Abstract
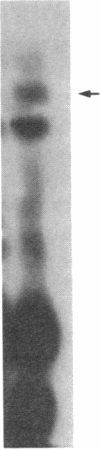
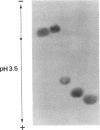
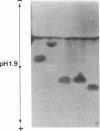
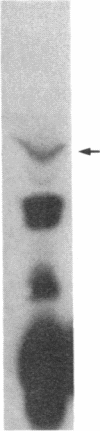
O5-Phosphohydroxylysine was chemically synthesized and techniques were established for its identification by combined use of cation-exchange chromatography, thin-layer electrophoresis at pH 1.9 and 3.5, and thin-layer chromatography. Clean separation of phosphohydroxylysine from the other phospho amino acids, phosphoethanolamine, and phosphocholine was achieved. Conditions were also determined to permit hydrolysis of proteins in 2 M HCl without loss of the phosphono group of phosphohydroxylysine residues. Experiments were then performed showing that 32P was incorporated into the hydroxylysine residues of cell-associated collagens when cultured calf aorta medial smooth muscle cells were incubated with [32P]orthophosphate. In other experiments, the cells incorporated [3H]lysine into hydroxylysine residues of cell-associated collagen and then 32P into phosphohydroxylysine residues. The doubly labeled phosphohydroxylysine subsequently isolated showed nearly 1:1 stoichiometry with respect to incorporation of precursor lysine and phosphorus. Finally, in preliminary experiments done with a cell-free extract of the smooth muscle cells, 32P was transferred from [gamma-32P]ATP to hydroxylysine residues in several kinds of collagenous substrates. Thus, this work shows that smooth muscle cells have the capacity to phosphorylate hydroxylysine residues in their cell-associated collagens and provides preliminary evidence that a protein kinase is involved.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eckhart W., Hutchinson M. A., Hunter T. An activity phosphorylating tyrosine in polyoma T antigen immunoprecipitates. Cell. 1979 Dec;18(4):925–933. doi: 10.1016/0092-8674(79)90205-8. [DOI] [PubMed] [Google Scholar]
- Fietzek P. P., Kühn K. The primary structure of collagen. Int Rev Connect Tissue Res. 1976;7:1–60. doi: 10.1016/b978-0-12-363707-9.50007-1. [DOI] [PubMed] [Google Scholar]
- Gallop P. M., Blumenfeld O. O., Seifter S. Structure and metabolism of connective 801 tissue proteins. Annu Rev Biochem. 1972;41:617–672. doi: 10.1146/annurev.bi.41.070172.003153. [DOI] [PubMed] [Google Scholar]
- Hiles R. A., Henderson L. M. The partial purification and properties of hydroxylysine kinase from rat liver. J Biol Chem. 1972 Feb 10;247(3):646–651. [PubMed] [Google Scholar]
- Hiles R. A., Triebwasser K. C., Henderson L. M. The degradation of hydroxy-L-lysine in liver via its phosphate ester. Biochem Biophys Res Commun. 1970 Nov 9;41(3):662–668. doi: 10.1016/0006-291x(70)90064-1. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kresina T. F., Miller E. J. Isolation and characterization of basement membrane collagen from human placental tissue. Evidence for the presence of two genetically distinct collagen chains. Biochemistry. 1979 Jul 10;18(14):3089–3097. doi: 10.1021/bi00581a028. [DOI] [PubMed] [Google Scholar]
- Schwartz E., Adamany A. M., Blumenfeld O. O. Extracellular proteins of the calf aortic media smooth muscle cells in culture. Biochim Biophys Acta. 1980 Aug 21;624(2):531–544. doi: 10.1016/0005-2795(80)90094-x. [DOI] [PubMed] [Google Scholar]
- Shinkai H., Yonemasu K. Hydroxylysine-linked glycosides of human complement subcomponent C1q and various collagens. Biochem J. 1979 Mar 1;177(3):847–852. doi: 10.1042/bj1770847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro R. G., Spiro M. J. Studies on the biosynthesis of the hydroxylysine-liked disaccharide unit of basement membranes and collagens. I. Kidney glucosyltransferase. J Biol Chem. 1971 Aug 25;246(16):4899–4909. [PubMed] [Google Scholar]
- Ushiro H., Cohen S. Identification of phosphotyrosine as a product of epidermal growth factor-activated protein kinase in A-431 cell membranes. J Biol Chem. 1980 Sep 25;255(18):8363–8365. [PubMed] [Google Scholar]
- Wu G. Y., Pereyra B., Seifter S. Specificity of trypsin and carboxypeptidase B for hydroxylysine residues in denatured collagens. Biochemistry. 1981 Jul 21;20(15):4321–4324. doi: 10.1021/bi00518a013. [DOI] [PubMed] [Google Scholar]