Abstract
The virome contains the most abundant and fastest-mutating genetic elements on Earth. The mammalian virome is constituted of viruses that infect host cells, virus-derived elements in our chromosomes, and viruses that infect the broad array of other types of organisms that inhabit us. Virome interactions with the host cannot be encompassed by a monotheistic view of viruses as pathogens. Instead, the genetic and transcriptional identity of mammals is defined in part by our co-evolved virome, a concept with profound implications for understanding health and disease.
This Essay describes the importance of the virome in mammalian biology and emerging concepts of virome-host interactions and their relationship to host genetics (Figure 1). The virome is one part of the microbiome, which constitutes all of the organisms that inhabit us, including bacterial and archaeal organisms as well as the mycobiome and other members of the meiofauna such as protists and metazoans. Studies of the virome are in their infancy because it has only recently become possible to ‘see’ the virome in large sequence datasets using bioinformatic tools that can detect relationships between viruses despite extreme nucleotide sequence diversity (Angly et al., 2005; De Vlaminck et al., 2013; Handley et al., 2012; Lysholm et al., 2012; Minot et al., 2012; Mokili et al., 2012; Reyes et al., 2010; Reyes et al., 2012; Willner et al., 2009). Studies of the virome have lagged behind analyses of the bacterial microbiome, which is detectable through inexpensive sequencing of conserved bacterial ribosomal genes, a luxury not available to virome investigators. Nevertheless, it appears that, as for bacteria, there are chronic viruses that may be considered commensals, the penetrance of overt disease in virus-infected persons is low in some cases, and viruses alter disease susceptibility via significant physiologic effects on the host independent of their role as pathogens. Given these advances, it is timely to consider the role of the virome in phenotypic and disease variations among individuals – termed here the genotype/phenotype relationship.
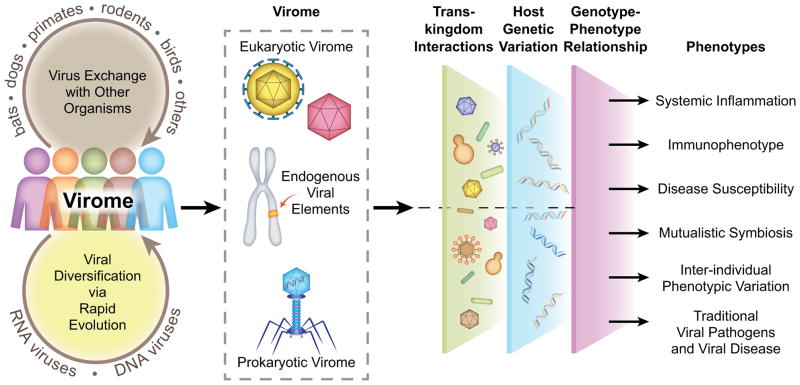
Figure 1. Components of the virome and their relationship to the genotype/phenotype relationship.
The mammalian virome changes rapidly over time through both exchange of viruses with other organisms and through evolution of the individual viruses that constitute the virome. The major components of the virome are the eukaryotic virome, endogenous viral elements, and the prokaryotic virome which includes viruses of both bacteria and archaea. Members of the virome influence the phenotype of the host in a combinatorial manner by interacting with other members of the microbiome (such as other members of the virome itself, the bacterial microbiome, the mycobiome, or the meiofauna) and by interacting with individual variations in host genetics. Together these interactions may influence a range of phenotypes, shown on the right, important for health and disease.
The mammalian virome (Virgin et al., 2009) includes viruses that infect eukaryotic cells (eukaryotic virome); bacteriophages that infect bacteria (bacterial virome); viruses that infect archaea (archaeal virome), and virus-derived genetic elements in host chromosomes that can change host gene expression, express proteins or even generate infectious virus (pro-phages, endogenous retroviruses, endogenous viral elements) (Figure 1). Of the different types of viruses found in mammals, the least is known about the archaeal virome, although these viruses have some interactions with their host cells similar to those observed between bacteriophages and bacteria (Gill and Brinkman, 2011; Prangishvili, 2013). It is useful to consider all of these viruses and virus-related sequences as members of the virome since they can generate infectious viruses under at least some circumstances and encode proteins that participate in viral replication or that have evolved from such proteins. One might consider prions as part of the virome, but herein we will consider elements with nucleic acid genomes.
Eukaryotic viruses and bacterial viruses differ in many regards, but similarly utilize both ‘lytic’ life cycles in which the host cell is destroyed during viral replication and ‘latent’ life cycles in which the virus stably resides within a living cell. Latency is the survival of the viral genome either integrated into the host chromosome (e.g. prophages) or as an episome (e.g. herpesviruses) until the need arises to reactivate and become infectious. This variety of life styles allows the virome to survive, evade defenses, diversify, and evolve amazingly complex, sometimes mutualistic, symbiotic relationships with the host. Importantly, these relationships are often ‘trans-kingdom’ in nature, defined as involving virome interactions with organisms within the microbiome from different kingdoms of life (for example virome interactions with bacteria).
Members of the virome fill a variety of ecological niches in their hosts. Members of the eukaryotic virome that chronically infect mammals, including humans, have significant effects on host physiology outside of the simple paradigm of invasion and tissue destruction that has been the focus of much work on viruses (Foxman and Iwasaki, 2011; Stelekati and Wherry, 2012; Virgin et al., 2009). This suggests that, as the virome is further characterized and annotated, new pathogens, symbiotes, mutualistic symbiotes, and virus-related genetic elements in host chromosomes will be identified.
In mammals the virome inhabits (in addition to the chromosomes themselves) all mucosal surfaces. After clearance of acute viral infection, viruses can also persist in systemic niches in neurons, hematopoietic cells, stem cells, vascular endothelial cells, and likely many additional cell types. For example, after recovery from acute infection, herpesviruses can maintain life-long latent infection in neurons, hematopoietic progenitors, and long-lived lymphocytes. This is a significant difference between the virome and the bacterial microbiome, members of which are not known to persist systemically. The systemic nature of the virome permits it to influence the host in ways that other members of the microbiome do not.
Membership In the Virome
The Earth’s DNA and RNA virome is enormous with an estimated 1031 members when sources such as the ocean are included. This extrapolated estimate is uncertain, but indicates the scale and potential complexity of the virome. One estimate is that we have now explored about 1% of the virome at the sequence level (Mokili et al., 2012), but the biological analysis of the virome is far behind even that low estimate. The size of the mammalian virome is not known. While our own cells are outnumbered about 10-fold by our bacterial microbiome, the number of viruses may be 10-fold higher still (Mokili et al., 2012). Human feces alone contain ca. 108–109 viruses per gram (Mokili et al., 2012; Reyes et al., 2012). Additionally, the mammalian virome is continuously ‘updated’ through rapid evolution of viruses and through exposure to viruses in the environment or in other mammals (Figure 1).
The eukaryotic virome has likely been underestimated because methods for sequencing metagenomes have, surprisingly, often ignored RNA viruses. Some of the most important human and veterinary pathogens are RNA viruses (e.g. influenza, rotavirus, arboviruses, hepatitis C virus) and so the identification of the RNA virome is an important priority. Evidence that the RNA virome in mammals is much more extensive than currently recognized comes from deep sequencing of samples from humans, pigs, bats, sea lions, wild rodents, and macaques with AIDS (Finkbeiner et al., 2008; Handley et al., 2012; Li et al., 2011; Phan et al., 2011; Shan et al., 2011; Smith and Wang, 2013) (Figure 1). Multiple RNA viruses that are human pathogens including SARS, Nipah virus, Hendra virus, and Ebola have arisen in bats alone (Smith and Wang, 2013). Another contributor to underestimating the eukaryotic virome is likely the use of harsh isolation procedures that may result in a failure to detect giant viruses in mammalian virome samples (Colson et al., 2013; Popgeorgiev et al., 2013).
The great majority of sequences present in purified ‘virus’ samples defy annotation because they are not related to currently identified viruses at all, or have extensively diversified from known viruses through rapid evolution. Importantly our definition of ‘virus’ as yet relies on similarities to known viruses at the protein sequence level because of the enormous variability of these organisms at the nucleic acid level and the inadequacy of reference databases. Thus the virome may include novel virus types that have not yet been identified in addition to those with a detectable similarity to known viruses.
As the virome is better defined, viral signatures in our chromosomes will increasingly be possible to identify (Figure 1). There are now ‘fossil’ remains of many known DNA and RNA virus types in our genomes (Feschotte and Gilbert, 2012; Holmes, 2011; Patel et al., 2011), reflecting the role of the eukaryotic virome in gene transfer and evolution. Importantly, these endogenous viral elements may regulate gene expression, participate in mutagenic events, and encode mRNAs for proteins with important functions (Feschotte and Gilbert, 2012; Holmes, 2011; Patel et al., 2011). The best described of these ‘domesticated’ viral genes are the syncitins which are derivative of endogenous retroviral genes and now participate in placentation. Further analysis of the virome is likely to provide unique insights into the structure and evolution of our own genomes by identifying the potential origins and functions of a subset of mammalian genes and proteins.
Invaders From the Virome As Traditional Pathogens
The virome has an important impact on the health of mammals as the source for traditional viral pathogens. From the recognition of viral diseases such as polio or yellow fever in ancient times, to the discovery that filterable agents cause disease, to the linkage of individual viruses to specific diseases such as rabies or influenza, this dominant paradigm has had an enormous impact on human health. Most of these studies relied on detection of viruses through the capacity to grow them in cultured cells or animals. However, culture-dependent approaches have failed to reveal a causative agent for many apparently ‘infectious’ diseases or to definitively link individual viruses to autoimmune diseases. Nevertheless, new pathogenic viruses continue to be discovered at a high rate. Between 1980 and 2005 an estimated 87 pathogens were discovered, about 67% of which were viral (Rosenberg et al., 2013; Woolhouse et al., 2008). Discovery of new pathogenic viruses is likely to continue, and even increase for a period of time, as next generation sequencing of RNA and DNA libraries plummets in cost, bioinformatic tools improve and become generally available, and as viral databases are expanded and more accurately curated. Thereafter, new viral pathogens will likely continue to appear through mutation, recombination, zoonotic transmission, and engineering of viruses for therapeutic or military purposes. The role of the virome as a source of pathogens that cause infectious diseases will continue for the foreseeable future.
The Virome Is More Than a Source of Pathogens
The virome is far more than a source of deadly threats to human and animal welfare. In fact asymptomatic hosts carry multiple viruses and interactions between the virome and the host do not always involve the death of virus-infected cells. This concept has led to emerging paradigm that the virome can influence the host in profound ways independent of classical viral disease. As systemic viruses are frequently inherited from parents very early in life, either by vertical or horizontal transmission soon after birth, the virome may be properly seen as a significant part of our individual genetic identity, whether or not the virus is integrated into our chromosomes (Virgin et al., 2009). Data supporting these emerging concepts is only now appearing, making this an exciting area of virology and immunology. Evolution of our view of viruses to encompass symbiosis, sometimes mutualistic symbiosis, as well as pathogenesis and virulence has the potential to significantly impact our understanding of our own genetics, health and disease.
The immune system is not a static entity- it is in a constant state of activation in between episodic infectious or oncogenic threats. The immune system is in dynamic equilibrium with all components of the microbiome, including the virome, in the asymptomatic state. This ‘normal’ state may vary from person to person, or change over time, and is termed herein the host immunophenotype. Many of the processes in the immune system that are activated in asymptomatic individuals are regulated by cytokines emanating from immune cells that recognize antigens or pathogen associated molecular patterns (PAMPS) including those from viruses. These cytokines act at a distance on bystander cells not actively involved in threat recognition. As many ‘non-immune’ cells have receptors for these cytokines, there is the potential for ongoing inflammatory processes to have broad effects on physiology. For example, interferon-γ is a key cytokine regulating many aspects of both innate and adaptive immunity through interaction with its receptor. The interferon-γ receptor is located on all cells in the body with the exception of erythrocytes, and therefore this cytokine can have both immune and non-immune effects (Farrar and Schreiber, 1993).
It is likely that many components of the mucosal microbiome, such as bacteria and fungi, stimulate low-level immune responses in asymptomatic individuals. Recent work shows that the virome can continuously stimulate the immune system in a similar way. We are constantly re-infected by common low-virulence viruses that repeatedly stimulate mucosal host responses, with potential consequences for host resistance to other infections and susceptibility to diseases such as asthma and Type 1 diabetes (Foxman and Iwasaki, 2011). Increasingly, these ‘acute’ viruses are recognized to persistently infect a proportion of our population, a phenomenon familiar to bacteriologists who understand that pathogens such as Staphylococcus aureus and pneumococcus are carried in the nasopharynx of healthy individuals (Bosch et al., 2013; Foxman and Iwasaki, 2011). This chronic carrier state for viruses has likely been underestimated due to the lack of inexpensive and high quality tools to detect and quantify extremely diverse members of the virome. As the virome is further defined in more healthy people, the proportion and number of viruses that regulate the immunophenotype by both causing acute disease and persisting in some asymptomatic individuals will likely increase.
In addition to these mucosal components of the virome, the immune system is continuously stimulated by chronic systemic viruses. This aspect of host-microbiome interactions appears specific to the virome. It is estimated that an individual healthy human harbors >10 permanent chronic systemic viral infections, but this number may be far higher since the virome has not yet been fully defined (Virgin et al., 2009). These include herpesviruses, polyomaviruses, anelloviruses, adenoviruses, papillomaviruses, and, for many of us, additional viruses such as hepatitis B virus, hepatitis C virus, and HIV. These infections drive continuous activation of the immune system in response to PAMPs and antigens generated as they reactivate from latency or continuously replicate. The role of such active immune surveillance in control of these systemic additions to our individual genetic identities is clear from the fact that these viruses often cause disease when immunosurveillance fails, as for example in the setting of acquired immunodeficiency.
It is easy to understand how inter-individual variations in this asymptomatic systemic virome may contribute to phenotypic variations between hosts, as discussed in more detail below. This applies also to experimental animals housed in different environments, potentially contributing to variation in experiments in animal models (Basic et al., 2014; Cadwell et al., 2010; Young et al., 2012). In this vision of our metagenome, variations in the systemic virome may contribute to phenotypic variation by regulating immunophenotype rather than by acting as pathogens (Figures 1, 2). It is interesting in this regard that idiopathic systemic inflammation has been linked to many of the most severe public health threats of western society including diabetes, cardiovascular disease, and the metabolic syndrome. The actual drivers of this inflammation are not yet fully defined, but the systemic and mucosal virome is certainly a candidate. Even ageing may be influenced by the continuous stimulation of the immune system by the virome. This is exemplified by the role of chronic infection with cytomegalovirus, and perhaps other herpesviruses, in immune senescence of T cell responses, perhaps leading to decreased resistance to infection and cancer in the elderly (Fulop et al., 2013).
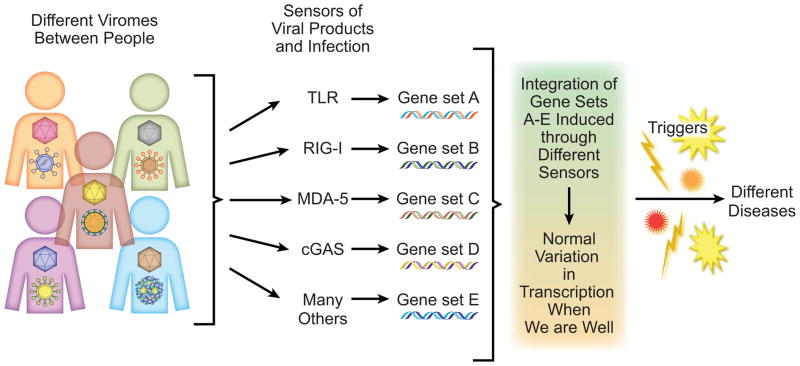
Figure 2. One possible mechanism for the influence of the virome on host phenotype.
Since the virome differs between individuals, and since multiple sensors of viral infection can induce overlapping but distinct transcriptional effects, the virome of asymptomatic individuals may play an important role in regulating the transcriptional state of healthy people. This transcriptional state is referred to as the immunophenotype of the host in the text. Individuals may then respond to triggers of disease in different ways in a manner dependent on the nature of their individual viromes and genetic constitution.
The Bacterial Virome Interacts With the Mammalian Host
The enteric bacterial virome is highly variable between individuals, incredibly diverse, and responsive to environmental stimuli (Minot et al., 2012; Reyes et al., 2010). Bacteriophages regulate the bacterial microbiome via gene transfer, killing competing bacteria to allow invasion of prophage-containing bacteria to fill a partly emptied niche, or by encoding toxins that alter the host intestine to foster bacterial pathogenesis (Duerkop and Hooper, 2013). Given the importance of the bacterial microbiome to our physiology, these effects of the bacterial virome may have a profound impact on health and disease.
In addition to the accepted role of the bacterial virome in regulating the bacterial microbiome, there are tantalizing hints that this part of the virome may also directly interact with the immune system (Figure 2). Free bacteriophage particles in the intestine can contact epithelial cells and, via breaks in the intestinal mucosa or dendritic cell transport, may access the lamina propria and spread systemically (De Vlaminck et al., 2013; Duerkop and Hooper, 2013). Bacteriophages are immunogenic enough to generate antibodies in human infants (Uhr et al., 1962). Additionally endotoxin-free bacteriophage particles are reported to stimulate production of IL-1β and TNF-α by macrophages (Eriksson et al., 2009). CpG motifs in bacteriophage genomes, which stimulate interferon production, may protect against vaccinia virus infection (Mori et al., 1996). Bacteriophage proteins have been reported to enhance DNA vaccine potency (Cuesta et al., 2006). An interesting concept is that tailed DNA bacteriophages, about 25% of which bind mucins via immunoglobulin-like domains, may confer ‘innate’ resistance to bacterial infection by populating the mucin layer over mucosal surfaces awaiting unsuspecting phage-susceptible bacteria (Barr et al., 2013). Bacteriophages can also benefit a multicellular organism through infection of a mutualistic bacterial endosymbiont. For example, aphids are protected against the injection of a parasitic wasp larva by bacteria that produce a toxin that kills the implanted larvae. The toxin is supplied by a bacteriophage that infects the bacterial endosymbiont (Roossinck, 2011). Together these data indicate that the bacterial, and likely archaeal, viromes are an underexplored part of our individual genetic identities that may confer resistance or susceptibility to disease in ways independent of their day job as shepherds of the bacterial or archaeal microbiome.
The Eukaryotic Virome- New Roles in Host-Virus Interactions
An emerging concept is that the virome may contain mutualistic symbiotes with some effects benefiting the host while others may be harmful (Figure 1). In an example of mutualistic symbiosis, chronic infection of mice with a γ-herpesvirus increases resistance to Listeria monocytogenes and Yersinia pestis (Barton et al., 2007), and activates NK cells resulting in increased resistance to tumor grafts (White et al., 2010). We should not be surprised by such emerging observations as they simply bring mammals into a world of viral mutualistic symbiosis that has been observed in many other organisms. For instance, cucumber mosaic virus is a common plant pathogen that decreases crop biomass; however plants infected by this virus and others are resistant to drought (Bao and Roossinck, 2013). Curvularia thermal tolerance virus confers heat tolerance to plants via infection of a fungus that lives in the plant (Bao and Roossinck, 2013; Marquez et al., 2007). Importantly for conceptualizing how the mammalian virome may function in disease and normal physiology, this mutualistic symbiosis appears related to viral infection changing the expression of genes in the fungus such that it can confer heat tolerance to the plant (Bao and Roossinck, 2013; Marquez et al., 2007; Morsy et al., 2010). In another remarkable process in which gene expression changes during viral mutualistic symbiosis, Dysaphis plantagenea densovirus infection of aphid nymphs promotes differentiation to a winged form that promotes spread of both the aphid and virus (Roossinck, 2011).
Virome Interactions With Other Kingdoms of Life
The virome can influence the host through interactions with other types of organisms within the microbiome (trans-kingdom interactions herein) (Figure 1). Perhaps because of the complexity of these interactions and the difficulty in defining mechanisms for them, the current approach to understanding the virome and other components of the microbiome is in some ways similar to the earth-centric view of the universe before the Copernican revolution. We tend to view the interactions between components of the microbiome as revolving around us as the center of our metagenomic universe. The real truth is that complex interactions between kingdoms are formative for physiology, and that co-infections are the rule, not the exception. This complexity is very poorly understood.
The difficulty of analyzing trans-kingdom interactions involving the virome is increased by the fact that our scientific culture tends to limit interchange between groups with expertise in the different components of our metagenome. Many outstanding studies of the bacterial microbiome interpolate that effects are due to alterations in bacteria, without measuring or acknowledging the archaea, the virome, or the mycobiome and other components of the meiofauna (Norman et al., 2014). Virologists are as narrow in their weltanschauung, most frequently attributing viral virulence to viral genes alone when there are numerous examples of virus-induced disease being mediated by bacteria. Furthermore, neither bacteriologists nor virologists properly include the mycobiome or the meiofauna in their considerations (Findley et al., 2013; Iliev et al., 2012; Norman et al., 2014). A non-sectarian approach recognizing that diseases and physiologic processes are reasonably viewed as ecological problems of interactions between groups of organisms, including the virome, and their environment may provide important tools for understanding mammalian biology.
Perhaps the best studies of trans-kingdom interactions have been of infections of the respiratory tract where there is extensive evidence for both bacteria-bacteria and virus-bacteria interactions (Bosch et al., 2013; Foxman and Iwasaki, 2011). A classic example of a trans-kingdom effect of the virome is the increase in pneumococcal and staphylococcal pneumonia seen during influenza pandemics. Trans-kingdom interactions in which viruses worsen bacterial infection in the respiratory system are increasingly well studied (Bosch et al., 2013; Foxman and Iwasaki, 2011; Lysholm et al., 2012; Willner et al., 2009). There is growing evidence that the nasopharynx is chronically infected with viruses, often multiple viruses, in normal individuals. It will be interesting to consider how this respiratory virome (Lysholm et al., 2012; Willner et al., 2009) relates to lung transplant rejection and inflammatory diseases such as asthma, chronic obstructive pulmonary disease, and chronic bronchitis.
In a fascinating example of trans-kingdom interactions involving the virome, recent work has shown that the bacterial microbiome regulates the anti-viral T cell and macrophage responses to influenza and lymphocytic choriomeningitis virus (LCMV) (Abt et al., 2012; Ichinohe et al., 2011). Furthermore, bacterial products contribute to the infectivity of retroviruses and enteroviruses (Kane et al., 2011; Kuss et al., 2011). Other examples of critically important trans-kingdom interactions involving the virome include the inhibitory effects of Schistosoma mansoni infection on T cell responses to vaccinia and hepatitis C virus (Actor et al., 1993; Kamal et al., 2001), and the synergistic effects of malaria and HIV infections in enhancing the spread of both infections (Abu-Raddad et al., 2006). A novel recent example of trans-kingdom interactions involving the virome comes from the observation that RNA viruses infecting organisms such as the pathogens Leishmania and Trichomonas increase the inflammatory response in humans by triggering TLR3 signaling (Fichorova et al., 2012; Ives et al., 2011).
‘Virus-plus-host-gene’ Effects of the Virome
The virome, and trans-kingdom interactions involving the virome, can interact with genetic variation in the host to determine phenotypes (Figure 1). In the simplest case, severe virus-induced disease occurs when specific antiviral host pathways are genetically disrupted, demonstrating that human genetic diversity in responses to virus infection, and by extension the virome, may be responsible for significant phenotypic variation (Sancho-Shimizu et al., 2011). Similarly, both innate immune sensors and the adaptive immune system are important for control of endogenous retroviruses (Young et al., 2012; Yu et al., 2012).
Specific interactions between a viral strain and host allelic variation can lead to phenotypes not observed with either the virus or the host variation alone. In this way ‘virus-plus-host-gene’ combinations can contribute to host fitness or disease risk in a manner that could contribute to understanding the as-yet-unexplained genetic ‘dark matter’ that prevents host genotype from fully explaining disease risk (Figure 1) (Virgin and Todd, 2011). For example, persistent murine norovirus infection interacts with low expression of Atg16L1, an autophagy gene with allelic variants that predispose to Crohn’s disease, to induce disease not observed with either virus infection or Atg16L1 mutation alone (Cadwell et al., 2010). Remarkably this ‘virus-plus-host-gene’ triggered inflammatory response is mitigated by antibiotic treatment, indicating that trans-kingdom interactions with bacteria play a role in ‘virus-plus-host-gene’ induced pathology (Cadwell et al., 2010). The ‘virus-plus-host-gene’ concept, including the role of bacteria in virus-induced pathology in the setting of a specific host mutation, has been confirmed in an independent study of norovirus infection of mice mutant for IL10 (Basic 2014). This relationship is reciprocal as variations in the host genome can define the phenotypes of viral mutants, including by host immunodeficiency specifically complementing attenuation of viruses with mutations in immune evasion genes (Leib et al., 1999; Leib et al., 2000).
Components of the virome can also interact in physiologically important ways with each other. HIV infection leads to changes in expression of endogenous retroviral elements (Contreras-Galindo et al., 2013; Li et al., 2013). Further, expression of Epstein-Barr virus latency genes can increase the expression of a human endogenous retrovirus-related superantigen that prunes the T cell repertoire (Sutkowski et al., 2004; Sutkowski et al., 2001). These data indicate that interactions that involve the virome have, in combination with variations in our host genomes, a potentially significant role in generating phenotypes that cannot be predicted when considering host genes or viruses independently (Figure 1).
The existence of these trans-kingdom interactions with the virome means that understand some aspects of mammalian biology, including treatment or prevention of disease, will likely require defining the virome and its role in mammalian physiology in detail. A major approach to developing therapeutics is based on the concept that diseases are single entities that can effectively be targeted by identifying mechanisms within the mammalian host. This reductionist view of complex disease is highly valuable, but does not account for the role of the metagenome and its components including the virome (Virgin and Todd, 2011). We are all too often surprised when a therapeutic or vaccine ‘fails’ without obvious cause. There are certainly many causes for this (e.g. off-target effects of drugs in human cells), but one might speculate that therapeutic development is over-focused on a single part of the metagenome driven by a human-centric view of what is really a more complex metagenetic organism. In this view, the virome, perhaps through control of the immunophenotype, may be a primary determinant of human variation in drug and vaccine responses either by its direct effects on transcription of the host (see below), through trans-kingdom interactions, or through ‘virus-plus-host-gene’ driven phenotypes.
The Virome As a Determinant of Our ‘Normal’ Transcriptional State
Is there an underlying principle that might explain the effects of the virome on the host that are outside of the traditional role of viruses as pathogens? One possible unifying concept is that the virome acts by altering the transcriptional state of the host in both infected and bystander cells (Figure 2). An initial example of this came from the demonstration that persistent LCMV infection can cause runting of mice by altering growth hormone gene expression without damaging the cells that secrete the hormone (de la Torre and Oldstone, 1992; Valsamakis et al., 1987). A few studies have directly assessed the effects of chronic herpesvirus infection on expression of host genes in the liver, spleen and brain, revealing substantial effects that are both virus and organ-specific (Canny et al., 2013; White et al., 2012). Many of the observed changes in gene expression overlap with interferon-induced transcriptional programs, suggesting a role for virus-induced cytokine expression. These studies are notable since humans carry multiple chronic herpesvirus infections for life. Proof that the virome alters transcription in uninfected cells comes from studies of enteric norovirus infection, which alters epithelial cell gene expression in uninfected cells in a manner dependent on autophagy gene expression (Cadwell et al., 2010). Furthermore, recent single-cell studies in rotavirus-infected mice reveal changes in gene expression in both infected cells and uninfected bystander cells (Sen et al., 2012). Virus infection can trigger changes in bystander cells through inducing release of interferons, other cytokines, or cGAMP(2′–5′) (Ablasser et al., 2013; Canny et al., 2013; Sen et al., 2012; White et al., 2012). It is likely that many such mechanisms remain undiscovered.
While such transcriptional imprinting by the virome may provide mutualistic benefit in some cases, there are untoward effects as well. For example, chronic infection of mice with a single herpesvirus can increase resistance to Listeria monocytogenes and Yersinia pestis (Barton et al., 2007), activate NK cells and increase resistance to tumor grafts (White et al., 2010), delay onset of Type 1 Diabetes in NOD mice (Smith et al., 2007) and decrease adenoviral infection (Nguyen et al., 2008). However, the same infection can increase susceptibility to auto-antigen driven experimental allergic encephalomyelitis (Peacock et al., 2003), and either increase or decrease malaria lethality depending on the kinetics of herpesvirus infection (Haque et al., 2004). Therefore the complexity of the effects of the virome’s role in determining the transcriptional state of the asymptomatic host is likely to be high, with cost versus benefit for the host balanced on a knife’s edge.
Virotype Hypothesis
The concept that viruses within the virome regulate transcription in asymptomatic hosts in variable ways depending on host genetics presents a very complicated view of individual variation and disease mechanisms. The underlying complexity of mechanisms implicit in this conceptualization is daunting (although perhaps correct) and at a minimum offends the sensibility that there should be simple underlying rules that we can decipher and leverage to our own benefit. How then can we reduce this complexity to manageable and testable hypotheses? Understanding the role of the virome in health and disease will certainly require annotating the structure of the virome itself, but this alone does not present an obvious avenue to defining how the virome relates to host biology.
In this regard it is notable that there are many different host molecules that sense the presence of viruses and elicit transcriptional responses. These ever-better-understood molecules differ in the nature of the virus types and virus-derived ligand(s) recognized (Figure 2). Further, the transcriptional programs induced by these different sensors overlap but are distinct and cell type-specific. This allows consideration of what is in common between transcriptional responses to different viruses. Groups of viruses that share cell tropism and the capacity to activate a given sensor or set of sensors, termed herein ‘virotypes’, might be predicted to elicit a predictable host transcriptional response. The concept of virotypes is similar to that of an ecological guild, with the unification of different species into a virotype based on host responses rather than into a guild by exploitation of shared resources. A virotype eliciting a shared host response might reasonably be considered as a potential cause of disease or an alteration in immunophenotype. The presence of a virotype might thereby set up the host to respond in either a positive or negative fashion to environmental or infectious triggers of disease (Figure 2).
This concept differs from current methods for phylogenetic grouping of viruses used to seek new pathogens because a virotype might include different kinds of viruses unified by their capacity to induce a specific host response. If a virotype-elicited host transcriptional response predisposed to disease, then standard approaches to linking a single virus to the disease, for example by PCR or serologic analysis, would be fruitless (Figure 2). However, identifying the relationship between a virotype and a disease might reveal a causative role for components of the virome. This is attractive since evidence that infection triggers autoimmunity and inflammation has been collected for many years, but it has been difficult to link individual viruses to specific inflammatory diseases by classical methods. The use of metagenomic sequencing to characterize the virome during disease, and in healthy individuals, would be an important first step testing this hypothesis.
Coda
The virome is an enormous and fast-evolving component of our metagenome whose effects are only now coming to light. Our understanding of the nature of the virome itself is incomplete and rapidly changing as deep sequencing efforts progress. Major questions for future analysis of the virome include the quantitative and qualitative description of the virome itself, the effects of the virome on organs and tissues outside of the immune system, the nature of trans-kingdom (and inter-virome) interactions involving the virome, identification of PAMPS and antigens within the virome that stimulate innate and adaptive immunity, whether there are virotypes that share effects on the host, how age-at-acquisition influences the impact of the virome on the host, and how we can leverage understanding of the virome to our own benefit.
It is clear that we are in for quite a ride as the capacity to detect and quantify the virome becomes more widespread through decreasing sequencing costs, improved annotation of virome databases, and discovery of additional viral types. The potential importance of the virome indicates the need for a major effort to sequence and annotate the viruses that inhabit us. A Human Virome Project is therefore needed to define the genotype/phenotype relationship. The challenges for such an effort, including preservation and sequencing of RNA and DNA viruses of all types and annotating highly divergent viral genomes, can be met by effectively linking existing basic virology expertise to rapidly evolving sequencing and bioinformatic technologies.
The virome is only one component of our metagenome, and one that must not be studied in isolation from other parts of the microbiome and our own genetics. The early findings from studies of the virome, and other aspects of the microbiome, point to the need for a new science of metagenetics, defined as the integrative analysis of the impact of genes of the host in the context of the genes within the microbiome including the virome. To be effective, practitioners in this field must not be divided into silos that separate scientists with expertise in components of, or responses to, the metagenome. The virome is in us and on us, and is likely to be recognized through metagenetic analysis in the future as a friend at times, and, at other times, as a more complex enemy than we supposed. Both camaraderie and enmity are likely to emanate from the virome in currently unrecognized and unsuspected ways.
Acknowledgments
HWV is supported by NIH grants AI054483, U54 AI057160, AI084887, OD011170, Crohn’s and Colitis Foundation grants 3132 and 274415, and grant IBD-0357 from the Broad Medical Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ablasser A, Schmid-Burgk JL, Hemmerling I, Horvath GL, Schmidt T, Latz E, Hornung V. Cell intrinsic immunity spreads to bystander cells via the intercellular transfer of cGAMP. Nature. 2013;503:530–534. doi: 10.1038/nature12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, Paley MA, Antenus M, Williams KL, Erikson J, et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37:158–170. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Raddad LJ, Patnaik P, Kublin JG. Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa. Science. 2006;314:1603–1606. doi: 10.1126/science.1132338. [DOI] [PubMed] [Google Scholar]
- Actor JK, Shirai M, Kullberg MC, Buller RM, Sher A, Berzofsky JA. Helminth infection results in decreased virus-specific CD8+ cytotoxic T-cell and Th1 cytokine responses as well as delayed virus clearance. Proc Natl Acad Sci U S A. 1993;90:948–952. doi: 10.1073/pnas.90.3.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angly F, Rodriguez-Brito B, Bangor D, McNairnie P, Breitbart M, Salamon P, Felts B, Nulton J, Mahaffy J, Rohwer F. PHACCS, an online tool for estimating the structure and diversity of uncultured viral communities using metagenomic information. BMC Bioinformatics. 2005;6:41. doi: 10.1186/1471-2105-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X, Roossinck MJ. A life history view of mutualistic viral symbioses: quantity or quality for cooperation? Curr Opin Microbiol. 2013;16:514–518. doi: 10.1016/j.mib.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Barr JJ, Auro R, Furlan M, Whiteson KL, Erb ML, Pogliano J, Stotland A, Wolkowicz R, Cutting AS, Doran KS, et al. Bacteriophage adhering to mucus provide a non-host-derived immunity. Proc Natl Acad Sci U S A. 2013;110:10771–10776. doi: 10.1073/pnas.1305923110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton ES, White DW, Cathelyn JS, Brett-McClellan KA, Engle M, Diamond MS, Miller VL, Virgin HWt. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature. 2007;447:3267–329. doi: 10.1038/nature05762. [DOI] [PubMed] [Google Scholar]
- Basic M, Keubler LM, Buettner M, Achard M, Breves G, Schroder B, Smoczek A, Jorns A, Wedekind D, Zschemisch NH, et al. Norovirus Triggered Microbiota-driven Mucosal Inflammation in Interleukin 10-deficient Mice. Inflammatory bowel diseases. 2014 doi: 10.1097/01.MIB.0000441346.86827.ed. [DOI] [PubMed] [Google Scholar]
- Bosch AA, Biesbroek G, Trzcinski K, Sanders EA, Bogaert D. Viral and bacterial interactions in the upper respiratory tract. PLoS Pathog. 2013;9:e1003057. doi: 10.1371/journal.ppat.1003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadwell K, Patel KK, Maloney NS, Liu TC, Ng AC, Storer CE, Head RD, Xavier R, Stappenbeck TS, Virgin HW. Virus-plus-susceptibility gene interaction determines Crohn’s disease gene Atg16L1 phenotypes in intestine. Cell. 2010;141:1135–1145. doi: 10.1016/j.cell.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canny SP, Goel G, Reese TA, Zhang X, Xavier RJ, Virgin HW. Latent gammaherpesvirus 68 infection induces distinct transcriptional changes in different organs. J Virol. 2013 doi: 10.1128/JVI.02708-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colson P, Fancello L, Gimenez G, Armougom F, Desnues C, Fournous G, Yoosuf N, Million M, La Scola B, Raoult D. Evidence of the megavirome in humans. J Clin Virol. 2013;57:191–200. doi: 10.1016/j.jcv.2013.03.018. [DOI] [PubMed] [Google Scholar]
- Contreras-Galindo R, Kaplan MH, He S, Contreras-Galindo AC, Gonzalez-Hernandez MJ, Kappes F, Dube D, Chan SM, Robinson D, Meng F, et al. HIV infection reveals widespread expansion of novel centromeric human endogenous retroviruses. Genome Res. 2013;23:1505–1513. doi: 10.1101/gr.144303.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuesta AM, Suarez E, Larsen M, Jensen KB, Sanz L, Compte M, Kristensen P, Alvarez-Vallina L. Enhancement of DNA vaccine potency through linkage of antigen to filamentous bacteriophage coat protein III domain I. Immunology. 2006;117:502–506. doi: 10.1111/j.1365-2567.2006.02325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre JC, Oldstone MB. Selective disruption of growth hormone transcription machinery by viral infection. Proc Natl Acad Sci U S A. 1992;89:9939–9943. doi: 10.1073/pnas.89.20.9939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vlaminck I, Khush KK, Strehl C, Kohli B, Luikart H, Neff NF, Okamoto J, Snyder TM, Cornfield DN, Nicolls MR, et al. Temporal response of the human virome to immunosuppression and antiviral therapy. Cell. 2013;155:1178–1187. doi: 10.1016/j.cell.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerkop BA, Hooper LV. Resident viruses and their interactions with the immune system. Nature immunology. 2013;14:654–659. doi: 10.1038/ni.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson F, Tsagozis P, Lundberg K, Parsa R, Mangsbo SM, Persson MAA, Harris RA, Pisa P. Tumor-specific bacteriophages induce tumor destruction through activation of tumor-associated macrophages. The Journal of Immunology. 2009;182:3105–3111. doi: 10.4049/jimmunol.0800224. [DOI] [PubMed] [Google Scholar]
- Farrar MA, Schreiber RD. The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- Feschotte C, Gilbert C. Endogenous viruses: insights into viral evolution and impact on host biology. Nat Rev Genet. 2012;13:283–296. doi: 10.1038/nrg3199. [DOI] [PubMed] [Google Scholar]
- Fichorova RN, Lee Y, Yamamoto HS, Takagi Y, Hayes GR, Goodman RP, Chepa-Lotrea X, Buck OR, Murray R, Kula T, et al. Endobiont viruses sensed by the human host - beyond conventional antiparasitic therapy. PLoS One. 2012;7:e48418. doi: 10.1371/journal.pone.0048418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findley K, Oh J, Yang J, Conlan S, Deming C, Meyer JA, Schoenfeld D, Nomicos E, Park M, Program NISCCS, et al. Topographic diversity of fungal and bacterial communities in human skin. Nature. 2013;498:367–370. doi: 10.1038/nature12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner SR, Allred AF, Tarr PI, Klein EJ, Kirkwood CD, Wang D. Metagenomic analysis of human diarrhea: viral detection and discovery. PLoS Pathog. 2008;4:e1000011. doi: 10.1371/journal.ppat.1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxman EF, Iwasaki A. Genome-virome interactions: examining the role of common viral infections in complex disease. Nat Rev Microbiol. 2011;9:254–264. doi: 10.1038/nrmicro2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T, Larbi A, Pawelec G. Human T Cell Aging and the Impact of Persistent Viral Infections. Frontiers in immunology. 2013;4:271. doi: 10.3389/fimmu.2013.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill EE, Brinkman FS. The proportional lack of archaeal pathogens: Do viruses/phages hold the key? Bioessays. 2011;33:248–254. doi: 10.1002/bies.201000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley SA, Thackray LB, Zhao G, Presti R, Miller AD, Droit L, Abbink P, Maxfield LF, Kambal A, Duan E, et al. Pathogenic simian immunodeficiency virus infection is associated with expansion of the enteric virome. Cell. 2012;151:253–266. doi: 10.1016/j.cell.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque A, Rachinel N, Quddus MR, Haque S, Kasper LH, Usherwood E. Co-infection of malaria and gamma-herpesvirus: exacerbated lung inflammation or cross-protection depends on the stage of viral infection. Clin Exp Immunol. 2004;138:396–404. doi: 10.1111/j.1365-2249.2004.02652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes EC. The evolution of endogenous viral elements. Cell Host Microbe. 2011;10:368–377. doi: 10.1016/j.chom.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliev ID, Funari VA, Taylor KD, Nguyen Q, Reyes CN, Strom SP, Brown J, Becker CA, Fleshner PR, Dubinsky M, et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science. 2012;336:1314–1317. doi: 10.1126/science.1221789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives A, Ronet C, Prevel F, Ruzzante G, Fuertes-Marraco S, Schutz F, Zangger H, Revaz-Breton M, Lye LF, Hickerson SM, et al. Leishmania RNA virus controls the severity of mucocutaneous leishmaniasis. Science. 2011;331:775–778. doi: 10.1126/science.1199326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal SM, Rasenack JW, Bianchi L, Al Tawil A, El Sayed Khalifa K, Peter T, Mansour H, Ezzat W, Koziel M. Acute hepatitis C without and with schistosomiasis: correlation with hepatitis C-specific CD4(+) T-cell and cytokine response. Gastroenterology. 2001;121:646–656. doi: 10.1053/gast.2001.27024. [DOI] [PubMed] [Google Scholar]
- Kane M, Case LK, Kopaskie K, Kozlova A, MacDearmid C, Chervonsky AV, Golovkina TV. Successful transmission of a retrovirus depends on the commensal microbiota. Science. 2011;334:245–249. doi: 10.1126/science.1210718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuss SK, Best GT, Etheredge CA, Pruijssers AJ, Frierson JM, Hooper LV, Dermody TS, Pfeiffer JK. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science. 2011;334:249–252. doi: 10.1126/science.1211057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib DA, Harrison TE, Laslo KM, Machalek MA, Moorman NJ, Virgin HW. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J Exp Med. 1999;189:663–672. doi: 10.1084/jem.189.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib DA, Machalek MA, Williams BR, Silverman RH, Virgin HW. Specific phenotypic restoration of an attenuated virus by knockout of a host resistance gene. Proc Natl Acad Sci U S A. 2000;97:6097–6101. doi: 10.1073/pnas.100415697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Deng X, Linsuwanon P, Bangsberg D, Bwana MB, Hunt P, Martin JN, Deeks SG, Delwart E. AIDS alters the commensal plasma virome. J Virol. 2013;87:10912–10915. doi: 10.1128/JVI.01839-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Shan T, Wang C, Cote C, Kolman J, Onions D, Gulland FM, Delwart E. The fecal viral flora of California sea lions. J Virol. 2011;85:9909–9917. doi: 10.1128/JVI.05026-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysholm F, Wetterbom A, Lindau C, Darban H, Bjerkner A, Fahlander K, Lindberg AM, Persson B, Allander T, Andersson B. Characterization of the viral microbiome in patients with severe lower respiratory tract infections, using metagenomic sequencing. PLoS One. 2012;7:e30875. doi: 10.1371/journal.pone.0030875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez LM, Redman RS, Rodriguez RJ, Roossinck MJ. A virus in a fungus in a plant: three-way symbiosis required for thermal tolerance. Science. 2007;315:513–515. doi: 10.1126/science.1136237. [DOI] [PubMed] [Google Scholar]
- Minot S, Grunberg S, Wu GD, Lewis JD, Bushman FD. Hypervariable loci in the human gut virome. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:3962–3966. doi: 10.1073/pnas.1119061109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokili JL, Rohwer F, Dutilh BE. Metagenomics and future perspectives in virus discovery. Curr Opin Virol. 2012;2:63–77. doi: 10.1016/j.coviro.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Kubo T, Kibayashi Y, Ohkuma T, Kaji A. Anti-vaccinia virus effect of M13 bacteriophage DNA. Antiviral research. 1996;31:79–86. doi: 10.1016/0166-3542(96)00951-5. [DOI] [PubMed] [Google Scholar]
- Morsy MR, Oswald J, He J, Tang Y, Roossinck MJ. Teasing apart a three-way symbiosis: transcriptome analyses of Curvularia protuberata in response to viral infection and heat stress. Biochem Biophys Res Commun. 2010;401:225–230. doi: 10.1016/j.bbrc.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Nguyen Y, McGuffie BA, Anderson VE, Weinberg JB. Gammaherpesvirus modulation of mouse adenovirus type 1 pathogenesis. Virology. 2008;380:182–190. doi: 10.1016/j.virol.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman JM, Handley SA, Virgin HW. Kingdom-agnostic Metagenomics: The Importance of Complete Characterization of Enteric Microbial Communities. Gastroenterology. 2014 doi: 10.1053/j.gastro.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MR, Emerman M, Malik HS. Paleovirology - ghosts and gifts of viruses past. Curr Opin Virol. 2011;1:304–309. doi: 10.1016/j.coviro.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock JW, Elsawa SF, Petty CC, Hickey WF, Bost KL. Exacerbation of experimental autoimmune encephalomyelitis in rodents infected with murine gammaherpesvirus-68. Eur J Immunol. 2003;33:1849–1858. doi: 10.1002/eji.200323148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan TG, Kapusinszky B, Wang C, Rose RK, Lipton HL, Delwart EL. The fecal viral flora of wild rodents. PLoS Pathog. 2011;7:e1002218. doi: 10.1371/journal.ppat.1002218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popgeorgiev N, Temmam S, Raoult D, Desnues C. Describing the silent human virome with an emphasis on giant viruses. Intervirology. 2013;56:395–412. doi: 10.1159/000354561. [DOI] [PubMed] [Google Scholar]
- Prangishvili D. The wonderful world of archaeal viruses. Annu Rev Microbiol. 2013;67:565–585. doi: 10.1146/annurev-micro-092412-155633. [DOI] [PubMed] [Google Scholar]
- Reyes A, Haynes M, Hanson N, Angly FE, Heath AC, Rohwer F, Gordon JI. Viruses in the faecal microbiota of monozygotic twins and their mothers. Nature. 2010;466:334–338. doi: 10.1038/nature09199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A, Semenkovich NP, Whiteson K, Rohwer F, Gordon JI. Going viral: next-generation sequencing applied to phage populations in the human gut. Nat Rev Microbiol. 2012;10:607–617. doi: 10.1038/nrmicro2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roossinck MJ. The good viruses: viral mutualistic symbioses. Nat Rev Microbiol. 2011;9:99–108. doi: 10.1038/nrmicro2491. [DOI] [PubMed] [Google Scholar]
- Rosenberg R, Johansson MA, Powers AM, Miller BR. Search strategy has influenced the discovery rate of human viruses. Proc Natl Acad Sci U S A. 2013;110:13961–13964. doi: 10.1073/pnas.1307243110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho-Shimizu V, Perez de Diego R, Jouanguy E, Zhang SY, Casanova JL. Inborn errors of anti-viral interferon immunity in humans. Curr Opin Virol. 2011;1:487–496. doi: 10.1016/j.coviro.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A, Rothenberg ME, Mukherjee G, Feng N, Kalisky T, Nair N, Johnstone IM, Clarke MF, Greenberg HB. Innate immune response to homologous rotavirus infection in the small intestinal villous epithelium at single-cell resolution. Proc Natl Acad Sci U S A. 2012;109:20667–20672. doi: 10.1073/pnas.1212188109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan T, Li L, Simmonds P, Wang C, Moeser A, Delwart E. The fecal virome of pigs on a high-density farm. J Virol. 2011;85:11697–11708. doi: 10.1128/JVI.05217-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I, Wang LF. Bats and their virome: an important source of emerging viruses capable of infecting humans. Curr Opin Virol. 2013;3:84–91. doi: 10.1016/j.coviro.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KA, Efstathiou S, Cooke A. Murine gammaherpesvirus-68 infection alters self-antigen presentation and type 1 diabetes onset in NOD mice. J Immunol. 2007;179:7325–7333. doi: 10.4049/jimmunol.179.11.7325. [DOI] [PubMed] [Google Scholar]
- Stelekati E, Wherry EJ. Chronic bystander infections and immunity to unrelated antigens. Cell Host & Microbe. 2012;12:458–469. doi: 10.1016/j.chom.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutkowski N, Chen G, Calderon G, Huber BT. Epstein-Barr virus latent membrane protein LMP-2A is sufficient for transactivation of the human endogenous retrovirus HERV-K18 superantigen. J Virol. 2004;78:7852–7860. doi: 10.1128/JVI.78.14.7852-7860.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutkowski N, Conrad B, Thorley-Lawson DA, Huber BT. Epstein-Barr virus transactivates the human endogenous retrovirus HERV-K18 that encodes a superantigen. Immunity. 2001;15:579–589. doi: 10.1016/s1074-7613(01)00210-2. [DOI] [PubMed] [Google Scholar]
- Uhr JW, Dancis J, Franklin EC, Finkelstein MS, Lewis EW. The antibody response to bacteriophage phi-X 174 in newborn premature infants. The Journal of clinical investigation. 1962;41:1509–1513. doi: 10.1172/JCI104606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsamakis A, Riviere Y, Oldstone MB. Perturbation of differentiated functions in vivo during persistent viral infection. III. Decreased growth hormone mRNA. Virol. 1987;156:214–220. doi: 10.1016/0042-6822(87)90400-4. [DOI] [PubMed] [Google Scholar]
- Virgin HW, Todd JA. Metagenomics and personalized medicine. Cell. 2011;147:44–56. doi: 10.1016/j.cell.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- White DW, Keppel CR, Schneider SE, Reese TA, Coder J, Payton JE, Ley TJ, Virgin HW, Fehniger TA. Latent herpesvirus infection arms NK cells. Blood. 2010;115:4377–4383. doi: 10.1182/blood-2009-09-245464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DW, Suzanne Beard R, Barton ES. Immune modulation during latent herpesvirus infection. Immunol Rev. 2012;245:189–208. doi: 10.1111/j.1600-065X.2011.01074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner D, Furlan M, Haynes M, Schmieder R, Angly FE, Silva J, Tammadoni S, Nosrat B, Conrad D, Rohwer F. Metagenomic analysis of respiratory tract DNA viral communities in cystic fibrosis and non-cystic fibrosis individuals. PloS one. 2009;4:e7370. doi: 10.1371/journal.pone.0007370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolhouse ME, Howey R, Gaunt E, Reilly L, Chase-Topping M, Savill N. Temporal trends in the discovery of human viruses. Proceedings Biological sciences/The Royal Society. 2008;275:2111–2115. doi: 10.1098/rspb.2008.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young GR, Eksmond U, Salcedo R, Alexopoulou L, Stoye JP, Kassiotis G. Resurrection of endogenous retroviruses in antibody-deficient mice. Nature. 2012;491:774–778. doi: 10.1038/nature11599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Lubben W, Slomka H, Gebler J, Konert M, Cai C, Neubrandt L, Prazeres da Costa O, Paul S, Dehnert S, et al. Nucleic acid-sensing Toll-like receptors are essential for the control of endogenous retrovirus viremia and ERV-induced tumors. Immunity. 2012;37:867–879. doi: 10.1016/j.immuni.2012.07.018. [DOI] [PubMed] [Google Scholar]