ABSTRACT
The Lyme disease spirochete Borrelia burgdorferi senses and responds to environmental cues as it transits between the tick vector and vertebrate host. Failure to properly adapt can block transmission of the spirochete and persistence in either vector or host. We previously identified BBD18, a novel plasmid-encoded protein of B. burgdorferi, as a putative repressor of the host-essential factor OspC. In this study, we investigate the in vivo role of BBD18 as a regulatory protein, using an experimental mouse-tick model system that closely resembles the natural infectious cycle of B. burgdorferi. We show that spirochetes that have been engineered to constitutively produce BBD18 can colonize and persist in ticks but do not infect mice when introduced by either tick bite or needle inoculation. Conversely, spirochetes lacking BBD18 can persistently infect mice but are not acquired by feeding ticks. Through site-directed mutagenesis, we have demonstrated that abrogation of spirochete infection in mice by overexpression of BBD18 occurs only with bbd18 alleles that can suppress OspC synthesis. Finally, we demonstrate that BBD18-mediated regulation does not utilize a previously described ospC operator sequence required by B. burgdorferi for persistence in immunocompetent mice. These data lead us to conclude that BBD18 does not represent the putative repressor utilized by B. burgdorferi for the specific downregulation of OspC in the mammalian host. Rather, we suggest that BBD18 exhibits features more consistent with those of a global regulatory protein whose critical role occurs during spirochete acquisition by feeding ticks.
IMPORTANCE
Lyme disease, caused by Borrelia burgdorferi, is the most common arthropod-borne disease in North America. B. burgdorferi is transmitted to humans and other vertebrate hosts by ticks as they take a blood meal. Transmission between vectors and hosts requires the bacterium to sense changes in the environment and adapt. However, the mechanisms involved in this process are not well understood. By determining how B. burgdorferi cycles between two very different environments, we can potentially establish novel ways to interfere with transmission and limit infection of this vector-borne pathogen. We are studying a regulatory protein called BBD18 that we recently described. We found that too much BBD18 interferes with the spirochete’s ability to establish infection in mice, whereas too little BBD18 appears to prevent colonization in ticks. Our study provides new insight into key elements of the infectious cycle of the Lyme disease spirochete.
INTRODUCTION
The causative agent of Lyme disease, Borrelia burgdorferi (1–3), is maintained in an enzootic cycle involving Ixodes ticks and mammalian hosts (4). Successful completion of this cycle requires the spirochete’s ability to sense environmental changes and alter its gene expression and protein profiles. A key example of this adaptation occurs when infected ticks ingest a blood meal and a subset of spirochetes within the tick midgut begin to express the gene encoding outer surface protein C (OspC), which prepares B. burgdorferi for transmission to the mammalian host (5–7). Our laboratory previously showed that B. burgdorferi mutants lacking ospC are noninfectious by tick bite or needle inoculation (8–11). However, OspC is also a target for neutralizing antibodies, and ospC expression must be downregulated after B. burgdorferi establishes infection in order to avoid clearance by the host’s adaptive immune response (12–15).
As infected ticks ingest blood, spirochetes experience changes in temperature, pH, nutrients, and other host factors, which lead to induction of a novel regulatory cascade involving the alternative sigma factors RpoN and RpoS (16–19). This cascade is responsible for the increased expression of a number of B. burgdorferi genes associated with mammalian infection, including ospC (20–25). However, as mentioned above, ospC is only transiently expressed by spirochetes in an infected vertebrate host, whereas other RpoS-dependent genes continue to be expressed by the spirochete throughout mammalian infection (26, 27). These observations led to the hypothesis by Xu and colleagues that an unidentified repressor, distinct from the RpoN/RpoS regulatory cascade, is required for the specific downregulation of ospC by B. burgdorferi during persistent mammalian infection (28, 29). More recently, our laboratory identified bbd18, a plasmid-located gene of B. burgdorferi whose expression resulted in downregulation of ospC at the transcriptional level (30). The bbd18 gene is well conserved among B. burgdorferi isolates but lacks homologs outside the Borrelia genus (30). This initial work with BBD18 was conducted in an avirulent strain of B. burgdorferi (B312), so it was important to demonstrate that BBD18 could downregulate OspC in wild-type B. burgdorferi, as well as to investigate the in vivo role of BBD18 during an experimental mouse-tick infectious cycle. Related to this, we wished to determine if BBD18 was the ospC-specific repressor required for B. burgdorferi persistence in the vertebrate host (28).
In this study, we have utilized a molecular genetic approach to define the functional significance of BBD18 as a regulatory protein during the infectious cycle of B. burgdorferi. We have determined the outcome in mice and ticks of engineered expression of bbd18 alleles that vary in OspC regulatory activity. We have also utilized direct and indirect methods to investigate the mechanism of OspC suppression by BBD18. Finally, we have determined the point during the infectious cycle when BBD18-mediated regulation is critical for spirochete adaptation and survival. Our results indicate that BBD18 does not function as an ospC-specific repressor during persistent infection but likely plays a broader role at a different stage of the infectious cycle. In a concurrent study, we have demonstrated that BBD18 indirectly modulates ospC expression through posttranscriptional regulation of RpoS (43). Taken together, these data lead us to propose that BBD18 functions as a global regulator of the adaptive response of spirochetes as they exit the vertebrate host and are acquired by feeding ticks. These data demonstrate that synthesis of key regulatory proteins, such as BBD18, must be tightly controlled and illustrate the careful orchestration of gene expression by the Lyme disease spirochete throughout its natural infectious cycle.
RESULTS
Constitutive bbd18 expression abrogates B. burgdorferi infection in the mouse model.
To address the role of BBD18 during the infectious cycle, we first undertook to determine whether constitutive expression of bbd18 in an infectious B. burgdorferi clone interfered with infection or transmission. Since OspC is an essential virulence factor for B. burgdorferi infection in mice, we hypothesized that spirochetes constitutively expressing an ospC repressor would be unable to upregulate OspC and therefore would be noninfectious in the mouse model. For this purpose, we utilized wild-type strain S9, a virulent clone of the B31 type strain of B. burgdorferi with enhanced shuttle vector transformation efficiency (31–33), and introduced pBSV2*flaBp-bbd18 (see Table S1 in the supplemental material) (30), a shuttle vector harboring the bbd18 open reading frame expressed from the constitutive flaB promoter. We have previously demonstrated that this 767-bp fragment, which encompasses only the bbd18 open reading frame and 100 bp of the 3′ flanking sequence, does not alter ospC expression in B. burgdorferi when introduced without a promoter (30). For brevity, we will refer to strain S9 as the wild type (WT) and S9 carrying pBSV2*flaBp-bbd18 as WT+cBBD18. To confirm that any observed differences from the WT were caused by overexpression of bbd18, we subsequently displaced pBSV2*flaBp-bbd18 from WT+cBBD18 by introducing an incompatible plasmid without an insert (pBSV2G). The resulting clone, termed S9/chase-pBSV2G (WT/chase), retained the same endogenous plasmid profile as WT+cBBD18 and yet lacked the constitutively expressed copy of bbd18 on the shuttle vector. Southern blot analysis confirmed the presence of the correct shuttle vectors in these strains (data not shown).
Groups of mice were injected with WT, WT+cBBD18, or WT/chase. After 3 weeks, mice inoculated with WT or WT/chase were seropositive for B. burgdorferi proteins, whereas mice inoculated with WT+cBBD18 remained seronegative (Table 1). In addition, spirochetes were subsequently isolated from tissues (ear, bladder, and ankle joint) of mice inoculated with WT or WT/chase but not from any tissues of any mice inoculated with WT+cBBD18 (Table 1). These results demonstrate that constitutive expression of bbd18 renders B. burgdorferi noninfectious by needle inoculation. Restoration of infectivity in WT/chase corroborated these findings and indicated that the inappropriate expression of bbd18 was solely responsible for the loss of infectivity by WT+cBBD18.
TABLE 1 .
Constitutive expression of bbd18 prevents B. burgdorferi infection in mice by needle inoculation
| Straina | No. of seropositive mice/no. of mice injectedb | No. of tissue isolates/no. of mice injected (ear, bladder, joint)c |
|---|---|---|
| WT | 5/5 | 5/5, 5/5, 5/5 |
| WT+cBBD18 | 0/10d | 0/10, 0/10, 0/10d |
| WT/chase | 4/5 | 4/5, 4/5, 4/5 |
Mice were inoculated with B. burgdorferi strains S9 (WT), S9/pBSV2*flaBp-bbd18 (WT+cBBD18), or S9/chase-pBSV2G (WT/chase).
Seroconversion to B. burgdorferi whole-cell lysates was assessed by immunoblotting 3 weeks after needle inoculation of 104 spirochetes.
Isolation of spirochetes from mouse tissues was attempted 5 weeks after inoculation.
P value of <0.0005 compared to WT and P value of <0.005 compared to WT/chase, using Fisher’s exact test.
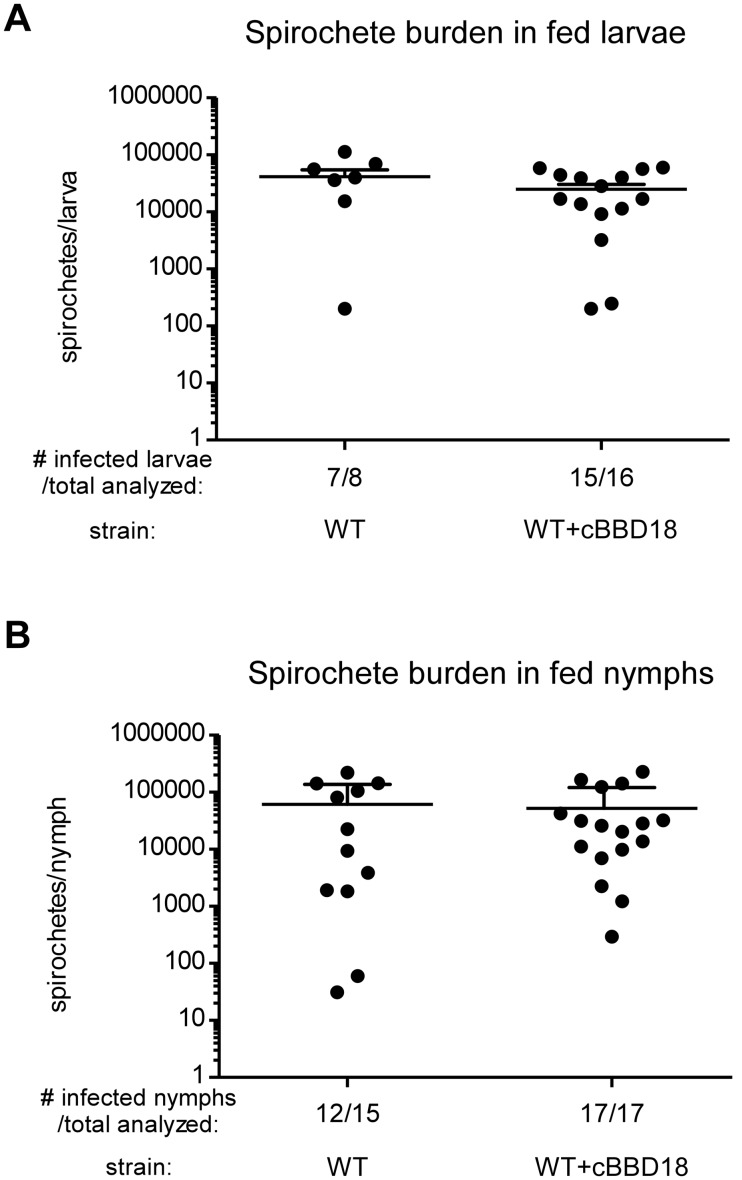
Spirochetes expressing bbd18 were noninfectious by needle inoculation, but in nature B. burgdorferi is transmitted by Ixodes ticks (4). We wanted to investigate the potential impact of a constitutively expressed bbd18 gene on spirochetes within ticks and during natural transmission to the mammalian host. This would typically be determined by feeding larval Ixodes scapularis ticks on infected mice and confirming acquisition of B. burgdorferi, before allowing the larvae to molt and feed as nymphs, to assess transmission. However, this approach was not possible because mice inoculated with WT+cBBD18 were not infected (Table 1). Therefore, we artificially infected I. scapularis larvae with WT or WT+cBBD18 spirochetes (34). Groups of larval ticks were analyzed 7 days after feeding, and similar numbers of viable spirochetes were present in both tick cohorts (Fig. 1A), indicating that artificial infection was successful with both WT and WT+cBBD18. The remaining fed larval I. scapularis ticks were allowed to molt and recover (~12 weeks after larval feeding), before feeding on naive mice.
FIG 1 .
Spirochete burden in artificially infected ticks. (A) Spirochete burden in fed larvae. I. scapularis larvae were artificially infected (34) with S9 (WT) or S9/pBSV2*flaBp-bbd18 (WT+cBBD18) and fed to repletion on naive mice. Each point denotes the number of viable spirochetes per tick, as determined by the CFU in solid medium, when fed larval ticks were individually crushed and plated 7 days after drop off, and bars represent means plus standard deviations. There was no significant difference in spirochete burden between the two groups (P value = 0.1939) based on unpaired Student’s t test. (B) Spirochete burden in fed nymphs. The remaining artificially infected larvae were allowed to molt into nymphs and then fed to repletion on naive mice (~5 ticks per mouse). Each point denotes the number of viable spirochetes per nymph when fed ticks were individually crushed and plated 7 days after drop off, and bars represent means plus standard deviations. There was no significant difference in spirochete burden between the two groups (P value = 0.7444) based on unpaired Student’s t test.
Infected nymphs (~5 ticks per mouse) were allowed to feed to repletion to determine whether bacteria constitutively expressing bbd18 were infectious in mice by tick transmission (Table 2). WT spirochetes were transmitted by infected nymphs and could establish infection in mice, as measured by both seroconversion and isolation of spirochetes from mouse tissues. In contrast, WT+cBBD18 spirochetes were not isolated from any of the mice fed on by infected ticks nor did these mice seroconvert to B. burgdorferi proteins. Although WT+cBBD18 spirochetes could not establish infection in mice by needle inoculation (Table 1) or tick transmission (Table 2), constitutive expression of bbd18 did not affect persistence or replication within ticks, as the number of viable spirochetes in infected nymphs after feeding was comparable between strains (Fig. 1B).
TABLE 2 .
Constitutive expression of bbd18 prevents B. burgdorferi infection of mice by tick transmission
| Straina | No. of infected mice/no. of mice fed on by infected larvaeb | No. of infected mice/no. of mice fed on by infected nymphsb |
|---|---|---|
| WT | 2/2 | 5/5 |
| WT+cBBD18 | 0/6c | 0/7c |
Mice were fed upon by ticks infected with B. burgdorferi strains S9 (WT) or S9/pBSV2*flaBp-bbd18 (WT+cBBD18).
Mouse infection determined by seroconversion to B. burgdorferi whole-cell lysates and isolation of spirochetes from mouse tissues 3 weeks after tick feeding.
P value of <0.05 compared to WT using Fisher’s exact test.
Constitutive expression of bbd18 prevents synthesis of OspC by spirochetes in feeding ticks.
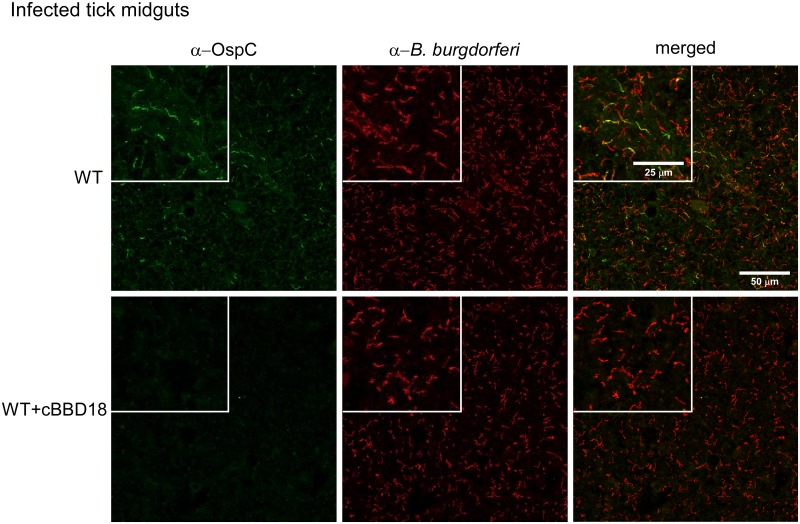
After demonstrating that spirochetes constitutively expressing bbd18 could not infect mice by needle inoculation (Table 1) or tick bite (Table 2), we investigated the basis for this attenuated phenotype. We have previously shown that BBD18 suppresses the synthesis of OspC by spirochetes grown in vitro (30) and that OspC is required by B. burgdorferi for infection of the mammalian host (8–10). We presumed that the observed loss of infectivity by WT+cBBD18 resulted from the inability of spirochetes to induce ospC expression during tick feeding or after transmission to a mouse. Therefore, we visualized the OspC phenotype of WT and WT+cBBD18 spirochetes in infected nymphs after they had fed to repletion on a mouse, approximately 96 h postattachment. We used a monoclonal antibody to identify spirochetes that were synthesizing OspC (Fig. 2, left) and convalescent-phase serum from an infected rabbit to visualize the entire B. burgdorferi population (Fig. 2, middle) within infected tick midguts. As anticipated (5–7), OspC was detected on a subset of spirochetes in fed ticks infected with WT B. burgdorferi, as shown in Fig. 2 (green compared to red spirochetes, top). Although we did not detect any OspC-positive spirochetes in ticks infected with WT+cBBD18 (Fig. 2, bottom left), spirochetes were clearly present within these ticks when analyzed using the anti-B. burgdorferi rabbit serum (Fig. 2, red spirochetes, middle, and merged images, right), and spirochete burdens within infected nymphs were similar between WT and WT+cBBD18 (Fig. 1B). These data demonstrate that constitutive expression of bbd18 prevents the synthesis of OspC by B. burgdorferi, even in the natural environment of a feeding tick when ospC is normally induced, and provides an explanation for why this strain is noninfectious in mice (Tables 1 and 2) (8–11).
FIG 2 .
Constitutive expression of bbd18 prevents induction of ospC in feeding nymphs. Immunofluorescence microscopy of spirochetes within replete I. scapularis nymphs. Spirochetes in fed nymphs, infected as larvae with S9 (WT, top) or S9/pBSV2*flaBp-bbd18 (WT+cBBD18, bottom), were assessed at detachment from the host. Crushed ticks were costained with a mouse monoclonal antibody recognizing OspC (left) and polyclonal rabbit serum that detects all B. burgdorferi (middle); merged images are shown in the right panels. Scale bars as shown in the upper right panel apply to all images, and inset boxes represent 2-fold magnification of a comparable area in all 3 panels for each strain.
BBD18 site-directed mutagenesis.
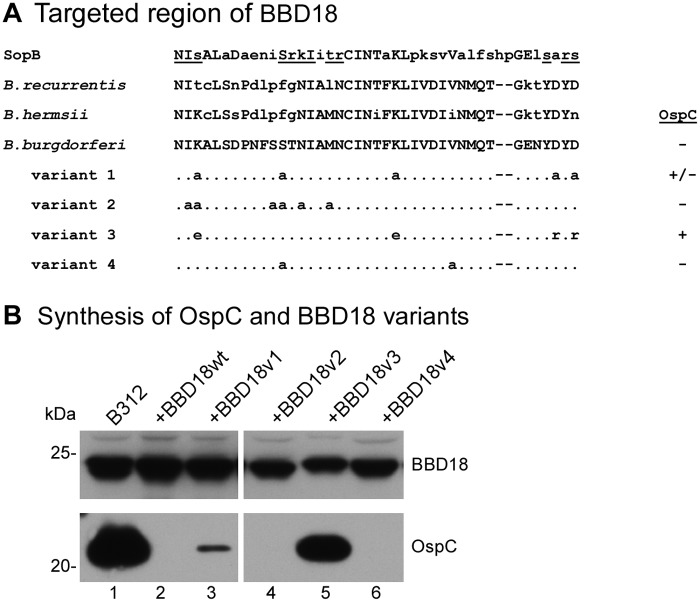
BBD18 is highly conserved in Lyme disease and relapsing fever spirochetes (30) (data not shown) but lacks homologs outside the genus Borrelia. As previously noted (30), the predicted BBD18 protein is very basic (pI of 9.39) and contains many positively charged residues. A BLAST search of the Protein Data Bank identified weak similarities between BBD18 and two proteins, the ribosome-inactivating pokeweed antiviral protein (BLAST E value 0.035) and a helix-turn-helix motif (HTH) of SopB (BLAST E value of 1.9), a DNA-binding protein of Escherichia coli involved in plasmid segregation (35). Focusing on the analogous residues that contact DNA in the SopB crystal structure (35) or were conserved in an alignment of the primary amino acid sequence of BBD18 in Lyme disease and relapsing fever Borrelia, we designed four BBD18 site-directed mutants, each with multiple amino acid substitutions (Fig. 3A and Materials and Methods). Synthetic genes encoding these BBD18 variants were inserted on the shuttle vector under control of the constitutive flaB promoter, creating pBSV2*flaBp-bbd18v1 to -v4 (see Table S1 in the supplemental material).
FIG 3 .
OspC regulatory activity of BBD18 site-directed mutants. (A) Alignment of amino acid residues 178 to 220 of the SopB helix-turn-helix motif, residues 52 to 92 of the BBD18 homolog in the relapsing fever spirochetes B. recurrentis and B. hermsii, and residues 56 to 96 of WT B. burgdorferi BBD18, with the same region of the four BBD18 variants (v1 to v4) with site-directed mutations. The underlined residues of the SopB sequence are those that have been shown to contact DNA (35). Amino acid residues that differ from the WT B. burgdorferi BBD18 sequence are shown in lowercase letters. Residues that have been changed in BBD18 variants are specified, whereas unchanged residues in this region are represented with dots; the remainder of the BBD18 sequence not depicted in this alignment is identical between the WT and variant BBD18 proteins. Synthesis of OspC by spirochetes coexpressing WT or variant bbd18 alleles (as shown in panel B) is indicated to the right of the alignment. (B) BBD18 (top) and OspC (bottom) immunoblots of B312 harboring WT or variant bbd18 alleles (variant 1 to variant 4 in panel A) expressed from the flaB promoter on a shuttle vector. Whole-cell lysates of B312 (lane 1), B312/pBSV2*flaBp-bbd18 (+BBD18wt, lane 2), and B312 harboring the bbd18 variants (+BBD18v1 to -v4, lanes 3 to 6) were analyzed by immunoblotting with antiserum recognizing BBD18 or OspC, as indicated. Lanes 1 to 3 and 4 to 6 were from nonadjacent regions of the same immunoblots. The molecular mass (kDa), based on the mobility of protein standards, is denoted on the left, and lane numbers are indicated beneath the panel.
We next tested whether these BBD18 variants were similar to wild-type BBD18 in their ability to prevent ospC expression. After stably introducing each of the bbd18 variants into avirulent strain B312, which produces OspC in vitro (36), we analyzed whole-cell lysates for OspC and BBD18 (Fig. 3B). By immunoblot analysis, we were able to detect comparable levels of BBD18 protein in all of the B312 derivatives harboring a bbd18 gene on the shuttle vector (Fig. 3B, top blot, lanes 2 to 6), regardless of the site-directed mutations. As expected, the parental strain B312 (which lacks lp17 and thus does not make BBD18) produced abundant OspC (Fig. 3B, bottom blot, lane 1), while no OspC was made by B312 harboring a shuttle vector carrying a constitutively expressed wild-type bbd18 gene (Fig. 3B, lane 2). BBD18v2 and BBD18v4 were also able to fully suppress OspC production in B312 (Fig. 3B, lanes 4 and 6, respectively), indicating that the respective mutations in these bbd18 alleles did not diminish their impact on ospC expression. In contrast, BBD18v3 completely lacked regulatory activity, as demonstrated by the continued synthesis of OspC protein when this BBD18 variant was introduced into B312 (Fig. 3B, lane 5). OspC was also detected in B312 synthesizing BBD18v1 (Fig. 3B, lane 3), although at a lower level than with BBD18v3. Importantly, identification of a bbd18 allele (bbd18v3) that yielded a comparable amount of BBD18 protein but did not suppress OspC synthesis (Fig. 3B, lane 5) provided a tool with which to further investigate the in vivo function of BBD18.
Spirochetes constitutively producing an inactive BBD18 variant remain infectious in mice.
We next determined whether constitutive synthesis of an inactive form of BBD18 in virulent B. burgdorferi would render spirochetes noninfectious. To this end, we introduced a shuttle vector carrying bbd18v3 into the infectious WT clone. In parallel, we introduced a shuttle vector carrying bbd18v4, which encodes a BBD18 variant that exhibits OspC regulatory activity (Fig. 3B). Three groups of 5 mice were inoculated with WT, WT+cBBD18v3, or WT+cBBD18v4. Significantly, we found that both WT and WT+cBBD18v3 spirochetes could infect mice, whereas WT+cBBD18v4 was noninfectious (Table 3). Thus, the in vivo phenotype of WT spirochetes constitutively expressing bbd18 was correlated with the OspC regulatory activity of the BBD18 protein they produced: BBD18v3 did not prevent OspC synthesis (Fig. 3B) nor did it render WT spirochetes noninfectious in mice (Table 3), whereas the opposite was true for BBD18v4. This experiment also demonstrated that constitutive synthesis of an inactive or irrelevant protein does not intrinsically render B. burgdorferi noninfectious, as only the BBD18 proteins with OspC regulatory activity (i.e., WT BBD18 or BBD18v4) gave rise to a noninfectious phenotype when inappropriately expressed. Although these data do not rule out the possibility of a separate or additional function for BBD18, they do indicate that the ability to suppress OspC synthesis in cultured spirochetes correlates with the ability to abrogate B. burgdorferi infectivity in mice.
TABLE 3 .
Constitutive production of an inactive BBD18 variant does not prevent B. burgdorferi infection in mice
| Straina | No. of seropositive mice/no. of mice injectedb | No. of tissue isolates/no. of mice injected (ear, bladder, joint)c |
|---|---|---|
| WT | 4/4 | 4/4, 4/4, 4/4 |
| WT+cBBD18v3 | 4/5 | 4/5, 4/5, 4/5 |
| WT+cBBD18v4 | 0/5 | 0/5, 0/5, 0/5d |
Mice were inoculated with B. burgdorferi strains S9 (WT), S9/pBSV2*flaBp-bbd18v3 (WT+cBBD18v3), or S9/pBSV2*flaBp-bbd18v4 (WT+cBBD18v4).
Seroconversion to B. burgdorferi whole-cell lysates was assessed by immunoblotting 3 weeks after needle inoculation of 104 spirochetes.
Isolation of spirochetes from mouse tissues was attempted 32 days after inoculation.
P value of <0.005 compared to WT and P value of <0.05 compared to WT+cBBD18v3, using Fisher’s exact test.
B. burgdorferi strains lacking bbd18 are infectious in mice.
To test whether bbd18 is required during mammalian infection, we designed and utilized an allelic exchange construct to inactivate bbd18. After multiple transformation attempts, we obtained a single bbd18 mutant that retained the full plasmid content of the WT and yet was noninfectious in mice by needle inoculation (data not shown). However, we were unable to restore infectivity to this mutant by complementation with a WT copy of bbd18 at either the endogenous locus on lp17 or on a shuttle vector, indicating that additional mutation(s) rendered it noninfectious. Further attempts to inactivate bbd18 in a WT clone have been unsuccessful.
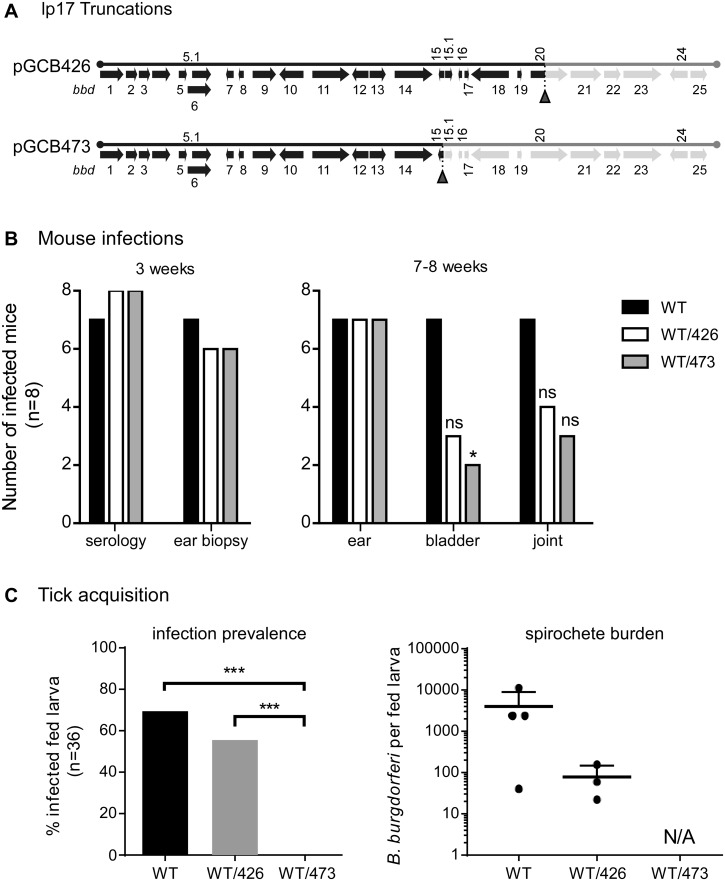
Given the difficulty of inactivating bbd18 in an otherwise infectious clone, we next opted to use deleted forms of lp17 (37) to investigate the role of bbd18 in B. burgdorferi during the infectious cycle. This represents the approach we used initially to identify BBD18 as an OspC repressor (30), as well as that subsequently used by Casselli et al. to investigate the role of lp17 genes during mouse infection by B. burgdorferi (38). To this end, we displaced the full-length plasmid from WT B. burgdorferi with two different truncated forms of lp17. One of these lp17 truncations, termed pGCB473 (37), harbors only bbd1 to bbd14 and thus lacks bbd18 (Fig. 4A). We also introduced pGCB426 (37), a truncated version of lp17 containing bbd1 to bbd19 (and thus retaining bbd18), as a control for WT/pGCB473 (Fig. 4A). We confirmed that full-length lp17 had been displaced from WT by introduction of pGCB473 or pGCB426, and the resulting strains retained an otherwise identical plasmid profile (data not shown).
FIG 4 .
Mouse infection and tick acquisition of B. burgdorferi harboring lp17 truncations. (A) Schematic diagram of pGCB426 and pGCB473, truncated forms of lp17 (37) that were introduced to displace full-length lp17 from wild-type S9. Pale-green shading indicates sequences that are deleted to the right of the synthetic telomere insertion points (red arrowheads beneath the diagram) in the respective lp17 truncations. This diagram is closely modeled after a figure by Sarkar et al. (30). (B) B. burgdorferi harboring lp17 truncations are infectious in mice. Three groups of 8 mice were injected with S9 (WT), S9/pGCB426 (WT/426), or S9/pGCB473 (WT/473). The ability of these B. burgdorferi strains to establish infection and persist in mice was assessed by seroconversion and isolation of spirochetes from ear biopsy specimens at 3 weeks postinfection and by isolation of spirochetes from ear, bladder, and joint tissues at 7 to 8 weeks postinfection, as indicated. *, P value of <0.05 compared to the WT using Fisher’s exact test; ns, no significant difference compared to the WT. (C) Acquisition of B. burgdorferi harboring lp17 truncations by larval ticks fed on infected mice. Three mice per strain that were positive for infection by both seroconversion and ear biopsy were used for larval tick feeding. The presence of spirochetes in fed larval ticks (left) was determined by culturing 36 individual ticks per strain in BSK medium. A subset of these fed larvae was also analyzed for spirochete burden, as determined by CFU in solid medium (right); each point denotes the number of viable spirochetes per tick. ***, P value of <0.0001 using Fisher’s exact test. There was no significant difference in prevalence of infection or spirochete burden between ticks infected with WT and those infected with WT/426. NA, not applicable since 0% of larvae acquired spirochetes from mice infected with WT/473.
Three groups of 8 mice were inoculated with WT or WT derivatives harboring the two different lp17 truncations. After 3 weeks, most mice from all 3 groups were infected, as indicated by seroconversion to B. burgdorferi proteins and isolation of spirochetes from ear punch biopsy specimens (Fig. 4B, left). These data demonstrate that spirochetes lacking approximately half of lp17, including bbd18, can establish infection in mice by needle inoculation. In addition, persistent infection by spirochetes in all 3 groups was demonstrated by isolation of B. burgdorferi from the ear tissues of most mice when the experiment was terminated at ~8 weeks postinoculation (Fig. 4B, right). Fewer isolates were obtained from bladder and joint tissues of mice inoculated with B. burgdorferi harboring either of the lp17 truncations (WT/pGCB426 and WT/pGCB473) than from mice inoculated with WT spirochetes (Fig. 4B, right). These results are in accord with the data from Casselli et al., in which they reported a defect in bladder colonization of mice infected with B. burgdorferi lacking bbd16 to bbd25 at 8 weeks postinoculation (38). However, because we observed a similar defect for spirochetes carrying either lp17 truncation, our results indicate that additional factors encoded in the bbd20 to bbd25 region of lp17 (and not just bbd18) likely contribute to the dissemination and/or persistent colonization of peripheral tissue by B. burgdorferi.
B. burgdorferi strains lacking bbd18 are not acquired by feeding Ixodes scapularis larvae.
Infected mice from this experiment were also used to feed larval ticks in order to assess the role of bbd18 and other lp17 genes in tick acquisition and transmission of B. burgdorferi. Naive I. scapularis larvae were allowed to feed to repletion on mice infected with WT, WT/pGCB426, and WT/pGCB473. Three mice per strain, which were positive by seroconversion and ear biopsy, were used for tick feeding approximately 3 weeks after initiation of infection. Fed larvae were crushed and either plated in solid Barbour-Stoenner-Kelly (BSK) medium to determine spirochete burden per tick or placed in liquid BSK medium to determine the prevalence of B. burgdorferi infection in larval ticks (Fig. 4C, right or left, respectively). Spirochetes were acquired by the majority of larvae that fed on mice infected with WT and WT/pGCB426, although the spirochete burden was slightly lower for WT/pGCB426. Strikingly, spirochetes were not present in any (n = 36) of the ticks that fed on WT/pGCB473-infected mice (Fig. 4C), suggesting that a factor encoded by bbd15 to bbd19 of lp17, potentially bbd18, contributes to efficient acquisition of B. burgdorferi by feeding larval ticks. Furthermore, spirochetes were subsequently isolated from the ear tissues of 2 out of 3 mice (~8 weeks postinoculation) on which these uninfected larvae had fed, indicating that the lack of acquisition of WT/pGCB473 by feeding ticks did not simply reflect the absence of spirochetes in the skin.
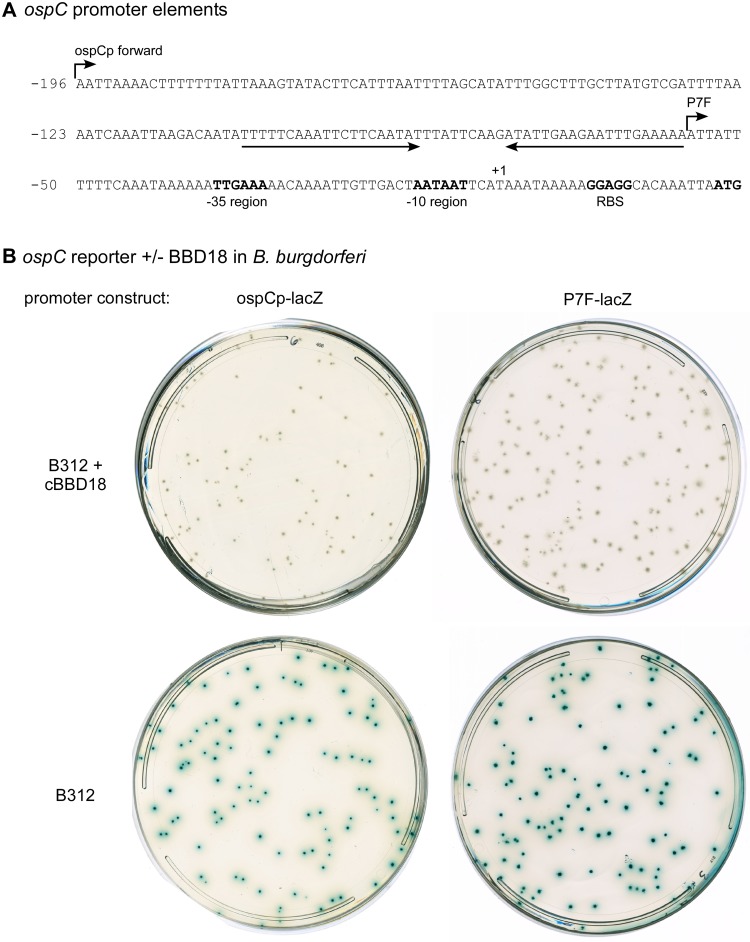
The ospC operator is not necessary for OspC regulation by BBD18.
The in vivo phenotype of spirochetes carrying lp17 truncations suggests that BBD18 plays a role at the host-to-vector interface rather than during infection of the mammalian host. Therefore, we questioned whether BBD18-mediated regulation of ospC could occur independently of the operator sequence described by Xu and colleagues, which is upstream of the core ospC promoter and required for persistent spirochete infection of mice (28). We utilized clone B312, which lacks lp17 (and bbd18), and a LacZ reporter system that we previously optimized for use in B. burgdorferi (lacZBb*) (30) to investigate the promoter elements required for regulation of ospC expression by BBD18.
We first transformed B312 with lacZBb* reporter constructs that carry either a full-length ospC promoter (ospCp-LacZ) or a minimal ospC promoter (P7F-lacZ) lacking the operator sequence (Fig. 5A). Xu et al. demonstrated that the minimal P7F promoter permitted ospC expression by B. burgdorferi during infection of a mouse but that these spirochetes were unable to subsequently downregulate ospC and thus were recognized and cleared by the acquired immune response of the host (28). In B312, both versions of the ospC promoter drove lacZ expression, as detected by β-galactosidase activity (blue colony color) in solid medium containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (Fig. 5B, bottom). We also introduced bbd18 into these clones on a compatible shuttle vector and found that BBD18 was able to prevent expression from both versions of the ospC promoter, regardless of whether the operator sequence was present or not (Fig. 5B, top). Furthermore, additional experiments demonstrated that the 5′ transcribed but untranslated region of ospC was not needed for regulation by BBD18 (data not shown). These data demonstrate that BBD18-mediated regulation of ospC in B312 occurs in an operator-independent manner, indicating that BBD18 is not the ospC-specific, in vivo repressor described by Xu et al. (28).
FIG 5 .
The ospC operator is not utilized by BBD18 for regulation in B. burgdorferi. (A) The nucleotide sequence of the ospC promoter and upstream regulatory regions. The palindromic operator sequence is indicated by a pair of convergent arrows. The −35 and −10 regions, the ribosome-binding site (RBS), and the start codon ATG of ospC are indicated in bold. The transcriptional start site of ospC (41) is designated +1. The 5′ ends of ospC promoter-lacZ reporter constructs, with or without the putative operator (ospCp forward and P7F, respectively), are indicated with arrows above the sequence. This diagram is closely modeled after a figure by Xu et al. (28). (B) BBD18-mediated repression of ospC promoter-lacZ fusions in B. burgdorferi. Strain B312, either with (top) or without (bottom) a constitutively expressed copy of the wild-type bbd18 gene (+cBBD18), also carried lacZ reporter constructs with different versions of the ospC promoter, as identified above the plates and described in panel A. LacZ gene expression in B. burgdorferi, as indicated by blue colony color, was visualized with X-Gal spread on plates after colony formation in BSK solid medium without phenol red.
DISCUSSION
In vivo regulation of OspC by BBD18.
We previously demonstrated a direct correlation between expression of bbd18 and suppression of OspC synthesis in an avirulent clone of B. burgdorferi (30). In the current study, we extended this analysis to WT B. burgdorferi in an experimental mouse-tick infectious cycle, with clear biological significance: engineered expression of bbd18 in otherwise WT B. burgdorferi blocked mouse infection by needle challenge and tick bite (Tables 1 and 2). Significantly, constitutive expression of bbd18 prevented the induction of ospC expression by spirochetes in feeding ticks (Fig. 2), providing one obvious reason for their loss of infectivity. We also observed continued synthesis of OspA by all spirochetes in fed ticks when bbd18 was constitutively expressed, indicating a reciprocal loss of ospA repression (data not shown). Despite profoundly detrimental consequences for spirochete viability in the mammalian host, constitutive expression of bbd18 did not impair B. burgdorferi colonization, replication, or persistence in the tick vector (Fig. 1), and all tick-derived spirochetes analyzed retained the shuttle vector carrying bbd18 (data not shown). Of note, two mice fed on by nymphs infected with WT+cBBD18 became infected, but all spirochetes recovered from these mice had lost the shuttle vector and thus reverted to WT; this outcome further illustrates the negative impact of constitutive bbd18 expression on mouse infection by B. burgdorferi and the strong selection imposed against it during tick transmission.
In the current study, we have also demonstrated a strict correlation between the ability of BBD18 to suppress OspC synthesis by spirochetes grown in vitro (Fig. 3B) and the ability of BBD18 to block infection of mice when overexpressed by spirochetes (Table 3). This was accomplished with a set of BBD18 variants with site-directed mutations in amino acid residues that are strictly conserved among BBD18 homologs in Lyme disease and relapsing fever Borrelia. These substitutions were clustered in a region of the BBD18 protein that has weak similarity with a DNA-binding motif of SopB (Fig. 3A), a protein involved in plasmid segregation in E. coli (35). We hypothesized that if BBD18 were indeed interacting with nucleic acids through a similar motif as a transcriptional regulator, we might be able to abrogate repressor function by site-directed mutagenesis within this region. There was no correlation, however, between the predicted DNA-binding residues based on homology with the SopB sequence (Fig. 3A) and the ability of these BBD18 variants to prevent synthesis of OspC (Fig. 3B), leading us to conclude that the significance of this weak similarity is unclear and may have no functional relevance.
Fortuitously, we found one variant, BBD18v3, which was not able to prevent OspC synthesis (Fig. 3B). Four amino acids substitutions distinguish BBD18v3 from wild-type BBD18 (Fig. 3A) and thus may be involved in BBD18 function. The charge reversals in BBD18v3 (K58E, K79E, D94R, and D96R) distinguish it from BBD18v1, in which these and an additional residue were mutated to alanine, with less effect on activity. However, single mutations for each of these residues yielded BBD18 variants that retained a full ability to suppress OspC synthesis by B312 (data not shown). The amino acid substitutions in BBD18v3 would not be predicted to disrupt function through a gross alteration of the overall structure, but they may impede critical interactions of BBD18v3 with other macromolecules. Further investigation will be required to determine the significance of this region and these particular residues for BBD18 structure and function.
BBD18 is not required for persistent infection of mice.
We have shown that constitutive expression of bbd18 in an otherwise WT clone prevents the synthesis of OspC by these spirochetes in feeding ticks and prevents subsequent infection in mice. We hypothesized that spirochetes lacking bbd18 would initially retain infectivity in mice, since OspC could still be made by these spirochetes when they were transmitted or inoculated. However, if BBD18 were the in vivo ospC repressor whose primary function is to downregulate OspC after the initial stage of mammalian infection, then spirochetes lacking BBD18 should not persist, as the mammalian immune system would target spirochetes that continue to make OspC.
During the course of these studies, Casselli et al. reported that a B. burgdorferi variant with an experimentally truncated form of lp17 that lacks bbd18 was able to establish chronic infection in mice but had delayed tissue colonization (38). We conducted similar experiments with two different wild-type derivatives carrying lp17 truncations that retained or lacked bbd18. Similar to Casselli et al., we found that spirochetes lacking bbd18 (as well as additional lp17 genes) were infectious by needle inoculation, but our data indicate that the previously described colonization defect of such B. burgdorferi variants does not stem entirely from the deletion of bbd18 (Fig. 4). Liang and colleagues reported that downregulation of OspC during B. burgdorferi infection of immunocompetent mice occurs from day 17 onward and that spirochetes that continue to express ospC are cleared (12, 14). However, both Casselli et al. (38) and we (Fig. 4B) found that spirochetes lacking bbd18 were able to persistently infect mice for at least 8 weeks.
The ability of these BBD18-null spirochetes to persist in mice beyond the point at which OspC would typically be downregulated does not support our original hypothesis that BBD18 functions as an essential in vivo OspC repressor (30). In addition, these data indicate that bbd15 to bbd25 of lp17 are not required for B. burgdorferi viability in vitro nor infection of mice in vivo. Thus, it remains unclear why inactivation of bbd18 in WT B. burgdorferi presented such a challenge and why, once isolated, the bbd18 mutant had accumulated secondary mutations that rendered it noninfectious in mice. We have determined that the sole bbd18 mutant we obtained in a WT background (and its complement) do not synthesize OspC when subjected to temperature or pH shift (data not shown), suggesting an explanation for the noninfectious phenotype of these clones. Furthermore, the ease with which we have been able to inactivate bbd18 in a noninfectious B. burgdorferi clone (data not shown), which also cannot be induced to synthesize OspC, indicates the complex role of bbd18, and possibly other lp17 genes, in wild-type bacteria.
Lp17 genes required for acquisition of B. burgdorferi by larval ticks.
None of the genes in the bbd15 to bbd25 region of lp17 were absolutely required for persistent and disseminated infection of mice. However, we observed a dramatic impact on spirochete acquisition by feeding larval ticks when the lp17 truncation extended through bbd18 (Fig. 4). We repeated this experiment and used a 10-fold-higher inoculum to optimize the spirochete load in infected mouse tissues and enhance subsequent acquisition by feeding larvae (data not shown). Again, all mice became infected, and both WT and WT/pGCB426 spirochetes (with bbd18) colonized all of the fed larval ticks that were analyzed (data not shown). However, as in the first experiment (Fig. 4C), WT/pGCB473 spirochetes (minus bbd18) were not present in any of the fed larvae analyzed, confirming our initial result. Together, these data indicate that something within the bbd15 to bbd19 region of lp17 (potentially bbd18) is required for successful colonization of feeding ticks by B. burgdorferi. We have observed delayed outgrowth and reduced spirochete loads in tissues of mice infected with WT/pGCB473 relative to WT or WT/pGCB426 (data not shown). However, the complete absence of WT/pGCB473 spirochetes in the relatively large number of fed larvae analyzed to date (121 ticks from 3 separate feedings) suggests a more specific defect at the point of larval tick acquisition and/or colonization, and preliminary studies indicate that WT/pGCB473 spirochetes do not colonize larval ticks even when introduced by artificial infection (data not shown). Additional experiments are required to determine what functional deficit accounts for this phenotype and whether loss of bbd18 and/or some other genetic element in the bbd15 to bbd19 region of lp17 is responsible. Most of the annotated genes in this region (bbd15, bbd15.01, bbd15.1, bbd16, bbd17, and bbd19) (39) encode very small and overlapping open reading frames that are poorly conserved among B. burgdorferi isolates (30). It is conceivable that these or other sequences in the bbd15 to bbd19 region of lp17 encode small regulatory RNAs. However, bbd18 is a relatively large, autonomous, and well-conserved gene and is the only annotated open reading frame in this region that has been shown to encode an actual protein.
Indirect BBD18-mediated regulation of ospC through RpoS.
The data presented in this study indicate that BBD18 does not represent the ospC-specific repressor required for persistent B. burgdorferi infection of a mammalian host. Xu and colleagues (28) described an operator sequence upstream of the core ospC promoter that was required for repression of ospC in vivo. We found that only the core ospC promoter, without the operator site, was required for BBD18-mediated suppression of ospC expression (Fig. 5). It is well documented that expression of ospC in wild-type B. burgdorferi is dependent on the alternative sigma factor RpoS and requires only the core ospC promoter (17, 20, 23, 25), and we confirmed that this was also the case in the B. burgdorferi strain in which these constructs were analyzed (see Fig. S1 in the supplemental material). In a parallel and concurrent study, we have demonstrated that bbd18 expression results in posttranscriptional negative regulation of RpoS (43). Together, these findings are wholly consistent with a model in which BBD18 plays a key role in the global repression of all RpoS-dependent genes of B. burgdorferi during larval tick acquisition, when the “on” environmental cues that induce rpoS expression are present and yet ospC and other RpoS-dependent genes (including the ospA repressor) are not expressed. In support of this model, preliminary analyses indicate that the bbd18 transcript is present at relatively high levels in fed larval ticks, whereas it is very low or undetectable in infected mouse tissues and fed nymphal ticks (data not shown). We propose that the strict conservation of bbd18 in both Lyme disease and relapsing fever spirochetes, which are transmitted by dissimilar tick vectors and exhibit dramatically different dynamics of infection, reflects a common purpose as an “off” regulatory switch in the critical adaptive response of Borrelia at the host-to-vector interface. Future experiments will be directed at elucidating the structure, function, and regulation of BBD18 during the infectious cycle of B. burgdorferi.
MATERIALS AND METHODS
Mouse infection.
All mouse studies were done in accordance with guidelines of the National Institutes of Health, and protocols were approved by the Rocky Mountain Laboratories Animal Care and Use Committee. The Rocky Mountain Laboratories are accredited by the International Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Infection studies were conducted with 6- to 8-week-old female RML mice, an outbred strain of Swiss-Webster mice reared at the Rocky Mountain Laboratories breeding facility. Mice were bled prior to injection with a target dose of 104 B. burgdorferi spirochetes (8 × 103 spirochetes intraperitoneally and 2 × 103 spirochetes subcutaneously). After 3 weeks, mice were bled to assess seroconversion to B. burgdorferi whole-cell lysates, and ear punch biopsy specimens were placed into BSK II medium to assess the presence of spirochetes in these tissues. At 4 to 8 weeks postinoculation, mice were euthanized and ear, bladder, and ankle joint tissues were cultured in BSK II medium for final assessment of infection.
Tick transmission studies.
Ixodes scapularis larvae were artificially infected as previously described (34). Briefly, I. scapularis larvae were partially dehydrated for 48 h at a relative humidity of 85% before submersion in a B. burgdorferi culture (~1 × 108 spirochetes/ml). The ticks were incubated with B. burgdorferi for 45 min at 35°C, washed twice with phosphate-buffered saline (PBS), and then allowed to recover for 48 h before feeding to repletion on naive mice (~100 ticks/mouse). Uninfected larvae were fed on infected mice to assess tick acquisition of different B. burgdorferi strains.
To assess colonization by B. burgdorferi, a subset of fed larvae were crushed 1 to 3 weeks after drop off and either plated in solid BSK medium or placed in liquid BSK medium. The remaining fed I. scapularis larvae were allowed to molt to nymphs and recover (approximately 12 weeks after larval feeding) before feeding on naive mice (~5 nymphs/mouse). Several I. scapularis nymphs from each infected tick cohort were prepared for immunofluorescence (see Text S1 in the supplemental material) shortly after feeding to repletion. The remaining fed nymphs (1 or 2/mouse) were crushed and plated in BSK solid medium at 7 days after drop off to enumerate spirochetes. Mice that were fed on by infected I. scapularis were euthanized at 3 weeks postfeeding and processed as described above to assess B. burgdorferi transmission via tick bite and ensuing infection.
Site-directed mutagenesis of BBD18.
Based on BLAST alignment of WT BBD18 (B. burgdorferi B31, GenBank accession no. AE000793.2) with amino acid residues contacting DNA in SopB (35) and conserved residues in other Borrelia species (B. recurrentis A1, plasmid plate 6, GenBank accession no. CP001000.1; B. hermsii MTW, plasmid contig 0014, GenBank accession no. CP005691.1), we designed four bbd18 variants, each with multiple amino acid substitutions (Fig. 3A). The changes are as follows: bbd18v1 has lysine 58 changed to alanine (K58A), S67A, K79A, D94A, and D96A; bbd18v2 has I57A, K58A, S66A, S67A, N69A, and M72A; bbd18v3 has K58E, K79E, D94R, and D96R; bbd18v4 has S67A and V85A. These BBD18 variants have predicted secondary structures similar to that of wild-type BBD18 (PSIPRED Protein Structure Prediction Server; available at http://bioinf.cs.ucl.ac.uk/psipred/). Variant bbd18 genes were synthesized by GenScript Corporation (Piscataway, NJ) with EcoRI recognition sites at both ends. These synthetic genes were excised from pUC57 with EcoRI-HF (New England Biolabs, Ipswich, MA) and ligated into appropriately digested and dephosphorylated pBSV2*flaBp (40) in a manner following the construction of pBSV2*flaBp-bbd18, which contains the wild-type bbd18 gene under expression of the constitutive flaB promoter (30). Resulting clones were screened by PCR to confirm orientation and the presence of the insert and then sequenced to confirm that no other mutations had arisen during cloning. These shuttle vectors were transformed into B312 and assessed by immunoblotting for their ability to suppress OspC synthesis. Two of these shuttle vectors, pBSV2*flaBp-bbd18v3 and pBSV2*flaBp-bbd18v4, were also transformed into S9.
SUPPLEMENTAL MATERIAL
Supplemental Materials and Methods includes information about bacterial strains and growth conditions, plasmid construction, β-galactosidase assays, protein analysis, immunoblotting, and fluorescence microscopy. Download
RpoS dependence of the ospC promoter varies between E. coli and B. burgdorferi. (A) OspC production in B. burgdorferi strain B312 requires RpoS. Whole-cell lysates of B312, A34 (negative control), and B312Δrpos, as indicated, were analyzed with antisera recognizing OspC or FlaB, which was used as an internal control for equivalent protein loading. Numbers on the left refer to molecular mass in kDa relative to the mobility of protein standards. The rpoS gene in B312 was inactivated with an allelic exchange construct previously used to inactivate rpoS in wild-type B. burgdorferi (42). OspC was present in B312, as anticipated, but not in B312ΔrpoS or A34, a noninfectious B31 clone that does not make OspC. These data confirm that ospC expression in B312 is RpoS dependent and validate using B312 to analyze ospC repression by BBD18. (B) Expression from the ospC promoter in E. coli is not RpoS dependent. Wild-type E. coli (wt) or an rpoS mutant strain (ΔrpoS) harboring lacZ reporter constructs without a promoter (no promoter, negative control), with a constitutive promoter (flaB promoter, positive control), or the ospC promoter, as indicated, were grown on LB agar containing gentamicin and X-Gal. The ospC and flaB promoters were active in both backgrounds, whereas the promoterless construct was inactive, indicating that transcription from the ospC promoter in E. coli does not require RpoS. This outcome confirms that BBD18 prevents ospC transcription through an RpoS-dependent mechanism in B. burgdorferi (A) and provides an explanation for the observed lack of repression in E. coli (30). These results also demonstrate the limited utility of E. coli as a heterologous host in which to investigate the regulation of ospC or other RpoS-dependent genes of B. burgdorferi. Download
Strains and plasmids used in this study.
Primers used in this study.
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
We thank Dave Dorward for assistance with confocal microscope analysis of tick samples and Sonja Best for advice and reagents for site-directed mutagenesis. We thank Tom Schwan for providing access to unpublished sequences of the relapsing fever spirochetes. We are grateful to George Chaconas, Roberto Kolter, Robert Gilmore, and Lamine Mbow for providing strains and antibodies. We thank Anita Mora, Heather Murphy, and Austin Athman for help with graphics. We are grateful to Paul Policastro, Frank Gherardini, Philip Stewart, Tom Schwan, Jorge Benach, and George Chaconas for constructive comments on the manuscript.
Footnotes
Citation Hayes BM, Dulebohn DP, Sarkar A, Tilly K, Bestor A, Ambroggio X, Rosa PA. 2014. Regulatory protein BBD18 of the Lyme disease spirochete: essential role during tick acquisition? mBio 5(2):e01017-14. doi:10.1128/mBio.01017-14.
REFERENCES
- 1. Benach JL, Bosler EM, Hanrahan JP, Coleman JL, Bast TF, Habicht GS, Cameron DJ, Ziegler JL, Burgdorfer W, Barbour AG, Edelman R, Kaslow RA. 1983. Spirochetes isolated from the blood of two patients with Lyme disease. N. Engl. J. Med. 308:740–742. 10.1056/NEJM198303313081302 [DOI] [PubMed] [Google Scholar]
- 2. Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. 1982. Lyme disease—a tick-borne spirochetosis? Science 216:1317–1319. 10.1126/science.7043737 [DOI] [PubMed] [Google Scholar]
- 3. Steere AC, Grodzicki RL, Kornblatt AN, Craft JE, Barbour AG, Burgdorfer W, Schmid GP, Johnson E, Malawista SE. 1983. The spirochetal etiology of Lyme disease. N. Engl. J. Med. 308:733–740. 10.1056/NEJM198303313081301 [DOI] [PubMed] [Google Scholar]
- 4. Lane RS, Piesman J, Burgdorfer W. 1991. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annu. Rev. Entomol. 36:587–609. 10.1146/annurev.en.36.010191.003103 [DOI] [PubMed] [Google Scholar]
- 5. Schwan TG, Piesman J, Golde WT, Dolan MC, Rosa PA. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. U. S. A. 92:2909–2913. 10.1073/pnas.92.7.2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ohnishi J, Piesman J, de Silva AM. 2001. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc. Natl. Acad. Sci. U. S. A. 98:670–675. 10.1073/pnas.98.2.670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schwan TG, Piesman J. 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38:382–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grimm D, Tilly K, Byram R, Stewart PE, Krum JG, Bueschel DM, Schwan TG, Policastro PF, Elias AF, Rosa PA. 2004. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. U. S. A. 101:3142–3147. 10.1073/pnas.0306845101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stewart PE, Wang X, Bueschel DM, Clifton DR, Grimm D, Tilly K, Carroll JA, Weis JJ, Rosa PA. 2006. Delineating the requirement for the Borrelia burgdorferi virulence factor OspC in the mammalian host. Infect. Immun. 74:3547–3553. 10.1128/IAI.00158-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tilly K, Bestor A, Jewett MW, Rosa P. 2007. Rapid clearance of Lyme disease spirochetes lacking OspC from skin. Infect. Immun. 75:1517–1519. 10.1128/IAI.01725-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tilly K, Krum JG, Bestor A, Jewett MW, Grimm D, Bueschel D, Byram R, Dorward D, Vanraden MJ, Stewart P, Rosa P. 2006. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect. Immun. 74:3554–3564. 10.1128/IAI.01950-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liang FT, Jacobs MB, Bowers LC, Philipp MT. 2002. An immune evasion mechanism for spirochetal persistence in Lyme borreliosis. J. Exp. Med. 195:415–422. 10.1084/jem.20011870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liang FT, Nelson FK, Fikrig E. 2002. Molecular adaptation of Borrelia burgdorferi in the murine host. J. Exp. Med. 196:275–280. 10.1084/jem.20020770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liang FT, Yan J, Mbow ML, Sviat SL, Gilmore RD, Mamula M, Fikrig E. 2004. Borrelia burgdorferi changes its surface antigenic expression in response to host immune responses. Infect. Immun. 72:5759–5767. 10.1128/IAI.72.10.5759-5767.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu Q, Seemanapalli SV, McShan K, Liang FT. 2006. Constitutive expression of outer surface protein C diminishes the ability of Borrelia burgdorferi to evade specific humoral immunity. Infect. Immun. 74:5177–5184. 10.1128/IAI.00713-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brooks CS, Hefty PS, Jolliff SE, Akins DR. 2003. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect. Immun. 71:3371–3383. 10.1128/IAI.71.6.3371-3383.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang X, Goldberg MS, Popova TG, Schoeler GB, Wikel SK, Hagman KE, Norgard MV. 2000. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol. Microbiol. 37:1470–1479. 10.1046/j.1365-2958.2000.02104.x [DOI] [PubMed] [Google Scholar]
- 18. Lybecker MC, Samuels DS. 2007. Temperature-induced regulation of RpoS by a small RNA in Borrelia burgdorferi. Mol. Microbiol. 64:1075–1089. 10.1111/j.1365-2958.2007.05716.x [DOI] [PubMed] [Google Scholar]
- 19. Smith AH, Blevins JS, Bachlani GN, Yang XF, Norgard MV. 2007. Evidence that RpoS (sigmas) in Borrelia burgdorferi is controlled directly by RpoN (sigma54/sigmaN). J. Bacteriol. 189:2139–2144. 10.1128/JB.01653-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hübner A, Yang X, Nolen DM, Popova TG, Cabello FC, Norgard MV. 2001. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc. Natl. Acad. Sci. U. S. A. 98:12724–12729. 10.1073/pnas.231442498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burtnick MN, Downey JS, Brett PJ, Boylan JA, Frye JG, Hoover TR, Gherardini FC. 2007. Insights into the complex regulation of rpoS in Borrelia burgdorferi. Mol. Microbiol. 65:277–293. 10.1111/j.1365-2958.2007.05813.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Caimano MJ, Iyer R, Eggers CH, Gonzalez C, Morton EA, Gilbert MA, Schwartz I, Radolf JD. 2007. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol. Microbiol. 65:1193–1217. 10.1111/j.1365-2958.2007.05860.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang XF, Lybecker MC, Pal U, Alani SM, Blevins J, Revel AT, Samuels DS, Norgard MV. 2005. Analysis of the ospC regulatory element controlled by the RpoN-RpoS regulatory pathway in Borrelia burgdorferi. J. Bacteriol. 187:4822–4829. 10.1128/JB.187.14.4822-4829.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Caimano MJ, Eggers CH, Hazlett KR, Radolf JD. 2004. RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants. Infect. Immun. 72:6433–6445. 10.1128/IAI.72.11.6433-6445.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eggers CH, Caimano MJ, Radolf JD. 2004. Analysis of promoter elements involved in the transcriptional initiation of RpoS-dependent Borrelia burgdorferi genes. J. Bacteriol. 186:7390–7402. 10.1128/JB.186.21.7390-7402.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crother TR, Champion CI, Whitelegge JP, Aguilera R, Wu XY, Blanco DR, Miller JN, Lovett MA. 2004. Temporal analysis of the antigenic composition of Borrelia burgdorferi during infection in rabbit skin. Infect. Immun. 72:5063–5072. 10.1128/IAI.72.9.5063-5072.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ouyang Z, Narasimhan S, Neelakanta G, Kumar M, Pal U, Fikrig E, Norgard MV. 2012. Activation of the RpoN-RpoS regulatory pathway during the enzootic life cycle of Borrelia burgdorferi. BMC Microbiol. 12:44. 10.1186/1471-2180-12-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu Q, McShan K, Liang FT. 2007. Identification of an ospC operator critical for immune evasion of Borrelia burgdorferi. Mol. Microbiol. 64:220–236. 10.1111/j.1365-2958.2007.05636.x [DOI] [PubMed] [Google Scholar]
- 29. Xu Q, McShan K, Liang FT. 2008. Verification and dissection of the ospC operator by using flaB promoter as a reporter in Borrelia burgdorferi. Microb. Pathog. 45:70–78. 10.1016/j.micpath.2008.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sarkar A, Hayes BM, Dulebohn DP, Rosa PA. 2011. Regulation of the virulence determinant OspC by bbd18 on linear plasmid lp17 of Borrelia burgdorferi. J. Bacteriol. 193:5365–5373. 10.1128/JB.01496-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lawrenz MB, Kawabata H, Purser JE, Norris SJ, Chaparro E, Pérez-Pastrana E, Vivo A, Anda P. 2002. Decreased electroporation efficiency in Borrelia burgdorferi containing linear plasmids lp25 and lp56: impact on transformation of infectious Borrelia. Infect. Immun. 70:4851–4858. 10.1128/IAI.70.9.4851-4858.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kawabata H, Norris SJ, Watanabe H. 2004. BBE02 disruption mutants of Borrelia burgdorferi B31 have a highly transformable, infectious phenotype. Infect. Immun. 72:7147–7154. 10.1128/IAI.72.12.7147-7154.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rego RO, Bestor A, Rosa PA. 2011. Defining the plasmid-encoded restriction-modification systems of the Lyme disease spirochete Borrelia burgdorferi. J. Bacteriol. 193:1161–1171. 10.1128/JB.01176-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Policastro PF, Schwan TG. 2003. Experimental infection of Ixodes scapularis larvae (acari: Ixodidae) by immersion in low passage cultures of Borrelia burgdorferi. J. Med. Entomol. 40:364–370. 10.1603/0022-2585-40.3.364 [DOI] [PubMed] [Google Scholar]
- 35. Schumacher MA, Piro KM, Xu W. 2010. Insight into F plasmid DNA segregation revealed by structures of SopB and SopB-DNA complexes. Nucleic Acids Res. 38:4514–4526. 10.1093/nar/gkq161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sadziene A, Wilske B, Ferdows MS, Barbour AG. 1993. The cryptic ospC gene of Borrelia burgdorferi B31 is located on a circular plasmid. Infect. Immun. 61:2192–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Beaurepaire C, Chaconas G. 2005. Mapping of essential replication functions of the linear plasmid lp17 of Borrelia burgdorferi by targeted deletion walking. Mol. Microbiol. 57:132–142. 10.1111/j.1365-2958.2005.04688.x [DOI] [PubMed] [Google Scholar]
- 38. Casselli T, Tourand Y, Bankhead T. 2012. Altered murine tissue colonization by Borrelia burgdorferi following targeted deletion of linear plasmid 17-carried genes. Infect. Immun. 80:1773–1782. 10.1128/IAI.05984-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Casjens S, Palmer N, van Vugt R, Huang WM, Stevenson B, Rosa P, Lathigra R, Sutton G, Peterson J, Dodson RJ, Haft D, Hickey E, Gwinn M, White O, Fraser CM. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490–516 [DOI] [PubMed] [Google Scholar]
- 40. Bestor A, Stewart PE, Jewett MW, Sarkar A, Tilly K, Rosa PA. 2010. Use of the Cre-lox recombination system to investigate lp54 gene requirement in the infectious cycle of Borrelia burgdorferi. Infect. Immun. 78:2397–2407. 10.1128/IAI.01059-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marconi RT, Samuels DS, Garon CF. 1993. Transcriptional analyses and mapping of the ospC gene in Lyme disease spirochetes. J. Bacteriol. 175:926–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Elias AF, Stewart PE, Grimm D, Caimano MJ, Eggers CH, Tilly K, Bono JL, Akins DR, Radolf JD, Schwan TG, Rosa P. 2002. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect. Immun. 70:2139–2150. 10.1128/IAI.70.4.2139-2150.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dulebohn DP, Hayes BM, Rosa PA. Global repression of host-associated genes of the Lyme disease spirochete through post-transcriptional modulation of the alternative sigma factor RpoS. PLoS ONE, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Materials and Methods includes information about bacterial strains and growth conditions, plasmid construction, β-galactosidase assays, protein analysis, immunoblotting, and fluorescence microscopy. Download
RpoS dependence of the ospC promoter varies between E. coli and B. burgdorferi. (A) OspC production in B. burgdorferi strain B312 requires RpoS. Whole-cell lysates of B312, A34 (negative control), and B312Δrpos, as indicated, were analyzed with antisera recognizing OspC or FlaB, which was used as an internal control for equivalent protein loading. Numbers on the left refer to molecular mass in kDa relative to the mobility of protein standards. The rpoS gene in B312 was inactivated with an allelic exchange construct previously used to inactivate rpoS in wild-type B. burgdorferi (42). OspC was present in B312, as anticipated, but not in B312ΔrpoS or A34, a noninfectious B31 clone that does not make OspC. These data confirm that ospC expression in B312 is RpoS dependent and validate using B312 to analyze ospC repression by BBD18. (B) Expression from the ospC promoter in E. coli is not RpoS dependent. Wild-type E. coli (wt) or an rpoS mutant strain (ΔrpoS) harboring lacZ reporter constructs without a promoter (no promoter, negative control), with a constitutive promoter (flaB promoter, positive control), or the ospC promoter, as indicated, were grown on LB agar containing gentamicin and X-Gal. The ospC and flaB promoters were active in both backgrounds, whereas the promoterless construct was inactive, indicating that transcription from the ospC promoter in E. coli does not require RpoS. This outcome confirms that BBD18 prevents ospC transcription through an RpoS-dependent mechanism in B. burgdorferi (A) and provides an explanation for the observed lack of repression in E. coli (30). These results also demonstrate the limited utility of E. coli as a heterologous host in which to investigate the regulation of ospC or other RpoS-dependent genes of B. burgdorferi. Download
Strains and plasmids used in this study.
Primers used in this study.