Significance
The developing brain is highly sensitive to ionizing radiation and DNA damage. Here we report that tumor suppressor breast cancer susceptibility gene 1 (BRCA1) plays a novel role in regulating the embryonic brain development and postnatal brain size. We found that loss of BRCA1 induces p53-dependent proapoptotic pathways in the CNS. BRCA1 possibly functions as a centrosomal factor in establishing the cellular polarity of the neural progenitors through the DNA damage sensor kinase ATM. Our data provide new insight in understanding the control of DNA damage sensitivity and brain size during development and evolution.
Abstract
Breast cancer susceptibility gene 1 (BRCA1) is a breast and ovarian cancer tumor suppressor whose loss leads to DNA damage and defective centrosome functions. Despite its tumor suppression functions, BRCA1 is most highly expressed in the embryonic neuroepithelium when the neural progenitors are highly proliferative. To determine its functional significance, we deleted BRCA1 in the developing brain using a neural progenitor–specific driver. The phenotype is characterized by severe agenesis of multiple laminated cerebral structures affecting most notably the neocortex, hippocampus, cerebellum, and olfactory bulbs. Major phenotypes are caused by excess apoptosis, as these could be significantly suppressed by the concomitant deletion of p53. Certain phenotypes attributable to centrosomal and cell polarity functions could not be rescued by p53 deletion. A double KO with the DNA damage sensor kinase ATM was able to rescue BRCA1 loss to a greater extent than p53. Our results suggest distinct apoptotic and centrosomal functions of BRCA1 in neural progenitors, with important implications to understand the sensitivity of the embryonic brain to DNA damage, as well as the developmental regulation of brain size.
The brain is the third most sensitive tissue to ionizing radiation (IR) as has been shown in victims of acute radiation exposure (1). Embryonic and adult neural stem cells (NSCs) are susceptible to DNA damage much more than postmitotic neurons (2). This susceptibility is most likely due to the high proliferative rate characteristic of NSCs. IR interferes with the embryonic development of many brain structures, such as the neocortex (3), cerebellum (4), and hippocampus (5). It has been shown that IR induces DNA double-strand breaks, leading to microcephaly and mental retardation via activation of p53-dependent apoptosis (3). The central orchestrator of cellular responses appears to be the ataxia-telangiectasia mutated (ATM) kinase that senses the DNA damage and ultimately leads to the execution of the proapoptotic program by p53 (6). It has been reported that the breast cancer susceptibility gene 1 (BRCA1), originally identified as a hereditary breast and ovarian cancer tumor suppressor (7), is highly expressed in embryonic neuroepithelium and adult neurogenic areas, which correspond to the niches of NSCs (8, 9). BRCA1 is ubiquitously expressed with a particular enrichment in the nuclei of proliferative cells (8).
Given the ascribed functions of BRCA1 related to DNA damage response checkpoints, we hypothesized that the enrichment of BRCA1 in the NSC niches might not be an epiphenomenon but may be of functional significance. The embryonic sensitivity to IR coincides with the active proliferation in embryos and parallels the highest levels of expression of embryonic BRCA1 (9). In addition to the DNA damage response, BRCA1 has also been shown to be required for centrosome and mitotic spindle functions (10). Cells devoid of BRCA1 undergo centrosome amplification and lose checkpoints in G2 (11). Consequently, BRCA1-deficient cells accumulate cytogenetic aberrations, such as chromosomal translocations and display aneuploidy (11). By deleting BRCA1 in the central nervous system, we observed widespread neuroanatomical abnormalities in the mutant animals, including severe proliferative defects accompanied with excessive apoptosis, leading to large cellular population losses in all laminated structures in the brain, including the neocortex, cerebellum, hippocampus, and olfactory bulb. These cellular losses can be rescued by the concomitant introduction of the p53 or ATM mutations that recover the apoptosis-related phenotype due to the loss of BRCA1 such as cell density and brain volume. However, the centrosome-associated phenotypes such as cell polarity are not rescued, indicating that it is possible to dissect DNA damage checkpoint-related functions of BRCA1 in the brain from those related to spindle and centrosomal defects.
Results
Widespread Deficiencies Associated with the BRCA1 Deletion from Neural Progenitors.
To confirm the expression pattern of BRCA1 in the embryonic brain (8, 12), we performed in situ hybridization (ISH) experiments at E12.5 to establish the localization of the BRCA1 mRNA (Fig. S1A). Peak levels of BRCA1 transcripts are mostly observed in the upper part of the ventricular zone (VZ), whereas the deeper areas present lower expression levels (Fig. S1A).
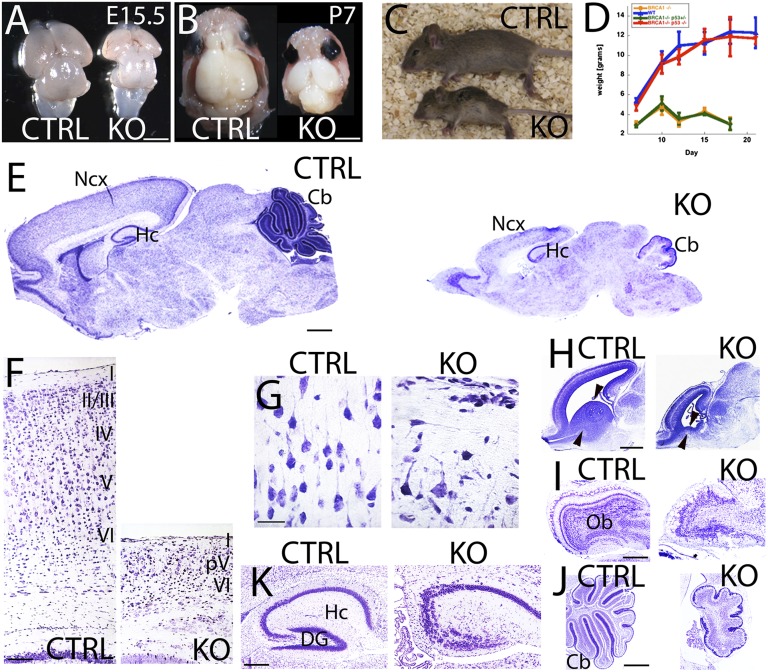
To study the role of BRCA1 in neuronal progenitors, we previously generated a mouse line that harbors a conditional null allele of BRCA1 that deletes exons 5–13 of BRCA1 (13) over a BRCA1-null allele (14). As a driver, we used the neural stem cell restricted Cre that contains a fragment of the mouse Nestin promoter with a rat neural specific enhancer (15). The onset of recombination for this Cre driver in telencephalic structures is at E11.5 (16). Recombination in CNS tissue was confirmed by PCR (Fig. S1 B and C). The BRCA1 KO animals show a slight reduction in brain volume mostly localized to the neocortex at E15.5 (Fig. 1A). However, at postnatal stages (P7), the overall brain volume is severely reduced, and it is particularly apparent in the neocortex, cerebellum, and olfactory bulbs (Fig. 1B). Presumably, this explains why the BRCA1 KO animals are ataxic and exhibit excess agitation and arousal on separation from the mother (Movie S1). BRCA1 KO animals are also smaller, and this difference becomes more pronounced with time (Fig. 1 C and D) up to their death at P19 (Fig. 1D).
Fig. 1.
Embryonic BRCA1 deletion from Nestin progenitors severely disrupts brain development. (A and B) Dorsal views of BRCA1 KO (Brca11+/−;Brca5-13cK+/−;Nestin-Cre+) and control (Brca11+/+;Brca5-13cK+/−;Nestin-Cre+, CTRL) brains at E15.5 (A) and P7 (B). (C) BRCA1 KO brains show smaller sizes and failure to thrive than their CTRL littermates. (D) Body weight curve at 2 weeks old showing one third of reduction in body size of BRCA1 KO compared with their littermates in the absence of food competition. (E–G) Nissl staining of BRCA1 KO and CTRL brains at P7. (E) Gross developmental defects are observed in most of the laminated structures in the BRCA1 KO brain. (F) Upper layers (II–IV) are not developed; layer V is only partially (pV) developed, and layer VI is poorly develop in the BRCA1 KO neocortex (Ncx). (G) Pyramidal neurons in the BRCA1 KO neocortex lack of any polarity and radial processes than CTRL. (H) Nissl staining of BRCA1 KO and CTRL brains at E15.5. Arrowheads point to the ganglionic eminence. (I–K) Nissl staining of BRCA1 KO and CTRL at P7 (I and K) and P21 (J). (I) Lamination defects are observed in the olfactory bulb (Ob) of the BRCA1 KO. (J) Reduced volume, lack of foliation, and lamination are present in the cerebellum (Cb) of the BRCA1 KO. (K) CA2-CA3 lamination is disorganized with agenesis of the dentate gyrus (DG) in the hippocampus (Hc) of the BRCA1 KO. I–VI, cortical layers 1–6. (Scale bars: A, B, and E, 1 mm; F, 100 μm; G, 50 μm; H, 200 μm; I and J, 500 μm; K, 100 μm.) Also see Figs. S2–S5.
A closer examination of the BRCA1 KO brains revealed severe defects in all of the laminated structures involved in cognition and motor learning, such as the neocortex, hippocampus, and cerebellum. The ganglionic eminences, which are the main source of GABAergic interneurons of the olfactory bulbs and spinal cord, were nearly absent at E15.5 (Fig. 1H).
The olfactory bulbs present a severe disorganized lamination most notable in the mitral cell layer (Fig. 1I and Fig. S4 C and D). The hippocampus presents a severe disorganization of the CA2-3 fields and dentate gyrus. In particular, the suprapyramidal blade of the dentate gyrus is absent (Fig. 1K and Fig. S4 E and F). In concordance with the absence of ganglionic eminences, we also see a severe reduction in Reelin-positive interneurons in the hippocampus (Fig. S4 E and F). In addition, the choroid plexus is hypertrophic from early embryonic stages on, suggesting some defects in the release of cerebrospinal fluid (Fig. 1H and Fig. S2 A–D).
To delineate the complex phenotype of the BRCA1 KO brains, we performed gene expression microarray analysis to help guide subsequent experiments (Tables S1–S5). Major features of differentially expressed genes suggest functions in neurogenesis, cortical differentiation, oligodendrocyte functions, oxidative stress, proliferation, interneuron function, extracellular matrix functions, and imprinting. Given these results and the observed histology, we decided to focus on the cortical differentiation, neurogenic, and interneuronal phenotypes that were the most striking from both datasets.
BRCA1 Deletion in Neural Progenitors Directly Impacts the Development of Centers of Cognition and Motor Learning in the Brain.
The mammalian neocortex is a laminated structure that consists of six layers. The cortical layers are generated in a well-controlled inside-out gradient with early born neurons located in deep layers (V–VI) and late-born neurons occupying upper layers (II–IV) (17–19). The neocortex is one of the most affected structures in the BRCA1 KO animals (Fig. 1E). Consistent with a neurogenic deficiency, the proliferative marker Ngn2, which marks future cortical neurons, is notably reduced in the VZ at E15.5 in the BRCA1 KO (Fig. S2 C and D).
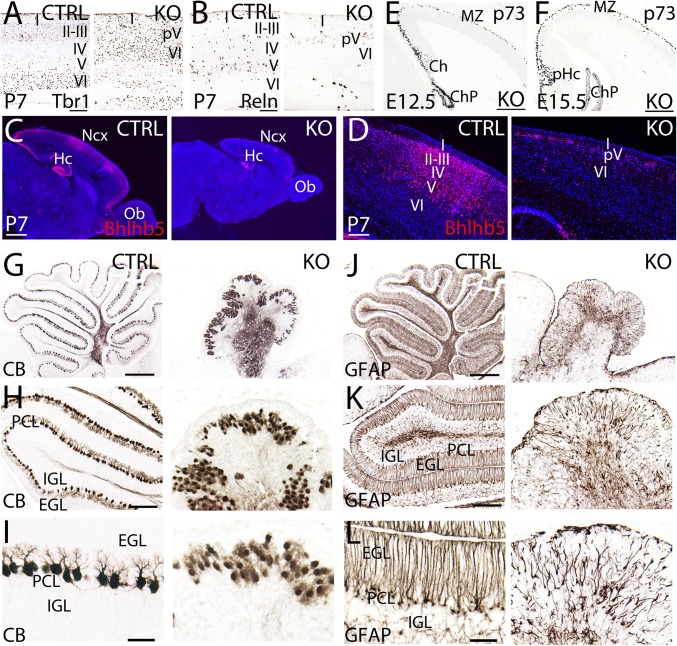
To characterize the laminar deficiencies of the BRCA1 KO cortex, we used a series of layer-specific markers to identify the surviving neurons at P7. The BRCA1 KO cortical lamination is severely disorganized as shown by the complete absence of upper layers (II–IV) and only some scattered neurons from layer V (Fig. 1F). Layer VI as shown by Tbr1 staining is formed but reduced and disorganized compared with controls (Fig. 2A). Layer V develops after layer VI and is the last layer that we were able to observe in the BRCA1 KO (Fig. 2 B and D). Reelin-immunoreactive layer V neurons are severely depleted in the BRCA1 KO (Fig. 2B), suggesting that the neuronal committed progenitor program was disrupted after initiation of layer V progenitor generation. Upper layer neurons (II–IV) are absent as observed by using the marker Bhlhb5 (labels layers II–V; Fig. 2 C and D). This observation implies that the progenitors destined to become upper layers were never generated or died before initiation of neuronal differentiation. Staining with both layer V markers (Bhlhb5 and Reelin) gave inconsistent patterns also consistent with an incomplete presence of layer V across the cortex in the BRCA1 KO (Fig. 2 B–D). Similarly, long pulses of BrdU (E15 injected, P8 harvested) also show (Fig. S3) a depleted BrdU+/Bhlhb5+ variegated pattern at P8 (cf. Fig. S3 H and J with Fig. S3 C and E). This pattern suggests that structures generated after E13.5 (time of generation of layer V neurons) are absent, which is within 2 days after the onset of the Nestin-Cre transcriptional induction.
Fig. 2.
Severe lamination defects associated to BRCA1 deletion from progenitors in the neocortex and cerebellum. The lamination of the neocortex (Ncx) (A–F) and cerebellum (G–L) is severely disrupted in the BRCA1 KO vs. controls (CTRL). (A–C) Immunostaining using specific markers for layer VI (Tbr1, A), layer V (Reelin, Reln; B), and layers II–V (Bhlhb5; C and D) at P7. BRCA1 KO neocortex lack of upper layers (II–IV), only partially developed in layer V (pV) and poorly developed in layer VI. (E and F) p73-labeling Cajal-Retzius cells in the embryonic BRCA1 KO neocortex at E12.5 (E) and E15.5 (F). (G–I) Calbindin (CB) labeling Purkinje cells in the cerebellum of the BRCA1 KO and CTRL at P7. Purkinje cells are confined in a monolayer in the CTRL; however, they form clusters in the BRCA1 KO. The typical dendritic tree of Purkinje cells in CTRL is not observed in the BRCA1 KO. (J–L) GFAP labeling Bergmann glia in the cerebellum of the BRCA1 KO and CTRL at P7. Bergmann glia is distributed in clusters presenting misaligned fibers with abnormal polarity. I–VI, cortical layers 1–6; Ch, cortical hem; ChP, choroid plexus; EGL, external granular layer; Hc, hippocampus; IGL, internal granular layer; MZ, marginal zone; Ob, olfactory bulb; PCL, Purkinje cells layer; pHc, primordium of the hippocampus. (Scale bars: A and B, 125 μm; C and D, 250 μm; E and F, 125 μm; G and J, 500 μm; H and K, 200 μm; I and L, 50 μm.) Also see Figs. S3–S5.
The Reelin signaling pathway establishes the inside-out gradient of cortical layers (20, 21). Loss of any of the main components of the Reelin pathway leads to an inversion of the cortical layers (20, 21). Therefore, to examine whether the Reelin pathway was affected by the deletion of BRCA1, we stained for p73-immunoreactive Cajal-Retzius cells that are the main source of embryonic Reelin (22). At E12.5 and E15.5, we did not observe any change in the distribution of p73-immunoreactive Cajal-Retzius cells, indicating that the BRCA1 phenotype is independent of the Reelin signaling pathway (Fig. 2 E and F).
The consequences of the lamination defects observed in the BRCA1 KO manifest themselves as a severe thinning of the cortex due to the complete loss of upper layers (Fig. 1F). A high-magnification view of the pyramidal neurons reveals that these neurons are not only reduced in number but also are frequently misoriented, losing the typical radial orientation of apical dendrites as observed in controls (Fig. 1G). Radial glia are the migratory guides for pyramidal neurons, along which newborn neurons migrate to their correct future cortical locations during corticogenesis. As stained by Nestin, the BRCA1 KO brains at E15.5 contained radial glia with an aberrant morphology and shortened processes (Fig. S5 A–D). Thus, the defect in radial glia is also potentially contributing to the complex cortical phenotype.
In addition to the neocortical phenotype, the cerebellum also suffers from severe proliferative and morphogenic defects. The cerebellar volume is reduced by more than 50% (Figs. 1 E and J and 2 G–L and Fig. S4 A and B). The main cellular components involved in the development of the cerebellum are severely affected in the BRCA1 KO animals. Purkinje cells are the main cellular type (23, 24) involved in the control of motor movement and are typically distributed forming a monolayer with an extensive arborization of their dendritic tree [Calbindin (CB); Fig. 2 G, H, and 2I). In the BRCA1 KO, Purkinje cells are clustered, presenting a very poor dendritic arborization and lack of polarity (Fig. 2 H and I). Bergmann glia serve as guidance cues for the granular cells migrating from the external to the internal granular layer (23, 24). In the control, the GFAP-positive Bergmann glia typically form radial processes perpendicular to the external granular layer (Fig. 2 J–L). In BRCA1 KO, the Bergmann glia are misaligned, reduced in number, and lack polarity (Fig. 2 J–L). Granular cells are the only known cell type that proliferate while migrating (24). Granular cells express the marker Reelin and are situated in the most internal part of the cerebellum (Fig. S4A). However, in the BRCA1 KO, those cells form clusters distributed in an apparent random fashion (Fig. S4B).
Staining with the astrocytic marker GFAP in postnatal brains to ascertain the status of the glial components in BRCA1 KO brains, unexpectedly revealed an extensive presence of reactive astrocytes indicative of a response to a physical insult within the neural tissue in the BRCA1 KO (Fig. S5 E and F). It is unknown what the contribution of reactive astrocytes is to the overall phenotype; however, given their function akin to inflammatory responses, it is plausible that they might play a role. Regardless of the level of contribution, this suggests that extensive apoptosis in early developmental stages can maintain a reactive astrocytic response long after the cessation of cell death.
Widespread Early Apoptosis Precedes Proliferative Defects in BRCA1 KO Embryonic Neural Progenitors.
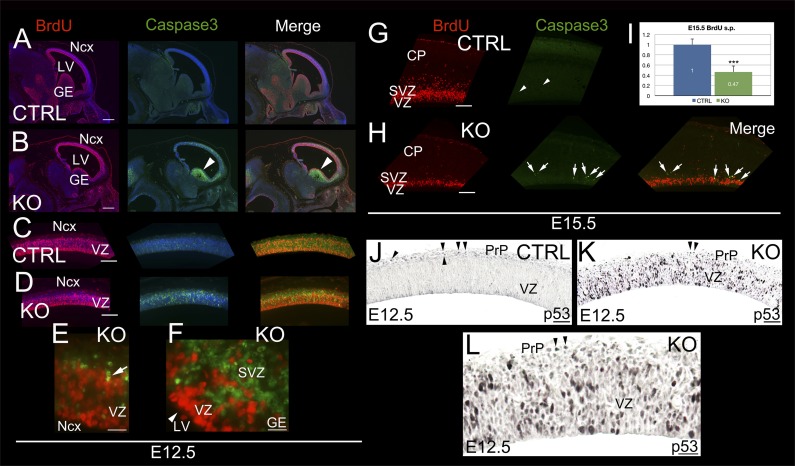
Due to the complexity of the BRCA1 conditional KO phenotype, which is dominated by hypocellularity, we needed to identify the onset of the first abnormalities that have contributed to what appears to be a cascade of catastrophic developmental failures. First, we reasoned that the expected consequence of the BRCA1 deletion from progenitors would be a decrease in proliferation at the earliest developmental time point, which was just 1 day after the onset of Cre expression. Unexpectedly, quantification of the cellular proliferation with a short pulse (2 hours) of BrdU incorporation did not reveal any statistically significant difference between controls (Fig. 3 A and C) and the BRCA1 KO animals (Fig. 3 B and D) at E12.5. Instead, we observed a strong increase in activated Caspase3 signal throughout all proliferative areas, suggesting substantial levels of apoptosis in presumed progenitor populations (Fig. 3 B, D, E, and F). The highest levels of apoptosis are observed in the ganglionic eminences, surpassing that in the neocortex (Fig. 3 B and F), which likely resulted in the absence of these structures at E15.5 (Fig. 1H). Indeed, the interneuron transcription factor Dlx5 is notably absent except in a remnant of the ganglionic eminences (Fig. S2 A and B).
Fig. 3.
p53-dependent apoptosis activation on BRCA1 deletion from neuronal progenitors. (A–H) Short pulses (2 hours) of BrdU in BRCA1 KO and control (CTRL) at E12.5 (A–F) and E15.5 (G–I). Immunostaining with BrdU (red), Caspase3 (green), and DAPI (blue) as counterstaining are shown. High power views of neocortex (Ncx) and ganglionic eminences (GE) are shown in E and G, respectively. Arrowheads in B and F indicate Caspase3+ GE. Arrow in E points to a BrdU+/Caspase3+ cell. Arrows in H indicate the same Caspase3+ cells in the same series. Arrowheads in G indicate Caspase3+ cells in the same series. (I) BrdU-positive cell quantification at E15.5 showing a pronounced significant reduction in proliferation in the KO brains vs. controls (n = 3, P = 0.0012). (J–L) Immunohistochemistry showing high levels of p53 in the ventricular zone (VZ) and preplate (PrP). p53 is normally expressed in Cajal-Retzius cells in control (arrowheads in J) and KO (arrowheads in K and L). CP, cortical plate; LV, lateral ventricle; SVZ, subventricular zone. (Scale bars: A and B, 250 μm; C and D, 125 μm; E and F, 50 μm; G and H, 100 μm; J and K, 100 μm; L, 50 μm.) Also see Fig. S1.
The observed pattern of apoptosis is reminiscent of BRCA1 expression as previously shown by ISH (Fig. S1A). Concurrent with the strong Caspase3 signal at E12.5, a p53 induction is observed (cf. Fig. 3 K and L with Fig. 3J), suggesting that the apoptosis program proceeds through a p53-dependent pathway. This finding would be consistent with previously established functions of BRCA1 in the DNA damage response pathway (11). These observations changed substantially at E15.5, where the BrdU incorporation decreased by more than 50% (cf. Fig. 3 G and H), suggesting that the defect in proliferation is a consequence of the preceding cell death first observed at E12.5. Consistent with this hypothesis, the levels of Caspase3 observed at E15.5 are still elevated in the BRCA1 KO brains compared with controls (cf. Fig. 3 H and G), albeit with a tapering difference compared with that of E12.5 (Fig. 3I). Of note, the observed pattern of activated Caspase3 is in close correlation with the expression of p53, suggesting a sequence of p53-mediated transcription and Caspase3 activation.
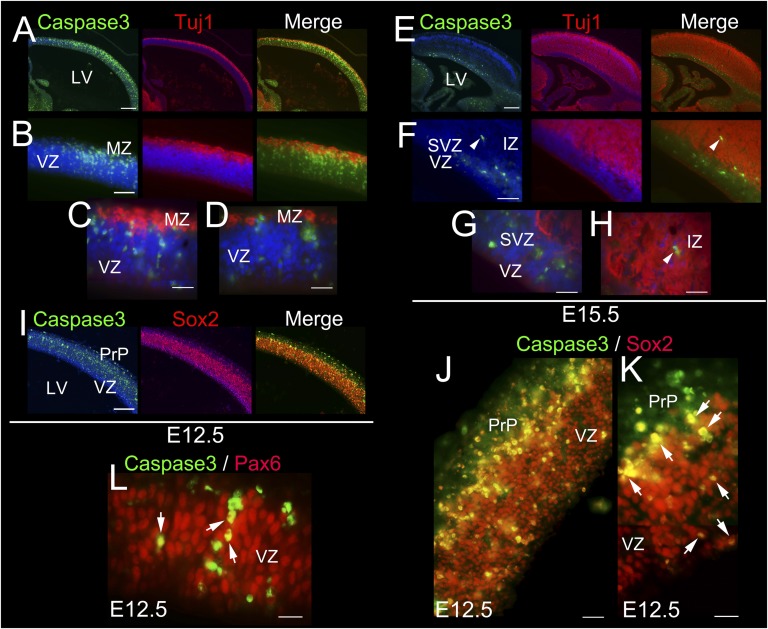
Because BRCA1 is presumed to play a major role in proliferative populations, we set out to test whether the apoptotic population was a progenitor or a committed neuronal population (Fig. 4). This determination was accomplished by immunostaining with the early neuronal marker Tuj1 (Fig. 4 A–H), in addition to the progenitor markers Sox2 (Fig. 4 I–K) and Pax6 (Fig. 4L) at E12.5 and E15.5. Our results show that the majority of the Caspase3 labeling occurs in Sox2- and Pax6-positive progenitors in the upper most part of the ventricular zone (VZ, Fig. 4 I–L), coincident with the BRCA1 ISH signal (Fig. S1A), with very few scattered cells colocalizing with early neuronal markers (Fig. 4 A–H). Collectively, these results suggest that loss of BRCA1 leads to an early p53-dependent apoptosis of neural progenitors that subsequently result in an observed proliferative defect due to cellular depletion.
Fig. 4.
Survival of embryonic neuronal progenitors is dependent of BRCA1. (A–H) BRCA1 KO brains immunostained with the early neuronal differentiated marker Tuj1 (red), Caspase3 (green), and DAPI (blue) as counterstaining at E12.5 (A–D) and E15.5 (E–H). C and D and G and H are high-power views. Arrowheads in F and H indicate the same cell. (I–L) BRCA1 KO brains immunostained with the neuronal progenitor markers Sox2 (I–K) or Pax6 (L), Caspase 3 (green), and DAPI (blue) as counterstaining at E12.5. Arrows point to Caspase3+ cells colabeled with Pax6 (L) and Sox2 (K). K is a high-power view. IZ, intermediate zone; MZ, marginal zone; PrP, preplate; LV, lateral ventricle; SVZ, subventricular zone; VZ, ventricular zone. (Scale bars: A, 250 μm; B, 100 μm; C and D, 75 μm; E, 250 μm; F–H, 75 μm; I, 125 μm; J, 75 μm; K and L, 50 μm.) Also see Fig. S1.
In Vitro Neural Progenitors Recapitulate a Cell Autonomous Deficiency.
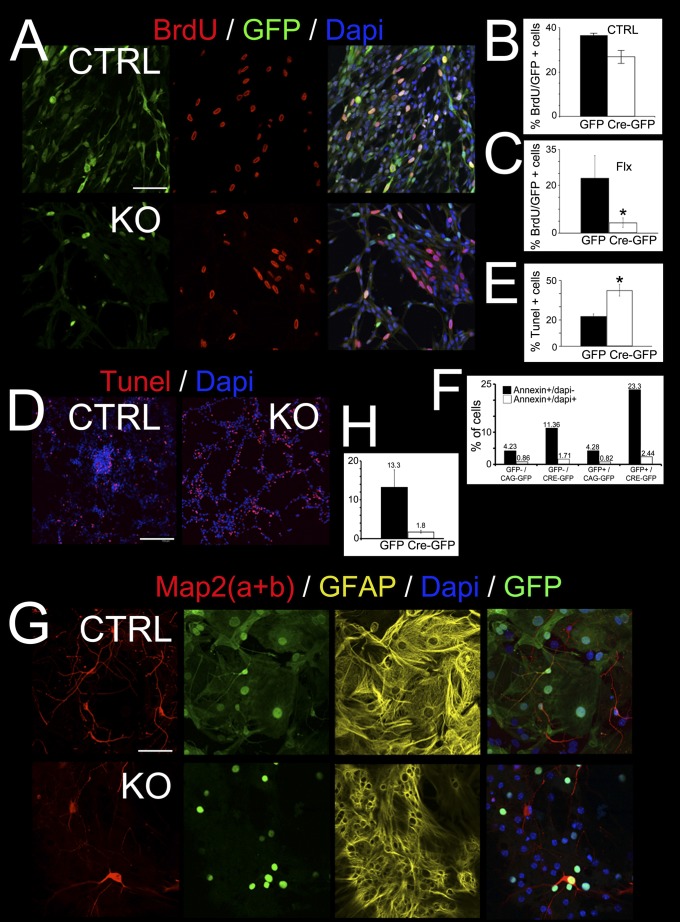
To test whether the apoptotic and proliferative phenotypes in the BRCA1 KO progenitors are cell autonomous, we isolated embryonic neural progenitor cells (ENPCs; Fig. 5). Cells either were floxed on one allele and wild-type for BRCA1 on the second allele (flox/+) for control cells or contained the floxed BRCA1 allele over a null allele (flox/−) (Fig. 5A). The floxed BRCA1 could be excised in these cells by retro or lentiviral delivery of Cre (13). Delivery of Cre-GFP fusion protein into control cells by viral infection and concomitant BrdU labeling shows that, in the presence of BRCA1 (flox/+), many cells could be simultaneously colabeled with BrdU and GFP (Fig. 5 A and B), whereas viral delivery of Cre-GFP into BRCA1 floxed over KO (flox/−) cells give virtually no GFP signal coincident with BrdU labeling. This result is indicative of proliferation failure in the absence of BRCA1 (Fig. 5 A and C). In both control (flox/+) and floxed over KO (flox/−) cells, delivery of GFP alone did not impair proliferation, as double labeling of BrdU and GFP was only limited by the infection efficiency (Fig. 5 B and C), indicating that viral infection did not affect proliferation.
Fig. 5.
Proliferation and apoptosis defects induced by BRCA1 loss are cell autonomous. In vitro assay of embryonic neural progenitor cells (ENPCs) isolated from P7 BRCA1 floxed forebrains. ENPCs were cultured and infected with retrovirus or lentivirus delivering the Cre-GFP fusion protein. (A) Concomitant labeling with BrdU indicates that ENPCs have a reduced proliferation on removal of BRCA1 (BRCA1 floxed over KO) as shown by the decreased amount of BrdU+/GFP+ cells in BRCA1 KO vs. control (BRCA1 floxed over control; CTRL). (B) Statistical analysis showing the BrdU incorporation efficiency in CTRL ENPCs infected with virus expressing GFP and Cre-GFP. Delivery of GFP alone did not have a significant effect in proliferation, whereas Cre-GFP showed about 20% decrease. (C) Statistically significant analysis (Student t test, P < 0.005) showing that BRCA1 KO ENPCs infected with Cre-GFP have a reduction of 80% in BrdU incorporation compared with GFP alone. (D) TUNEL assays showing an increase in twice as much apoptosis in BRCA1-deficient ENPCs than in CTRL (Student t test, P < 0.005) at 2 dpi. (E) Statistic showing a significant fivefold increase in the percentage of TUNEL+ cells in Cre-GFP infected ENPCs in comparison with GFP alone (Student t test, P < 0.005). (F) FACS analysis of apoptotic cells stained with Annexin V and DAPI at 3 dpi. (G and H) In vitro assay shows neuronal differentiation deficiency measured by the ratio of Map2(a+b) positivity over total GFP+ cells quantitated (H; Student t test, P < 0.005) in BRCA1 KO ENPCs infected with Cre-GFP or GFP alone. Astrocytic differentiation measured by GFAP was not affected. (Scale bars: A, D, and G, 45 μm.) Also see Fig. S6.
Furthermore, delivery of Cre-GFP into the BRCA1 floxed over KO (flox/−) cells gave substantially more TUNEL-positive cells than delivery of GFP in the same cells, indicating that deletion of BRCA1 induces apoptosis (Fig. 5 D and E). A similar experiment measuring apoptosis by Annexin-V staining was performed, by delivering Cre-GFP or GFP into cells to get a more quantitative measure of the apoptotic defect (Fig. 5F). The Annexin-V results corroborated the TUNEL results. In addition, on a complete loss of BRCA1, the ENPCs enriched the S and G2/M fractions in a manner consistent with an activation of intra-S phase and G2/M checkpoints similar to previous descriptions (Fig. S6). Both of these observations corroborate the in vivo phenotype and establish the cell autonomy of the loss of BRCA1 in apoptosis and proliferation.
We then tested whether BRCA1 affects neuronal and astrocytic differentiation from ENPCs (Fig. 5G). In vitro differentiation into neuronal lineages shows that on deletion of BRCA1, neuronal differentiation decreases ∼10-fold as evaluated by cells that stain MAP2(a+b) positive (Fig. 5 G and H), whereas astrocytic differentiation as shown by GFAP staining was not affected (Fig. 5G). These results suggest that neurons are more susceptible to a loss of BRCA1 during their differentiation than astrocytes.
Genetic Rescue of BRCA1 Phenotype by Suppression of the ATM/p53 Apoptotic Pathway.
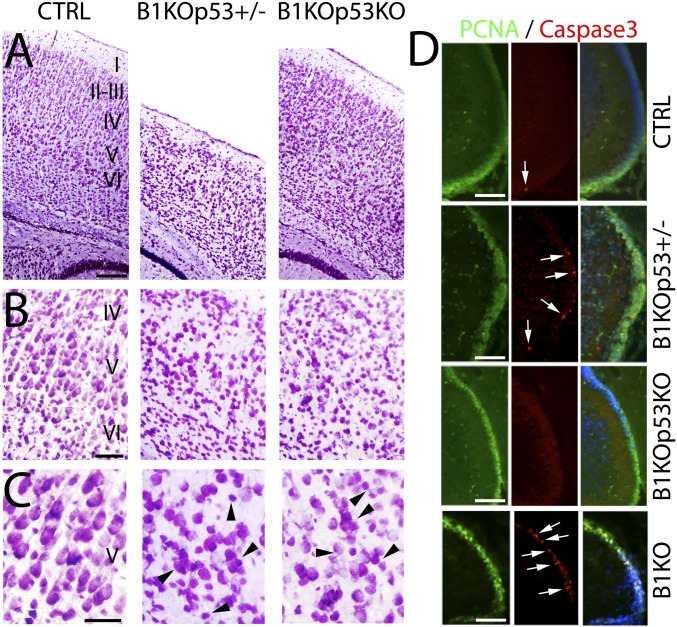
Previous studies have shown that the embryonic lethality of BRCA1 KO could be delayed by the concomitant loss of p53 (25). Occasionally, some mild hypomorphic alleles of BRCA1 can be rescued to obtain live births when crossed to a p53 KO (26). This observation has been reported to be due to suppression of p53-dependent apoptosis (26). To determine which aspects of the complex BRCA1 phenotype are due to the activation of the p53-dependent apoptotic pathway, we crossed our BRCA1 conditional KO into a p53 background (Fig. 6). The BRCA1/p53 double KOs (B1KOp53KO) were viable and showed no premature death or ataxic phenotype of the BRCA1 KO. Histological analysis of the neocortex (Fig. 6A) shows that the lamination in the double KO is largely rescued (Figs. 6A and 7B), whereas the p53 heterozygote (B1KOp53+/−) resembles the BRCA1 KO (cf. Fig. 6B with Fig. 1F). However, in the double KO, there are still subtle cortical layering abnormalities (Fig. 6B). Interestingly, the radial dendritic processes of the cortical pyramidal neurons were not rescued, irrespective of the presence of p53 (B1KOp53+/−, B1KOp53KO; Fig. 6 B and C), indicating that the disruption of the BRCA1-dependent cell polarity is not associated with a p53 mechanism. Rescue of BRCA1 phenotypes by p53 loss was observed in all analyzed brain structures (Figs. 6D and 7B).
Fig. 6.
Concomitant deletion of p53 in BRCA1 KO mice rescues apoptosis defects and restore lamination but not neuronal polarity. BRCA1 KO mice were crossed onto a p53 KO background. (A) Nissl staining at P7 showing cortical cytoarchitecture in control (CTRL), BRCA1 KO (B1KO) crossed with heterozygous p53 (B1KOp53+/−), and B1KO crossed with homozygous p53 (B1KOp53KO). B1KOp53+/− behaves as B1KO, whereas B1KOp53KO behaves more like a CTRL in terms of lamination in the cortex. (B) Series of high-power views of A. (C) Series of high-power views of B. Lamination is rescued in the B1KOp53KO but not polarity of the pyramidal neurons in the cortex that persist disoriented as in B1KOp53+/− or B1KO. Arrowheads in C indicate examples of pyramidal neurons not radially oriented. (D) Immunofluorescence in the cerebellum using proliferating cell nuclear antigen (green) to label dividing granular cells, Caspase3 (red, arrowheads), and DAPI as counterstaining. In the B1KOp53KO, the apoptosis defect is rescue and only present basal levels of apoptosis as in CTRL. However, the B1KOp53+/− presents high levels of apoptosis and behaves as in B1KO. I–VI, cortical layers 1–6. (Scale bars: A, 125 μm; B, 75 μm; C, 50 μm; D, 125 μm.) Also see Fig. S7.
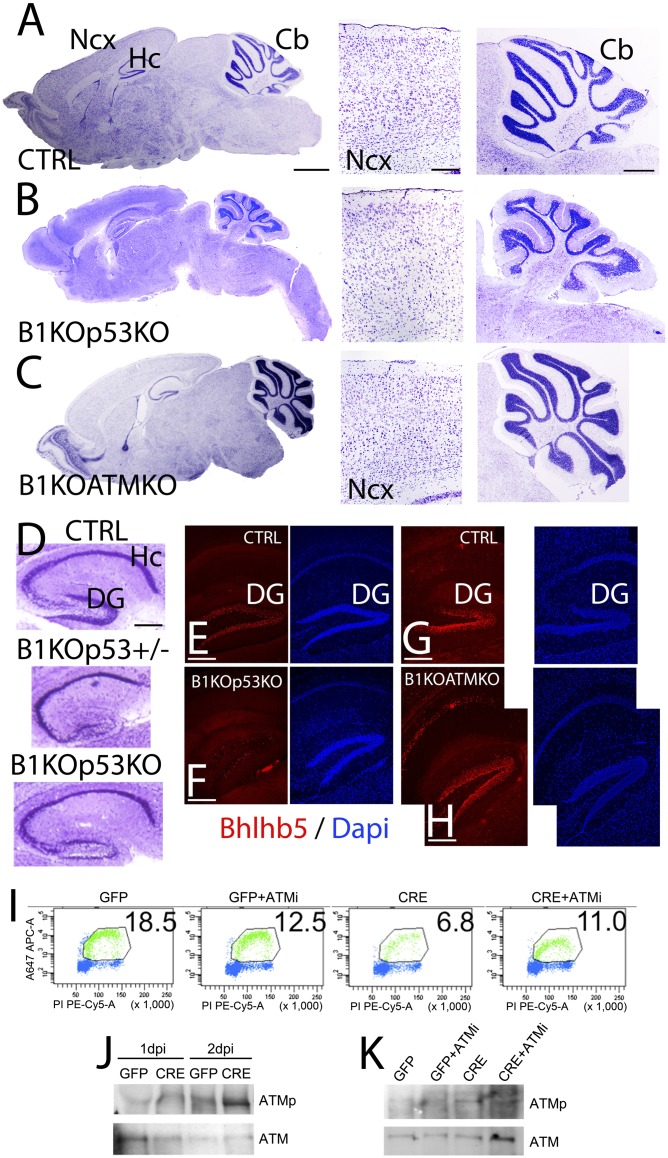
Fig. 7.
BRCA1 KO defects are fully rescued by codeletion with the tumor suppressor gene ATM. BRCA1 conditional KO (B1KO) mice crossed onto a p53 (B and D–F; B1KOp53KO) or ATM (C and G–K; B1KOATMKO) backgrounds. (A–C) Nissl staining showing P7 brain sections in control (CTRL; A), B1KOp53KO (B), and B1KOATMKO (C). Nissl sections at the level of the neocortex (Ncx) and cerebelllum (Cb) are also shown. Brain structures in B1KOATMKO resemble CTRL ones, whereas in the B1KOp53KO they are substantially recovered but still present some brain structural defects. (D) Nissl staining series showing a partial recovery of the hippocampal lamination in the B1KOp53KO compared with the defects still present in B1KOp53+/− (similar to B1KO) and CTRL. (E–H) Immunofluorescence in the hippocampus (Hc) showing Bhlhb5 labeling of granular cells in the dentate gyrus (DG) in the B1KOp53KO compared with B1KOATMKO and CTRL. DAPI is shown as counterstaining. Bhlhb5 expression is similar between B1KOATMKO and CTRL at difference than in B1KOp53KO. (I) Ku55933-induced ATM inhibition promotes the proliferation of BRCA1-deficient ENSCs. (J) Western blotting and detection of phosphorylated ATM (ATMp) or total ATM (ATM) protein showing that ATM is activated in BRCA1-deficient ENSCs. (K) Western blotting and detection of ATMp or total ATM protein after treatment with the ATM inhibitor Ku55933. ATM is inactivated after treatment with Ku55933. (Scale bars: A–C, panoramic at 1 mm, Ncx at 100 μm, and Cb at 500 μm; D, 100 μm; E–H, 100 μm.) Also see Fig. S7.
BRCA1 loss leads to both an increase in double-strand DNA breaks (DSBs) and centrosomal defects. The sensing of the resulting DSBs has been suggested to occur through the ATM kinase, which in turn activates the p53 pathway to execute the apoptotic program (6). Because ATM is upstream of p53 and also functions in the spindle checkpoint, we hypothesized that loss of ATM could be used to genetically dissect spindle checkpoint-related phenotypes compared with apoptotic events mediated by p53. Thus, we generated a double BRCA1/ATM KO (B1KOATMKO). Brains of the BRCA1/ATM double KO appear to be histologically indistinguishable from the controls (cf. Fig. 7 C with A), as opposed to the BRCA1/p53 double KO (B1KOp53KO; Fig. 7B). Although the BRCA1/p53 double KO rescues the brain volume, lamination, and cell density in the cortex (cf. Fig. 7 B with A), the cellular density in the internal granular layer of the cerebellum is still substantially diminished compared with controls (cf. Fig. 7 B with A). The hippocampal phenotype of the BRCA1 KO is characterized by a hypocellularity in the CA1-3 regions and virtual absence of the dentate gyrus (Fig. 7D). This defect is significantly ameliorated by the loss of the second p53 allele (B1KOp53KO; Fig. 7F). In the hippocampus of the double KO (B1KOp53KO), the cell density of the CA1-3 regions is partially recovered. However, both blades of the dentate gyrus exhibited decreased mature granular cell density (Fig. 7D) and the reduced Bhlhb5 labeling of the granular cells (cf. Fig. 7 E and F). Both observations from the cerebellum and dentate gyrus (Fig. 7 C and H) suggest that the BRCA1/ATM double KO achieves a greater degree of neuronal maturation, as demonstrated by Bhlhb5 staining (Fig. 7H), than the BRCA1/p53 double KO, which evades the apoptotic phenotype but is otherwise still not completely normal (Fig. 7 D and F).
To demonstrate the cell autonomy of ATM-dependent rescue of the proliferative defect in the BRCA1 KO brains, we performed experiments using ENSCs in culture. ENSCs with a floxed allele of BRCA1 were infected with retroviral vectors that either express Cre to delete the endogenous floxed BRCA1 allele or express GFP as controls. Deletion of BRCA1 led to a significant decrease in proliferation as measured by 5-ethynyl-2′deoxyuridine incorporation (Fig. 7I). This decrease could be largely restored by inclusion of a commonly used ATM inhibitor (Ku55933) (Fig. 7K). We also showed that deletion of BRCA1 in the ENSC leads to ATM activation as seen by its autophosphorylation (Fig. 7J). Similarly, ATM autophosphorylation mediated by BRCA1 deletion could be suppressed by using the ATM inhibitor (Ku55933) (Fig. 7K). Overall, these results are consistent with a model where loss of BRCA1 leads to activation of DNA damage checkpoints and centrosomal defects. p53 deletion can substantially rescue the induced apoptosis, whereas the centrosomal-associated defects appear to be p53 independent (Fig. S7). The ATM KO-mediated rescue appears to be more complete (Fig. S7), probably due to its more upstream position in the DNA damage response pathway and probably also due to its reported centrosomal checkpoint-sensing functions (27).
Discussion
Stem cell populations are generally very susceptible to IR (1). The teratogenic effect of IR is through the induction of double-strand breaks in the DNA. This effect is stronger in dividing cells due to the presence of numerous replication forks that are more difficult to repair once DNA breaks occur (11). Because proliferation rate is much higher during embryonic development, embryonic progenitors are more susceptible to DNA damage than adult cells (2). The cellular DNA replication machinery involves a plethora of genes to ensure the proper viability of the DNA at the progenitor level. Some of the genes involved in double-strand break repair such as NBS1 and ATRX (28, 29) have phenotypes characterized by high levels of apoptosis in the brain devoid of them. Some other genes, including ATM and the entire microcephaly complementation group, were found to regulate centrosomal functions (30). BRCA1 is at the intersection of these two functions and highly expressed in neurogenic areas (9). Because the loss of BRCA1 confers increased susceptibility to IR (31) and the expression pattern of BRCA1 is highest in embryonic and adult neurogenic areas (9), it was reasonable to hypothesize a neurodevelopmental function for BRCA1. In the present study, we show how the loss of BRCA1 in embryonic NPCs induces massive apoptosis of progenitors through a p53-dependent mechanism. This observation indicates that BRCA1 is a necessary key component during neurogenesis at a progenitor level and beyond. Consistent with this are our observations that deletion of BRCA1 in cultured neural progenitors affects neuronal differentiation much more than astrocytic differentiation, supporting the observations that the largest losses of cells in the BRCA1 KO are observed in neuron dense laminated structures.
To dissect the proapoptotic function of BRCA1 related to DNA damage from other possible functions, we suppressed apoptosis by genetic deletion of p53 and thus achieved a complete recovery of brain volume. However, several observable abnormalities persisted. Among these were the following: (i) the loss of cell polarity of pyramidal neurons in the neocortex, most likely due to the centrosome and spindle orientation roles attributed to BRCA1 (11, 32); and (ii) the hypocellularity observed in certain areas of the hippocampus and the granular cell layer in the cerebellum, probably associated with the proapoptotic functions of BRCA1, independent of p53 (see Fig. S7 for model). As previously mentioned, BRCA1 loss leads to an increase in double-strand DNA breaks (13, 14), which is sensed by the ATM kinase leading to activation of p53-dependent apoptotic program. As a result, our BRCA1/ATM double KO animals exhibited an apparent total recovery of the brain volume and cellular defects indistinguishable from controls. The more complete rescue of the BRCA1 phenotype by ATM compared with p53 is possibly not only due to its upstream position in the DNA damage response pathway but may also be due to its role in its centrosomal checkpoint-sensing functions (27).
Although BRCA1 has been reported to be expressed in neural stem cells (9), the complete extent of its involvement has been unclear as previous brain-specific KOs were hypomorphic mutants rather than true nulls (12). The null BRCA1 mutations in the whole animal exhibited phenotypes well before neural tube closure; hence, brain-specific phenotypes could not be assessed (33). A prior conditional KO of BRCA1 deleted just exon 11 that generates an in-frame fusion of BRCA1 in which the conserved domains of BRCA1, the N-terminal really interesting new gene (RING) finger, and the BRCA carboxy terminal repeats, at the C terminus, are preserved. Indeed, this hypomorph in combination with a p53 mutation in the whole animal can lead to viable animals in certain backgrounds (26). In contrast, the allele used in the present study never survived past E10.5 (14). We also generated a BRCA1 KO using the same Δ exon 11 allele (12) but using the CNS-specific Nestin-Cre driver instead of the cortically restricted Emx1 driver. We saw slight reductions in brain volume in otherwise normal animals (Fig. S8). The previous study showed a reduction in the early generated cortical layer VI, whereas we observe ubiquitous defects in all of the layers to varying degrees, with a complete absence of layers II–IV and a barely observable layer V. This observation is consistent with the inside-out gradient during the establishment of cortical layers where deeper layers are the first to develop (layer VI), and the upper ones (layers II and III) are the last ones (17–19) (Fig. S7). In addition, it is known that Emx1-Cre activation occurs earlier, at E10.5, compared with the Nestin-Cre that is induced 1 day later at E11.5 (16), and thus potentially explains the exclusivity of neurological phenotype in layer VI in the previous report.
Consistent with the more complete ATM rescue, BRCA1 probably plays some role in the evolution of brain size. Levels of BRCA1 are regulated by MCPH1, which is the first identified gene of the primary recessive microcephaly pathway (30). Primary microcephaly is a congenital birth defect for which patients afflicted have largely morphologically normal brains, but brain volume is severely reduced to sizes approximating those observed in chimpanzees and bonobos. Both BRCA1 and MCPH1 are phosphorylated and undergo transcriptional induction of their genes in response to DNA damage (34, 35). Furthermore, both genes have established functions in the regulation of the centrosome. Interestingly, all other mutations leading to microcephaly have functions in the regulation of the mitotic spindle and centrosome function, e.g., ASPM, CDK5RAP2, and CENPJ (30). BRCA1 is known to regulate centrosome functions, and loss of BRCA1 leads to centrosome amplification (11, 13). Notably, many proteins within the primary microcephaly complementation groups, such as MCPH1 and ASPM, show evidence for positive selection in lineages with large brain volume changes (36, 37). Therefore, it seems more than coincidental that a strong positive selection signal has been observed for BRCA1 in the primate lineage that parallels the increase in brain volume size (36–38).
BRCA1 binds to satellite repeats that make up centromeric DNA (13). These satellites repeats are among the fastest evolving DNA sequences in mammalian genomes, showing limited sequence conservation between human and chimps and no detectable sequence identity between humans and mice (39). Given that these regions encompass the mammalian centromere, this could explain the evolutionary pressure for positive selection given the fast evolution of centromeric sequences. We suggest that the proliferation has its rate-limiting step at the control of a centromere/spindle checkpoint. Because regulatory control is most efficiently exerted at the rate-limiting step of a process, it would be logical for both embryonic development and brain evolution to be driven by the regulation of this critical step. If brain size were to be determined by number of cells alone, a single additional round of cell division of the earliest neural progenitors could in theory double the brain size of an organism.
The focus of BRCA1 has historically been on breast and ovarian cancer, given that homozygous mutations of BRCA1 are most likely not viable because of the lethality observed in mice. However, two cases of patients that were heterozygous for BRCA1 have been reported that exhibit chimeric malformations in the brain that are most consistent with them arising by chimeric loss of heterozygosity of the wild-type BRCA1 allele (40, 41). These patients exhibited heterotopias consistent with migratory defects and aberrant lamination as observed from MRI images. These observations, although rare, point to the distinct possibility that the results in mice generated here are relevant for the understanding of the human brain and the pathologies associated with defects in genes involved in embryonic development.
Materials and Methods
Animals.
Mice were anesthetized with Avertin 2.5% (0.015 mL/g body weight) or ketamine/xylazine and perfused transcardially with cold 4% (wt/vol) buffered paraformaldehyde or Bouin’s fixative. See SI Materials and Methods for details. Animal work was done according with the established protocols of the Institutional Animal Care and Use Committee at The Salk Institute.
Tissue Preparation.
Brains were dissected out and prepared for cryostat or paraffin. Sections were cut at 20 μm (cryostat) and/or at 10 μm (microtome). Coronal and sagittal sections were used depending on the experimental procedure.
Immunohistochemistry.
Immunohistochemical staining was performed as described previously (16). See SI Materials and Methods for details.
ISH.
Antisense RNA probes for BRCA1, Dlx5, and Ngn2 were labeled using a DIG-RNA labeling kit (Roche). ISHs were performed as described previously (16).
Generation of CNS-Specific BRCA1 KO Mice.
CNS-specific BRCA1 KO mice were generated by crossing heterozygous BRCA1-null mice with Nestin-Cre transgenic mice followed to generate BRCA1-null and Nestin-Cre double heterozygotes. These mice were then crossed to the exon 5–13 floxed BRCA1 mice, which were maintained as a homozygous colony. The resulting BRCA1 KO contains a floxed allele over a conventional null KO allele. In the presence of Nestin-Cre, the floxed allele is deleted in a neurally restricted manner starting at E11.5 (16). Control mice were littermates that had a single floxed allele over a wild-type BRCA1 allele, essentially being CNS heterozygotes for BRCA1. PCR conditions used were as previously described (13).
Additional procedures are provided in the SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Barbara Miller for assistance in culturing NPCs, Eugene Ke for discussion and Affymetrix data analysis, and Audrey Egusa for assistance in mouse work. We also thank Mary Lynn Gage for editorial work in the preparation of the manuscript and Anton Berns for provision of floxed BRCA1 animals through the Mouse Models of Human Cancer bank and sustained interest in this work. G.M.P. was supported by California Institute for Regenerative Medicine Grant TR1-01273. Q.Z. was supported by Department of Defense Grant W81XWH-10-1-0963. I.M.V. is an American Cancer Society Professor of Molecular Biology and holds the Irwin and Joan Jacobs Chair in Exemplary Life Science. I.M.V. is supported in part by National Institutes of Health (NIH) Grant P30 CA014195-38, the Ipsen/Biomeasure, Sanofi Aventis, and the H. N. and Frances C. Berger Foundation. F.H.G. is supported by NIH Grants NS52842 and NS50217 and the Lookout fund. D.D.M.O. is supported by NIH Grant R37 NS31558.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1400783111/-/DCSupplemental.
References
- 1.Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8(9):955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 2.Yamazaki JN, Schull WJ. Perinatal loss and neurological abnormalities among children of the atomic bomb. Nagasaki and Hiroshima revisited, 1949 to 1989. JAMA. 1990;264(5):605–609. [PubMed] [Google Scholar]
- 3.Li H, et al. A role for endogenous and radiation-induced DNA double-strand breaks in p53-dependent apoptosis during cortical neurogenesis. Radiat Res. 2008;169(5):513–522. doi: 10.1667/RR1230.1. [DOI] [PubMed] [Google Scholar]
- 4.Tanori M, et al. Developmental and oncogenic radiation effects on neural stem cells and their differentiating progeny in mouse cerebellum. Stem Cells. 2013;31(11):2506–2516. doi: 10.1002/stem.1485. [DOI] [PubMed] [Google Scholar]
- 5.Okamoto M, et al. Effect of radiation on the development of immature hippocampal neurons in vitro. Radiat Res. 2009;172(6):718–724. doi: 10.1667/RR1741.1. [DOI] [PubMed] [Google Scholar]
- 6.Cao L, et al. ATM-Chk2-p53 activation prevents tumorigenesis at an expense of organ homeostasis upon Brca1 deficiency. EMBO J. 2006;25(10):2167–2177. doi: 10.1038/sj.emboj.7601115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miki Y, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266(5182):66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 8.Lane TF, et al. Expression of Brca1 is associated with terminal differentiation of ectodermally and mesodermally derived tissues in mice. Genes Dev. 1995;9(21):2712–2722. doi: 10.1101/gad.9.21.2712. [DOI] [PubMed] [Google Scholar]
- 9.Korhonen L, Brännvall K, Skoglösa Y, Lindholm D. Tumor suppressor gene BRCA-1 is expressed by embryonic and adult neural stem cells and involved in cell proliferation. J Neurosci Res. 2003;71(6):769–776. doi: 10.1002/jnr.10546. [DOI] [PubMed] [Google Scholar]
- 10.Hsu LC, White RL. BRCA1 is associated with the centrosome during mitosis. Proc Natl Acad Sci USA. 1998;95(22):12983–12988. doi: 10.1073/pnas.95.22.12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu X, et al. Centrosome amplification and a defective G2-M cell cycle checkpoint induce genetic instability in BRCA1 exon 11 isoform-deficient cells. Mol Cell. 1999;3(3):389–395. doi: 10.1016/s1097-2765(00)80466-9. [DOI] [PubMed] [Google Scholar]
- 12.Pulvers JN, Huttner WB. Brca1 is required for embryonic development of the mouse cerebral cortex to normal size by preventing apoptosis of early neural progenitors. Development. 2009;136(11):1859–1868. doi: 10.1242/dev.033498. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Q, et al. BRCA1 tumour suppression occurs via heterochromatin-mediated silencing. Nature. 2011;477(7363):179–184. doi: 10.1038/nature10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen SX, et al. A targeted disruption of the murine Brca1 gene causes gamma-irradiation hypersensitivity and genetic instability. Oncogene. 1998;17(24):3115–3124. doi: 10.1038/sj.onc.1202243. [DOI] [PubMed] [Google Scholar]
- 15.Tronche F, et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23(1):99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- 16.Chou SJ, Perez-Garcia CG, Kroll TT, O’Leary DD. Lhx2 specifies regional fate in Emx1 lineage of telencephalic progenitors generating cerebral cortex. Nat Neurosci. 2009;12(11):1381–1389. doi: 10.1038/nn.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angevine JB, Jr, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961;192:766–768. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- 18.Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145(1):61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- 19.Caviness VS, Jr, Takahashi T. Proliferative events in the cerebral ventricular zone. Brain Dev. 1995;17(3):159–163. doi: 10.1016/0387-7604(95)00029-b. [DOI] [PubMed] [Google Scholar]
- 20.Lambert de Rouvroit C, Goffinet AM. The reeler mouse as a model of brain development. Adv Anat Embryol Cell Biol. 1998;150:1–106. [PubMed] [Google Scholar]
- 21.Perez-Garcia CG, Tissir F, Goffinet AM, Meyer G. Reelin receptors in developing laminated brain structures of mouse and human. Eur J Neurosci. 2004;20(10):2827–2832. doi: 10.1111/j.1460-9568.2004.03733.x. [DOI] [PubMed] [Google Scholar]
- 22.Meyer G, Perez-Garcia CG, Abraham H, Caput D. Expression of p73 and Reelin in the developing human cortex. J Neurosci. 2002;22(12):4973–4986. doi: 10.1523/JNEUROSCI.22-12-04973.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellamy TC. Interactions between Purkinje neurones and Bergmann glia. Cerebellum. 2006;5(2):116–126. doi: 10.1080/14734220600724569. [DOI] [PubMed] [Google Scholar]
- 24.Hibi M, Shimizu T. Development of the cerebellum and cerebellar neural circuits. Dev Neurobiol. 2012;72(3):282–301. doi: 10.1002/dneu.20875. [DOI] [PubMed] [Google Scholar]
- 25.Hakem R, de la Pompa JL, Elia A, Potter J, Mak TW. Partial rescue of Brca1 (5-6) early embryonic lethality by p53 or p21 null mutation. Nat Genet. 1997;16(3):298–302. doi: 10.1038/ng0797-298. [DOI] [PubMed] [Google Scholar]
- 26.Xu X, Aprelikova O, Moens P, Deng CX, Furth PA. Impaired meiotic DNA-damage repair and lack of crossing-over during spermatogenesis in BRCA1 full-length isoform deficient mice. Development. 2003;130(9):2001–2012. doi: 10.1242/dev.00410. [DOI] [PubMed] [Google Scholar]
- 27.Yang C, et al. Aurora-B mediated ATM serine 1403 phosphorylation is required for mitotic ATM activation and the spindle checkpoint. Mol Cell. 2011;44(4):597–608. doi: 10.1016/j.molcel.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frappart PO, et al. An essential function for NBS1 in the prevention of ataxia and cerebellar defects. Nat Med. 2005;11(5):538–544. doi: 10.1038/nm1228. [DOI] [PubMed] [Google Scholar]
- 29.Bérubé NG, et al. The chromatin-remodeling protein ATRX is critical for neuronal survival during corticogenesis. J Clin Invest. 2005;115(2):258–267. doi: 10.1172/JCI22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thornton GK, Woods CG. Primary microcephaly: Do all roads lead to Rome? Trends Genet. 2009;25(11):501–510. doi: 10.1016/j.tig.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scully R, et al. Genetic analysis of BRCA1 function in a defined tumor cell line. Mol Cell. 1999;4(6):1093–1099. doi: 10.1016/s1097-2765(00)80238-5. [DOI] [PubMed] [Google Scholar]
- 32.Parvin JD. The BRCA1-dependent ubiquitin ligase, gamma-tubulin, and centrosomes. Environ Mol Mutagen. 2009;50(8):649–653. doi: 10.1002/em.20475. [DOI] [PubMed] [Google Scholar]
- 33.Gowen LC, Johnson BL, Latour AM, Sulik KK, Koller BH. Brca1 deficiency results in early embryonic lethality characterized by neuroepithelial abnormalities. Nat Genet. 1996;12(2):191–194. doi: 10.1038/ng0296-191. [DOI] [PubMed] [Google Scholar]
- 34.Xu X, Lee J, Stern DF. Microcephalin is a DNA damage response protein involved in regulation of CHK1 and BRCA1. J Biol Chem. 2004;279(33):34091–34094. doi: 10.1074/jbc.C400139200. [DOI] [PubMed] [Google Scholar]
- 35.Lin SY, Rai R, Li K, Xu ZX, Elledge SJ. BRIT1/MCPH1 is a DNA damage responsive protein that regulates the Brca1-Chk1 pathway, implicating checkpoint dysfunction in microcephaly. Proc Natl Acad Sci USA. 2005;102(42):15105–15109. doi: 10.1073/pnas.0507722102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mekel-Bobrov N, et al. Ongoing adaptive evolution of ASPM, a brain size determinant in Homo sapiens. Science. 2005;309(5741):1720–1722. doi: 10.1126/science.1116815. [DOI] [PubMed] [Google Scholar]
- 37.Evans PD, et al. Microcephalin, a gene regulating brain size, continues to evolve adaptively in humans. Science. 2005;309(5741):1717–1720. doi: 10.1126/science.1113722. [DOI] [PubMed] [Google Scholar]
- 38.Zhang G, et al. Comparative analysis of bat genomes provides insight into the evolution of flight and immunity. Science. 2013;339(6118):456–460. doi: 10.1126/science.1230835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salser W, et al. Investigation of the organization of mammalian chromosomes at the DNA sequence level. Fed Proc. 1976;35(1):23–35. [PubMed] [Google Scholar]
- 40.Eccles D, Bunyan D, Barker S, Castle B. BRCA1 mutation and neuronal migration defect: Implications for chemoprevention. J Med Genet. 2005;42(7):e42. [PMC free article] [PubMed] [Google Scholar]
- 41.Eccles DM, Barker S, Pilz DT, Kennedy C. Neuronal migration defect in a BRCA1 gene carrier: Possible focal nullisomy? J Med Genet. 2003;40(3):e24. doi: 10.1136/jmg.40.3.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.