC in various anatomical locations, rather than the oropharynx only (the most common location of HPV-associated HNSCC). Furthermore, robust tumor infiltration by CD3+ and CD8+ T cells correlated with improved clinical outcome in HPV− HNSCC patients only, whereas a modest benefit was observed in HPV+ individuals.4 Importantly, we failed to observe a significant difference in the lymphocytic infiltration between the HPV+ and HPV− patients included in our study. Mixed findings have been reported on the prognostic value of TILs according to HPV status in HNSCC patients.8,9 Thus, additional studies, preferably in patients affected by oropharyngeal tumors, are required to better elucidate the prognostic significance of TILs in HPV+ and HPV− patients (Fig. 1).
Figure 1. Tumor-infiltrating lymphocytes favor the response to chemoradiotherapy of head and neck cancer. Patients with head and neck squamous cell carcinoma (HNSCC) manifesting a robust tumor infiltration by CD8+ T cells before therapy (baseline) had a favorable clinical outcome, whereas patients with reduced amount of CD8+ (and CD3+) tumor-infiltrating lymphocytes presented worse prognosis upon chemoradiotherapy (CRT). Interestingly, high levels of CD8+ TILs were associated with excellent clinical outcome in human papillomavirus (HPV)− patients, whereas a only moderate benefit was observed in HPV16+ individuals. In contrast, patients with limited CD8+ T-cell infiltration had an unfavorable prognosis, irrespective of their HPV status. Of note, our cohort included patients with HNSCC from various anatomical locations and not only the oropharynx, which is the common site of HPV-associated HNSCCs. Based on the levels of CD8+ T-cell infiltration at baseline, novel immunotherapeutic strategies could be explored in combination with CRT to improve disease outcome in HNSCC patients.
How can we explain our findings? Accumulating evidence suggests that the immune microenvironment can alter the response of various cancers to CRT. Indeed, several preclinical and clinical studies have demonstrated limited responses to radiotherapy in tumor-bearing hosts (be them laboratory animals or patients) lacking a normal CD8+ T-cell repertoire, suggesting that the immune system can boost the efficacy of anticancer treatment.3,5,10 Our findings lend further support to this hypothesis. In addition, the significantly reduced incidence of metastases observed in HNSCC patients manifesting a robust tumor infiltration by CD3+ and CD8+ T cells possibly reflects the existence of a systemic immunosurveillance mechanism that prevents metastatic dissemination.
Taken together, our results indicate that CD3+ and CD8+ T cells can be used as markers to predict disease progression and highlight the importance of TILs in determining the response to chemoradiation in HNSCC patients. Hence, the combination of CRT with novel immunotherapies that activate T cells with might be useful in HNSCC patients that are characterized low levels of CD8+ TILs at baseline, perhaps enhancing treatment responses and improving disease outcome. An in-depth understanding of the role of CD8+ T cells in correlation to HPV status is urgently required to better elucidate the contribution of TILs to tumor-targeting immune responses against HNSCC.
Disclosure of Potential Conflicst of Interest
No potential conflicts of interest were disclosed.
Citation: Balermpas P, Rödel F, Weiss C, Rödel C, Fokas E. Tumor-infiltrating lymphocytes favor the response to chemoradiotherapy of head and neck cancer. OncoImmunology 2013; 2:e27403; 10.4161/onci.27403
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/27403
References
- 1.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371:1695–709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lesterhuis WJ, Haanen JB, Punt CJ. Cancer immunotherapy--revisited. Nat Rev Drug Discov. 2011;10:591–600. doi: 10.1038/nrd3500. [DOI] [PubMed] [Google Scholar]
- 3.Bhardwaj N. Harnessing the immune system to treat cancer. J Clin Invest. 2007;117:1130–6. doi: 10.1172/JCI32136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balermpas P, Michel Y, Wangenblast J, Seitz O, Weiss C, Rödel F, Rödel C, Fokas E. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br J Cancer. 2013 doi: 10.1038/bjc.2013.640. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–13. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 7.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nordfors C, Grün N, Tertipis N, Ahrlund-Richter A, Haeggblom L, Sivars L, Du J, Nyberg T, Marklund L, Munck-Wikland E, et al. CD8(+) and CD4(+) tumour infiltrating lymphocytes in relation to human papillomavirus status and clinical outcome in tonsillar and base of tongue squamous cell carcinoma. Eur J Cancer. 2013 doi: 10.1016/j.ejca.2013.03.019. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 9.Kong CS, Narasimhan B, Cao H, Kwok S, Erickson JP, Koong A, Pourmand N, Le QT. The relationship between human papillomavirus status and other molecular prognostic markers in head and neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 2009;74:553–61. doi: 10.1016/j.ijrobp.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnette B, Weichselbaum RR. Radiation as an immune modulator. Semin Radiat Oncol. 2013;23:273–80. doi: 10.1016/j.semradonc.2013.05.009. [DOI] [PubMed] [Google Scholar]