Abstract
The association of early‐onset wheezing with common viral and bacterial infections has raised significant interest in the role of infections in childhood asthma inception. This article serves to review these relationships among infections, host factors, and asthma inception in childhood.
Keywords: asthma, childhood, genetics, infection, inception, virus, wheezing
Asthma is a common chronic respiratory disease, responsible for a significant amount of morbidity and mortality in the Western world. Understanding the origins of asthma inception and risk factors for severity of disease can potentially help elucidate modes of early intervention that could alter the disease course, with an aim at primary prevention. The majority of children with a history of asthma and impaired lung function at school age have a history of recurrent wheezing illnesses in early life 1, 2, 3. Additionally, throughout childhood and beyond, viral illnesses are the most common triggers of asthma exacerbations 4. The timing of early‐onset wheezing with common viral infections has raised significant interest in the role of infections in childhood asthma inception. Furthermore, studies using molecular diagnostics suggest that the airway microbiome may be involved in complex infectious relationships that may be altering the disease course of both chronic asthma and associated exacerbations. Host factors such as allergic sensitization, antiviral immune responses, and environmental exposures are additionally thought to modify these relationships 3, 5, 6, 7, 8. In this article, we review these relationships among infections, host factors, and asthma inception in childhood (Fig. 1).
Figure 1.
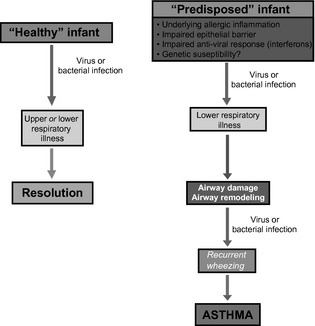
Pathogenesis of asthma inception. Adapted from Jackson et al., 73. This figure highlights relationships among infections, environmental, and host factors in the inception of asthma.
Epidemiology of wheezing and asthma
Asthma, the most common chronic disease of childhood, often presents during the preschool years with wheezing viral respiratory infections. Viral wheezing illnesses during childhood are pervasive, with up to 50% of children having at least one episode of wheezing prior to school age 1. Viral pathogens have been demonstrated in up to 90% of acute wheezing episodes within the first 3 yrs of life 2. Improvements in molecular diagnostic techniques, through the advent of PCR, have enhanced our ability to recognize novel species and types of viruses previously undocumented or underestimated in the wheezing patient (Table 1). While respiratory syncytial virus (RSV) had long been recognized as a cause of lower respiratory tract infections (LRTIs) in infants, particularly during the winter season, human rhinovirus (HRV) has been detected in more than 60% of asthma exacerbations, making it a common culprit throughout the year 9. The previously difficult to culture HRV‐C species has now been identified, with data indicating that this particular virus may be intrinsically more virulent and likely to incite wheezing 10. Parainfluenza, coronavirus, influenza, adenovirus, bocavirus, and human metapneumovirus (hMPV) have also been identified in wheezing children, along with bacteria including non‐typeable haemophilus influenza, streptococcus pneumoniae, moraxella catarrhalis, mycoplasma pneumoniae, and chlamydophila pneumoniae 11, 12, 13, 14, 15.
Table 1.
Etiology of infection with various outcomes
| Outpatient early life wheezing illnesses | Illnesses associated with hospitalization | Illnesses associated with increased asthma risk | |
|---|---|---|---|
| Viruses |
RSV 29 Influenza 40 HBoV 43 hMPV 47 |
Influenza 40 |
|
| Bacteria |
S. pneumonia H. influenza |
Mycoplasma 13, 15 |
S. pneumonia H. influenza Moraxella catarrhalis49 |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Viral infections: the major players
HRV and asthma inception
HRV is the most frequent cause of the common cold and has been identified as a pathogen in both the upper and lower airways 10, 16, 17. HRVs are non‐enveloped, single‐stranded RNA viruses from the Picornaviridae family of viruses. Based upon sequence homology, three distinct species of HRV have been identified: HRV‐A, HRV‐B, and HRV‐C. HRV‐A is composed of >70 types and HRV‐B with >25 types. More recently, a 3rd distinct group of HRV‐C has been identified, with a rapidly expanding number of distinct serotypes. This particular species has proven difficult to grow in traditional culture, making their initial detection elusive. HRV‐C has been identified frequently from patients hospitalized for fever, respiratory illness, and asthma exacerbations 10. Children hospitalized with HRV infection tend to be older with a prior history of wheezing, as well as a personal history of atopy 18. The omnipresent nature of HRV infections throughout the year can be further categorized via species analysis. Data have shown peak incidence of HRV‐C types in the fall, timing that correlates with increased asthma exacerbations and hospitalization rates and with increased HRV‐A detection during the spring 10, 19. There is a relatively low level of HRV‐B burden throughout the year 10. The broad spectrum of illness generated by HRV infections, from asymptomatic to severe respiratory compromise necessitating hospitalization, strongly suggests that host factors modify the impact of infection. As such, allergic sensitization and innate immune response patterns to infection are thought to play a vital role 5, 6, 20.
Recent evidence has pointed to illnesses caused by HRV infection as a strong predictor of asthma development. In a prospective analysis of outcome in relation to asthma, Kotaniemi and colleagues investigated 100 children hospitalized for wheezing during the first 2 yrs of life, performing viral RT‐PCR from frozen nasal aspirates obtained from the index episode of wheezing 21. They found that while prior to 6 months of age, RSV was the most commonly isolated viral pathogen, and from 6 months onward, HRV was most prevalent in wheezing children. The risk for asthma development by school age was 4× greater in the HRV‐infected group than for children hospitalized with other respiratory viral illnesses 21. Kusel et al. demonstrated, in a high‐risk birth cohort, an association between outpatient wheezing with HRV in infancy and persistent wheezing at 5 yrs of age, particularly for children with early allergic sensitization 3. The Childhood Origins of ASThma study (COAST), also a high‐risk birth cohort, identified HRV‐induced wheezing during infancy as the most significant predictor of persistent wheezing at 3 yrs of life and a diagnosis of asthma at age 6 yrs 2, 6, 12. These associations were strongest for those with a history of early aeroallergen sensitization 2.
RSV and asthma
Respiratory syncytial virus, a negative‐stranded, enveloped, RNA virus of the Paramyxoviridae family, has long been recognized as an important viral pathogen during the winter months, frequently leading to early life wheezing. While RSV is not a predominant cause of asthma exacerbations in the older child or adult, debate has continued on the causal relationship to the subsequent development of asthma 22, 23, 24, 25, 26, 27. Several studies have demonstrated that RSV‐related severe LRTIs, particularly those requiring hospitalization, are associated with an increased risk of asthma diagnosis at school age 28, 29. A prospective cohort study conducted in Sweden by Sigurs and associates 28, 30 used a case–control design to compare 47 hospitalized infants with documented RSV infection in infancy to 97 healthy controls, measuring rates of asthma and allergic sensitization over time. By the 18‐yr follow‐up, they found a higher percentage of both current asthma diagnosis (39% vs. 9%, p < 0.001) and allergic sensitization (41% vs. 14%, p = 0.005) in the RSV group than in the control 30. Using a multivariate analysis approach, RSV infection was found to be the major risk factor in subsequent asthma development (OR 7.2). They concluded that severe early RSV bronchiolitis was associated with increased prevalence and persistence of allergic asthma into early adulthood. The Tucson Children's Respiratory Study (TCRS) reported persisting respiratory symptoms (wheeze) through early adolescence in subjects who experienced outpatient RSV LRTI during the first 3 yrs of life. Interestingly, the association to wheeze diminished with age and lost significance by 13 yrs of age 29. The Tennessee Asthma Bronchiolitis Study (TABS), a large unselected population‐based retrospective study of children identified through a Medicaid database, investigated the relationship between winter virus infection and asthma 31, 32. They found that the risk of asthma by age 5 increased with the timing of infant birth in relation to the peak of the winter virus season, with subjects born 120 days prior to winter virus peak having the highest risk 31.
These findings led to speculation that prevention of RSV infection could potentially reduce asthma outcomes. Simoes and colleagues compared a group of premature infants treated with palivizumab, an anti‐RSV monoclonal antibody, to an untreated aged‐matched cohort. They found on prospective follow‐up that the incidence of recurrent wheezing was significantly lower in the palivizumab‐treated group than for the untreated controls, suggesting that prevention of LRTI with RSV could reduce the development of persistent wheezing 33. A recent randomized controlled trial by Blanken et al. showed that otherwise healthy premature infants treated with palivizumab had a relative reduction of 61% (95% CI 56–65) in the number of wheezing days within the first year of life when compared with controls that received a placebo injection during the RSV season. Perhaps even more encouraging was the demonstration of the extended protective benefit from palivizumab, noting a persistent reduction in total wheezing days and respiratory‐related hospitalizations >2 months out from the last palivizumab injection 34. Whether these findings will translate to asthma prevention is not known and of interest moving forward.
Conversely, there are several studies that argue against the causality of RSV in asthma inception. Poorisrisak and colleagues looked at 37 pairs of monozygotic twins, differing with respect to hospitalization for RSV bronchiolitis during infancy, and found no difference with regard to subsequent development of asthma or allergic sensitization by 7 yrs of age within the twin pairs 35. While definitive conclusions are limited due to the small sample size of the study, the results argued against the role of severe RSV infection in the development of asthma, as earlier studies had implied. Similarly, a larger twin‐based study, conducted in Denmark by Thomsen and associates, identified an association between severe RSV illness and asthma, but not a causal relationship 36.
Other viruses
Influenza, an RNA virus in the Orthomyxoviridae family, is responsible for a significant amount of respiratory‐related illness in both children and adults; however, its effect specifically within the asthma population has been debated37. Studies have demonstrated significant morbidity associated with influenza infection for patients with underlying chronic conditions (such as asthma) when compared with healthy controls 38. A large prospective study of children 6 months to 59 months of age looked at rates of influenza‐attributable outpatient visits and hospitalizations for children with asthma, and compared this with otherwise healthy children over a 5‐yr period of time 39. They found that the average influenza‐associated hospitalization rate was 4× greater, with a twofold increase in outpatient visits, for the asthma group, than for the non‐asthmatic children 39. However, prospective studies looking to distinguish influenza's role in the setting of acute asthma exacerbations have failed to show a strong association 27.
Human bocavirus (HBoV), a member of the Parvoviridae family, is a relatively newly identified virus, first recognized in 2005 with the aid of molecular sequencing 40. It primarily infects the respiratory tract, and while the virulence in causing symptomatic respiratory symptoms has been debated, data have shown a high degree of co‐infection with other viral pathogens, namely HRV, RSV, and adenovirus, particularly in children under age 2 yrs 41, 42. These data suggest that its role in early life LRTI may be underappreciated; however, further investigation is needed.
Human metapneumovirus (hMPV), a Paramyxovirus closely related to RSV, has been linked to both asthma inception and exacerbations within the pediatric and adult populations 24, 43, 44, 45, 46, 47. A study conducted by Garcia‐Garcia and associates investigated the relationship between hMPV bronchiolitis and the subsequent development of wheezing. They compared this outcome, at ages 3 and 5 yrs, for 55 children who were hospitalized for either hMPV‐related bronchiolitis or RSV bronchiolitis during the first 2 yrs of life. They found, in a multivariate analysis, that hMPV was the most important risk factor for the development of asthma by the preschool years (OR = 15.9) 43. Additionally, data published by Williams et al. found a significant association between hMPV and wheezing among children less than 3 yrs of age; however, the relationship lost significance in children 3 yrs and older, indicating that hMPV may play an important role during early life, a critical period of lung growth and development 47.
Bacteria and the airway microbiome
Advances in molecular diagnostics have expanded our knowledge of the diversity of the human microbiome and its relation to human diseases. With improved ability to recognize and identify a growing number of bacteria within the airway, the natural progression to investigate their potential role in chronic pulmonary diseases has come to the forefront of focus. Bisgaard et al. published data from a large birth cohort study of high‐risk subjects identified at birth by maternal history of asthma, that noted positive associations between bacterial colonization with streptococcus pneumoniae, haemophilus influenzae, and moraxella catarrhalis (or a combination of these organisms) in the hypopharynx of asymptomatic neonates, and the subsequent development of asthma (prevalence in colonized 33%, not colonized 10%, OR 4.57) within the first 5 yrs of life 48. The association was found to be ‘time specific’, that is, present only for the infants colonized by 1 month but not at 12 months of age 48. A second study, performed on the same high‐risk population, looked at the frequency of bacteria and virus present in airway aspirates during acute wheezing illness. 49 They found that wheezing was significantly associated with bacterial infections (OR 2.9, p < 0.001), primarily streptococcus pneumoniae, haemophilus influenzae, and moraxella catarrhalis, and this relationship was similar but distinct from the association with viral infections (OR 2.8, p < 0.001) 49. Similar findings were noted by De Shutter et al., who retrospectively analyzed a population of recurrent wheezing children between the ages of 4 and 48 months, who underwent bronchoscopy and BAL after failing to respond to inhaled corticosteroids 50. A total of 48% of subjects had significant bacterial BAL cultures, with non‐typeable haemophilus influenza most commonly, followed by streptococcus pneumoniae and moraxella catarrhalis, respectively 50.
Progression from early life wheezing to asthma
Inflammation in preschool children
Krawiec and colleagues performed bronchoscopy and bronchoalveolar lavage on 20 wheezing children (median age 15 months) with recurrent/persistent wheezing. When compared with normal controls, they found that the wheezing children had 3 times the total BAL cells. The cellular increase was non‐specific, unlike the eosinophilic predominance seen in many older children and adults with asthma 51, 52. They additionally found increased levels of inflammatory mediators PGE2, LTE4, and LTB4, suggesting that ongoing inflammation is present in the airways of young wheezers 52. Saglani and colleagues performed endobronchial biopsies on both wheezing subjects (aged 3 months to 5 yrs) and age‐matched controls 53. Unlike the pathogenic hallmarks of asthma established for older children and adults, they found no evidence of eosinophilic inflammation nor reticular basement membrane thickening in the infant wheezers (median age 12 months), even in the presence of atopy 54, 55. However, these pathologic markers were noted in slightly older wheezing children (median age 29 months), suggesting that the time between 1 and 3 yrs of age is a critical period for asthmatic airway remodeling and that changes are not a congenital phenomenon, but more likely a consequence of environmental exposures and disease development over time 53.
Symptoms and lung function changes in early life
Guilbert and colleagues followed a cohort of preschool aged children at high risk of asthma and found that for children who did not outgrow their wheezing, their symptom burden progressively increased through their preschool years 56. Those early wheezers with persistent disease have additionally been shown to have diminished lung function by school age. Within the TCRS cohort, children with persistent wheezing beginning prior to age 3 had reductions in lung function at age 6 yrs that persisted over time 57. Similarly, the COAST study demonstrated an association between recurrent severe exacerbations in early life that required OCS and reduced lung function at school age 58. The persistent and progressive nature of disease course noted in these studies suggests that early life remodeling of the airway may be occurring.
Host factors and asthma development
Immunologic factors
Whether infection leads to asthma vs. the counter argument of an underlying predisposition to asthma and early life wheezing illnesses has long been debated, with the search for explanatory mechanisms a goal for many research groups. Multiple cohort studies have identified impaired IFN‐γ responses at birth or infancy with recurrent wheezing in early life, but these relationships have not generally persisted to childhood asthma risk 5, 7, 59. Sensitization to aeroallergens is a clear risk factor for asthma development, with recent data suggesting that children who develop sensitization to multiple allergens in early life are at particularly high risk of asthma inception and severe exacerbations. 60, 61, In fact, a sequential developmental relationship with allergic sensitization leading to HRV‐induced wheezing, and subsequent asthma, has recently been identified 6. This leads one to question: What mechanisms link allergic sensitization to HRV‐induced wheezing and asthma?
One potential mechanism involves impairment of antiviral immunity by allergic sensitization and exposure. Children and adults with allergic asthma have been reported to have reductions in peripheral blood mononuclear cell production of types I and III interferons in response to influenza and HRV infection 62, 63. These abnormalities are associated with expression of the high‐affinity IgE receptor (FcεRI) on plasmacytoid dendritic cells (pDCs) and are accentuated by FcεRI cross‐linking. Additionally, epithelial cell antiviral responses have been reported to be abnormal in patients with allergic asthma 8, 64, although not all groups have been able to replicate these findings 65.
Other potential pathways that may enhance HRV susceptibility in allergic individuals involve the effects of allergic inflammation on the airway leading to enhanced airway responsiveness, impaired barrier function, and increased mucous secretion 66. It has also been recently proposed that allergic asthmatics may have an impaired ability to ‘shut‐off’ HRV‐induced inflammation 67. Finally, HRV can directly lead to a number of airway changes critical to asthma development. Leigh and colleagues looked at the response of airway epithelial cells following infection with HRV and noted an overall up‐regulation of several growth factors important in airway remodeling including amphiregulin, activin A, and VEGF 68. Zhu and associates found HRV infection triggered mucin production via toll‐like receptor 3 and also caused up‐regulation of epidermal growth factor receptor, providing mechanisms for mucus plugging and epithelial remodeling 69.
Genetic susceptibility to HRV infections
Genetic mutations in a variety of single‐nucleotide polymorphisms (SNPs) related to innate signaling responses have been linked to asthma, leading to further interest in the complex relationship between a gene‐by‐environment interaction in asthma inception. Variation at the 17q21 locus is the most replicated asthma susceptibility region of the genome, but is not associated with the development of allergic sensitization 70, 71, 72. Caliskan and colleagues recently reported that the increased risk of asthma in the at‐risk ‘TT’ genotype at SNP rs7216389 was seen only in children who wheezed with HRV infections in early life 70. The mechanisms underlying these observations are unknown and of clear interest for further investigation.
Conclusions and future directions
Both viral and bacterial infections in early life cause a significant amount of morbidity, with potentially lifelong implications. Their identification during wheezing illnesses and their subsequent presence during exacerbations once disease has been established suggest an import role in airway inflammatory responses, which may lead to the development of various asthma phenotypes. The implications for proving a causal relationship could open new doors for asthma treatment and prevention through the use of vaccination and direct antimicrobials, or indirect means of enhancing the immune response in high‐risk individuals. Studies focusing on early prevention, such as the case with palivizumab and RSV, are encouraging in demonstrating that early intervention may modify subsequent risk and morbidity from recurrent wheezing illnesses. These findings foreshadow the potential therapeutic and preventative role for strategies against other respiratory pathogens, notably HRV. The relationships among infections, host responses, and asthma inception remain complex and an important area for future study.
Funding
Supported by NIH Grants: P01 HL70831, 5U10HL098090 and by the Clinical and Translational Science Award program through the National Institutes of Health National Center for Advancing Translational Sciences Grant UL1TR000427.
Thomas AO, Lemanske RF Jr, Jackson DJ. Infections and their role in childhood asthma inception. Pediatr Allergy Immunol 2014: 25: 122–128.
References
- 1. Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. N Engl J Med 1995: 332: 133–8. [DOI] [PubMed] [Google Scholar]
- 2. Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high‐risk children. Am J Respir Crit Care Med 2008: 178: 667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kusel MM, de Klerk NH, Kebadze T, et al. Early‐life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol 2007: 119: 1105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnston SL, Pattemore PK, Sanderson G, et al. The relationship between upper respiratory infections and hospital admissions for asthma: a time‐trend analysis. Am J Respir Crit Care Med 1996: 154: 654–60. [DOI] [PubMed] [Google Scholar]
- 5. Gern JE, Brooks GD, Meyer P, et al. Bidirectional interactions between viral respiratory illnesses and cytokine responses in the first year of life. J Allergy Clin Immunol 2006: 117: 72–8. [DOI] [PubMed] [Google Scholar]
- 6. Jackson DJ, Evans MD, Gangnon RE, et al. Evidence for a causal relationship between allergic sensitization and rhinovirus wheezing in early life. Am J Respir Crit Care Med 2012: 185: 281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stern DA, Guerra S, Halonen M, Wright AL, Martinez FD. Low IFN‐gamma production in the first year of life as a predictor of wheeze during childhood. J Allergy Clin Immunol 2007: 120: 835–41. [DOI] [PubMed] [Google Scholar]
- 8. Wark PA, Johnston SL, Bucchieri F, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med 2005: 201: 937–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johnston SL, Pattemore PK, Sanderson G, et al. Community study of role of viral infections in exacerbations of asthma in 9‐11 year old children. BMJ 1995: 310: 1225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miller EK, Edwards KM, Weinberg GA, et al. A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol 2009: 123: 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnston SL, Martin RJ. Chlamydophila pneumoniae and Mycoplasma pneumoniae: a role in asthma pathogenesis? Am J Respir Crit Care Med 2005: 172: 1078–89. [DOI] [PubMed] [Google Scholar]
- 12. Lemanske RF Jr, Jackson DJ, Gangnon RE, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol 2005: 116: 571–7. [DOI] [PubMed] [Google Scholar]
- 13. Lieberman D, Printz S, Ben Yaakov M, et al. Atypical pathogen infection in adults with acute exacerbation of bronchial asthma. Am J Respir Crit Care Med 2003: 167: 406–10. [DOI] [PubMed] [Google Scholar]
- 14. Rosenthal LA, Avila PC, Heymann PW, et al. Viral respiratory tract infections and asthma: the course ahead. J Allergy Clin Immunol 2010: 125: 1212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bezerra PG, Britto MC, Correia JB, et al. Viral and atypical bacterial detection in acute respiratory infection in children under five years. PLoS ONE 2011: 6: e18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Papadopoulos NG, Bates PJ, Bardin PG, et al. Rhinoviruses infect the lower airways. J Infect Dis 2000: 181: 1875–84. [DOI] [PubMed] [Google Scholar]
- 17. Miller EK, Lu X, Erdman DD, et al. Rhinovirus‐associated hospitalizations in young children. J Infect Dis 2007: 195: 773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jartti T, Lehtinen P, Vuorinen T, Ruuskanen O. Bronchiolitis: age and previous wheezing episodes are linked to viral etiology and atopic characteristics. Pediatr Infect Dis J 2009: 28: 311–7. [DOI] [PubMed] [Google Scholar]
- 19. Sears MR, Johnston NW. Understanding the September asthma epidemic. J Allergy Clin Immunol 2007: 120: 526–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heymann PW, Platts‐Mills TA, Johnston SL. Role of viral infections, atopy and antiviral immunity in the etiology of wheezing exacerbations among children and young adults. Pediatr Infect Dis J 2005: 24: S217–22, discussion. [DOI] [PubMed] [Google Scholar]
- 21. Kotaniemi‐Syrjanen A, Vainionpaa R, Reijonen TM, Waris M, Korhonen K, Korppi M. Rhinovirus‐induced wheezing in infancy–the first sign of childhood asthma? J Allergy Clin Immunol 2003: 111: 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Henderson J, Hilliard TN, Sherriff A, Stalker D, Al Shammari N, Thomas HM. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: a longitudinal birth cohort study. Pediatr Allergy Immunol 2005: 16: 386–92. [DOI] [PubMed] [Google Scholar]
- 23. Lacaze‐Masmonteil T, Roze JC, Fauroux B. Incidence of respiratory syncytial virus‐related hospitalizations in high‐risk children: follow‐up of a national cohort of infants treated with Palivizumab as RSV prophylaxis. Pediatr Pulmonol 2002: 34: 181–8. [DOI] [PubMed] [Google Scholar]
- 24. Manoha C, Espinosa S, Aho SL, Huet F, Pothier P. Epidemiological and clinical features of hMPV, RSV and RVs infections in young children. J Clin Virol 2007: 38: 221–6. [DOI] [PubMed] [Google Scholar]
- 25. Schauer U, Hoffjan S, Bittscheidt J, et al. RSV bronchiolitis and risk of wheeze and allergic sensitisation in the first year of life. Eur Respir J 2002: 20: 1277–83. [DOI] [PubMed] [Google Scholar]
- 26. Smyth RL, Openshaw PJ. Bronchiolitis. Lancet 2006: 368: 312–22. [DOI] [PubMed] [Google Scholar]
- 27. Khetsuriani N, Kazerouni NN, Erdman DD, et al. Prevalence of viral respiratory tract infections in children with asthma. J Allergy Clin Immunol 2007: 119: 314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sigurs N, Gustafsson PM, Bjarnason R, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med 2005: 171: 137–41. [DOI] [PubMed] [Google Scholar]
- 29. Stein RT, Sherrill D, Morgan WJ, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 1999: 354: 541–5. [DOI] [PubMed] [Google Scholar]
- 30. Sigurs N, Aljassim F, Kjellman B, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 2010: 65: 1045–52. [DOI] [PubMed] [Google Scholar]
- 31. Wu P, Dupont WD, Griffin MR, et al. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med 2008: 178: 1123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carroll KN, Wu P, Gebretsadik T, et al. The severity‐dependent relationship of infant bronchiolitis on the risk and morbidity of early childhood asthma. J Allergy Clin Immunol 2009: 123: 1055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Simoes EA, Groothuis JR, Carbonell‐Estrany X, et al. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr 2007: 151: 42. [DOI] [PubMed] [Google Scholar]
- 34. Blanken MO, Rovers MM, Molenaar JM, et al. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med 2013: 368: 1791–9. [DOI] [PubMed] [Google Scholar]
- 35. Poorisrisak P, Halkjaer LB, Thomsen SF, et al. Causal direction between respiratory syncytial virus bronchiolitis and asthma studied in monozygotic twins. Chest 2010: 138: 338–44. [DOI] [PubMed] [Google Scholar]
- 36. Thomsen SF, van der Sluis S, Stensballe LG, et al. Exploring the association between severe respiratory syncytial virus infection and asthma: a registry‐based twin study. Am J Respir Crit Care Med 2009: 179: 1091–7. [DOI] [PubMed] [Google Scholar]
- 37. Glezen WP. Asthma, influenza, and vaccination. J Allergy Clin Immunol 2006: 118: 1199–206. [DOI] [PubMed] [Google Scholar]
- 38. Glezen WP, Greenberg SB, Atmar RL, Piedra PA, Couch RB. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA 2000: 283: 499–505. [DOI] [PubMed] [Google Scholar]
- 39. Miller EK, Griffin MR, Edwards KM, et al. Influenza burden for children with asthma. Pediatrics 2008: 121: 1–8. [DOI] [PubMed] [Google Scholar]
- 40. Arden KE, McErlean P, Nissen MD, Sloots TP, Mackay IM. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J Med Virol 2006: 78: 1232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Allander T. Human bocavirus. J Clin Virol 2008: 41: 29–33. [DOI] [PubMed] [Google Scholar]
- 42. Allander T, Jartti T, Gupta S, et al. Human bocavirus and acute wheezing in children. Clin Infect Dis 2007: 44: 904–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Garcia‐Garcia ML, Calvo C, Casas I, et al. Human metapneumovirus bronchiolitis in infancy is an important risk factor for asthma at age 5. Pediatr Pulmonol 2007: 42: 458–64. [DOI] [PubMed] [Google Scholar]
- 44. Williams JV. Human metapneumovirus: an important cause of respiratory disease in children and adults. Curr Infect Dis Rep 2005: 7: 204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Williams JV, Crowe JE Jr, Enriquez R, et al. Human metapneumovirus infection plays an etiologic role in acute asthma exacerbations requiring hospitalization in adults. J Infect Dis 2005: 192: 1149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Williams JV, Harris PA, Tollefson SJ, et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med 2004: 350: 443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Williams JV, Tollefson SJ, Heymann PW, Carper HT, Patrie J, Crowe JE. Human metapneumovirus infection in children hospitalized for wheezing. J Allergy Clin Immunol 2005: 115: 1311–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bisgaard H, Hermansen MN, Buchvald F, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med 2007: 357: 1487–95. [DOI] [PubMed] [Google Scholar]
- 49. Bisgaard H, Hermansen MN, Bonnelykke K, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ 2010: 341: c4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. De Schutter I, Dreesman A, Soetens O, et al. In young children, persistent wheezing is associated with bronchial bacterial infection: a retrospective analysis. BMC Pediatr 2012: 12: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ferguson AC, Whitelaw M, Brown H. Correlation of bronchial eosinophil and mast cell activation with bronchial hyperresponsiveness in children with asthma. J Allergy Clin Immunol 1992: 90: 609–13. [DOI] [PubMed] [Google Scholar]
- 52. Krawiec ME, Westcott JY, Chu HW, et al. Persistent wheezing in very young children is associated with lower respiratory inflammation. Am J Respir Crit Care Med 2001: 163: 1338–43. [DOI] [PubMed] [Google Scholar]
- 53. Saglani S, Payne DN, Zhu J, et al. Early detection of airway wall remodeling and eosinophilic inflammation in preschool wheezers. Am J Respir Crit Care Med 2007: 176: 858–64. [DOI] [PubMed] [Google Scholar]
- 54. Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med 2001: 164: S28–38. [DOI] [PubMed] [Google Scholar]
- 55. Saglani S, Malmstrom K, Pelkonen AS, et al. Airway remodeling and inflammation in symptomatic infants with reversible airflow obstruction. Am J Respir Crit Care Med 2005: 171: 722–7. [DOI] [PubMed] [Google Scholar]
- 56. Guilbert TW, Morgan WJ, Zeiger RS, et al. Long‐term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med 2006: 354: 1985–97. [DOI] [PubMed] [Google Scholar]
- 57. Morgan WJ, Stern DA, Sherrill DL, et al. Outcome of asthma and wheezing in the first 6 years of life: follow‐up through adolescence. Am J Respir Crit Care Med 2005: 172: 1253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. O'Brian AL, Lemanske RF Jr, Evans MD, Gangnon RE, Gern JE, Jackson DJ. Recurrent severe exacerbations in early life and reduced lung function at school age. J Allergy Clin Immunol 2012: 129: 1162–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Copenhaver CC, Gern JE, Li Z, et al. Cytokine response patterns, exposure to viruses, and respiratory infections in the first year of life. Am J Respir Crit Care Med 2004: 170: 175–80. [DOI] [PubMed] [Google Scholar]
- 60. Simpson A, Tan VY, Winn J, et al. Beyond atopy: multiple patterns of sensitization in relation to asthma in a birth cohort study. Am J Respir Crit Care Med 2010: 181: 1200–6. [DOI] [PubMed] [Google Scholar]
- 61. Stoltz DJ, Jackson DJ, Evans MD, et al. Specific patterns of allergic sensitization in early childhood and asthma & rhinitis risk. Clin Exp Allergy 2013: 43: 233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Durrani SR, Montville DJ, Pratt AS, et al. Innate immune responses to rhinovirus are reduced by the high‐affinity IgE receptor in allergic asthmatic children. J Allergy Clin Immunol 2012: 130: 489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gill MA, Bajwa G, George TA, et al. Counterregulation between the FcepsilonRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol 2010: 184: 5999–6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Contoli M, Caramori G, Mallia P, Johnston S, Papi A. Mechanisms of respiratory virus‐induced asthma exacerbations. Clin Exp Allergy 2005: 35: 137–45. [DOI] [PubMed] [Google Scholar]
- 65. Bochkov YA, Hanson KM, Keles S, Brockman‐Schneider RA, Jarjour NN, Gern JE. Rhinovirus‐induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal Immunol 2010: 3: 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kloepfer KM, Gern JE. Virus/allergen interactions and exacerbations of asthma. Immunol Allergy Clin North Am 2010: 30: 553–63, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Subrata LS, Bizzintino J, Mamessier E, et al. Interactions between innate antiviral and atopic immunoinflammatory pathways precipitate and sustain asthma exacerbations in children. J Immunol 2009: 183: 2793–800. [DOI] [PubMed] [Google Scholar]
- 68. Leigh R, Oyelusi W, Wiehler S, et al. Human rhinovirus infection enhances airway epithelial cell production of growth factors involved in airway remodeling. J Allergy Clin Immunol 2008: 121: 1238–45. [DOI] [PubMed] [Google Scholar]
- 69. Zhu L, Lee PK, Lee WM, Zhao Y, Yu D, Chen Y. Rhinovirus‐induced major airway mucin production involves a novel TLR3‐EGFR‐dependent pathway. Am J Respir Cell Mol Biol 2009: 40: 610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Caliskan M, Bochkov YA, Kreiner‐Moller E, et al. Rhinovirus wheezing illness and genetic risk of childhood‐onset asthma. N Engl J Med 2013: 368: 1398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Moffatt MF, Gut IG, Demenais F, et al. A large‐scale, consortium‐based genomewide association study of asthma. N Engl J Med 2010: 363: 1211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Halapi E, Gudbjartsson DF, Jonsdottir GM, et al. A sequence variant on 17q21 is associated with age at onset and severity of asthma. Eur J Hum Genet 2010: 18: 902–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jackson DJ, Lemanske RF Jr. The role of respiratory infections in childhood asthma inception. Immunol Allergy Clin North Am 2010: 30: 513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]


