Abstract
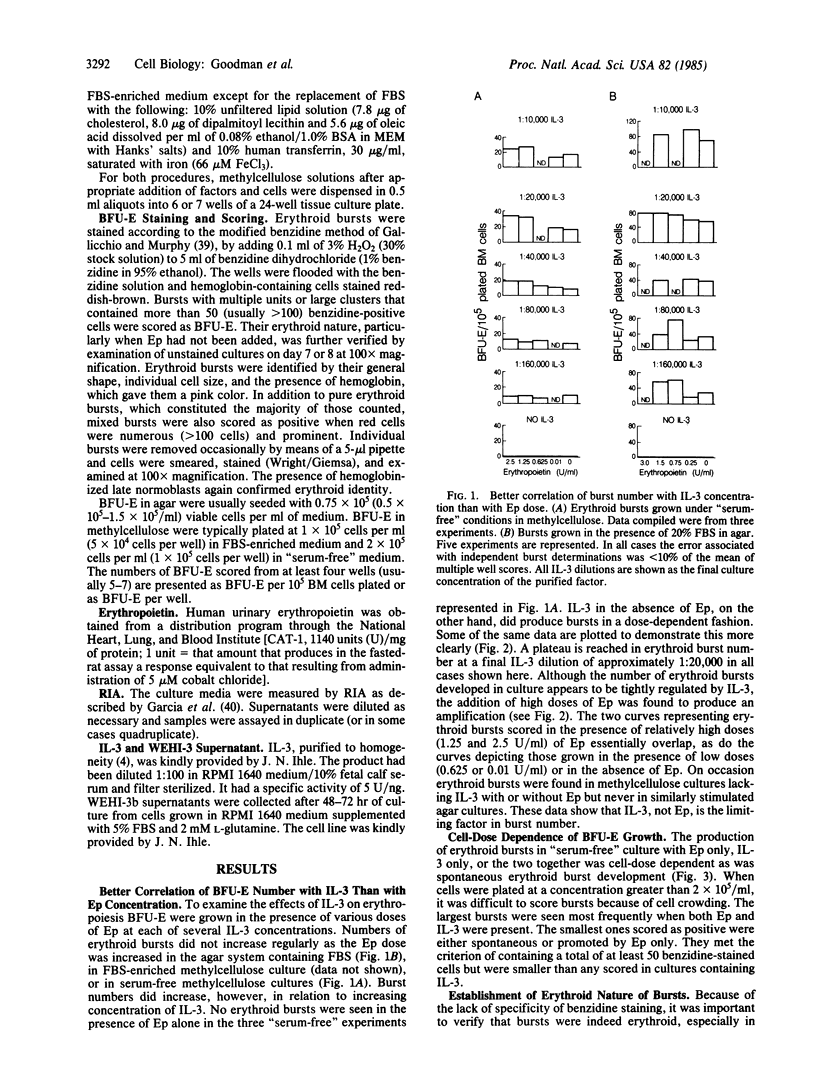
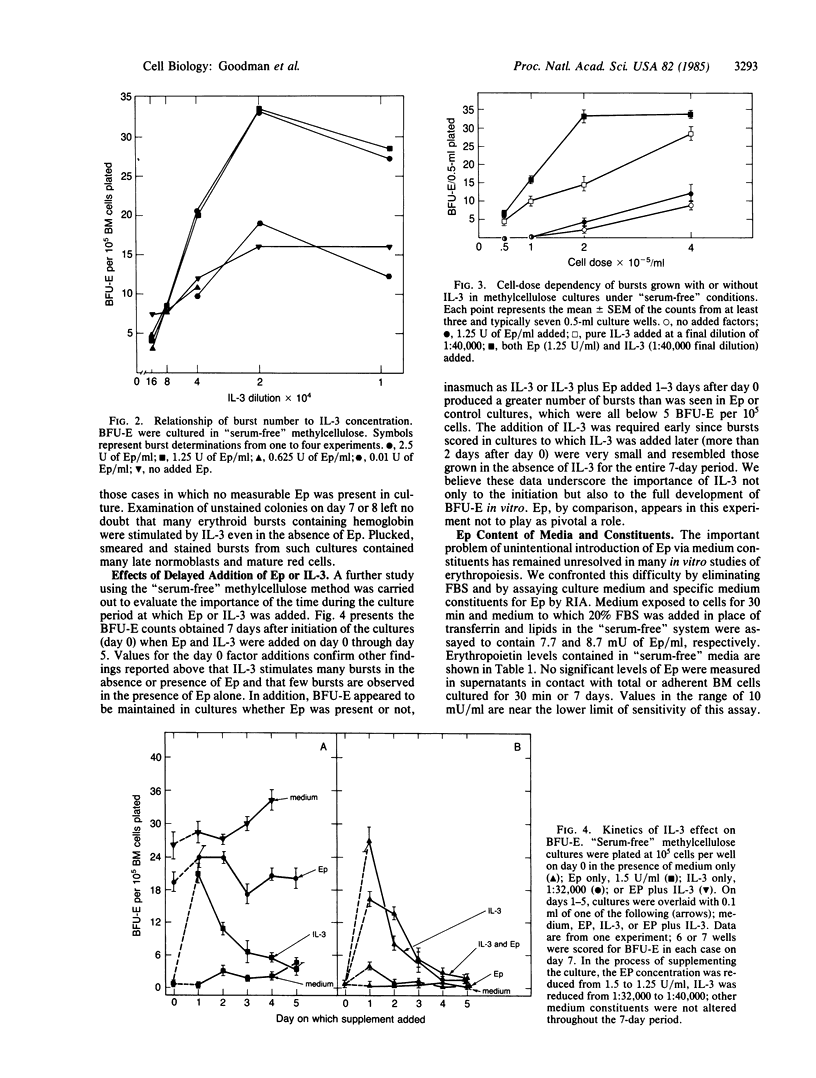
Erythroid burst-forming units (BFU-E) from mouse bone marrow were grown for 7 days in agar or serum-free methylcellulose cultures in the presence or absence of erythropoietin (Ep) and/or interleukin 3 (IL-3). It was found that IL-3, even in the absence of serum and detectable Ep, was able to stimulate the full development of many erythroid bursts. This IL-3 effect was cell-dose dependent and did not appear to correlate with Ep dose. Spontaneous bursts and those stimulated by Ep only were rare and when seen were very small relative to those produced by IL-3 or IL-3 plus Ep. When addition of IL-3 or Ep to 7-day cultures was delayed, IL-3 but not Ep was shown to maintain BFU-E. No evidence was found by radioimmunoassay that Ep was produced or released in 7-day, "serum-free" cultures of bone marrow nor was Ep activity detected in culture media except those to which it had been added deliberately.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aye M. T. Erythroid colony formation in cultures of human marrow: effect of leukocyte conditioned medium. J Cell Physiol. 1977 Apr;91(1):69–77. doi: 10.1002/jcp.1040910108. [DOI] [PubMed] [Google Scholar]
- Aye M. T., Seguin J. A., McBurney J. P. Erythroid and granulocytic colony growth in cultures supplemented with human serum lipoproteins. J Cell Physiol. 1979 May;99(2):233–238. doi: 10.1002/jcp.1040990210. [DOI] [PubMed] [Google Scholar]
- Casadevall N., Vainchenker W., Lacombe C., Vinci G., Chapman J., Breton-Gorius J., Varet B. Erythroid progenitors in polycythemia vera: demonstration of their hypersensitivity to erythropoietin using serum free cultures. Blood. 1982 Feb;59(2):447–451. [PubMed] [Google Scholar]
- Cashman J., Henkelman D., Humphries K., Eaves C., Eaves A. Individual BFU-E in polycythemia vera produce both erythropoietin dependent and independent progeny. Blood. 1983 May;61(5):876–884. [PubMed] [Google Scholar]
- Dessypris E. N., Krantz S. B. Effect of pure erythropoietin on DNA-synthesis by human marrow day 15 erythroid burst forming units in short-term liquid culture. Br J Haematol. 1984 Feb;56(2):295–306. doi: 10.1111/j.1365-2141.1984.tb03957.x. [DOI] [PubMed] [Google Scholar]
- Dukes P. P., Ma A., Clemons G. K., Meytes D. Measurement of human erythroid burst-promoting activity by a specific cell culture assay. Exp Hematol. 1985 Jan;13(1):59–66. [PubMed] [Google Scholar]
- Dy M., Lebel B., Kamoun P., Hamburger J. Histamine production during the anti-allograft response. Demonstration of a new lymphokine enhancing histamine synthesis. J Exp Med. 1981 Feb 1;153(2):293–309. doi: 10.1084/jem.153.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliason J. F., Van Zant G. Erythropoietin-dependent and erythropoietin-independent enhancement of colony formation by immature erythroid progenitors (BFUe). Cell Tissue Kinet. 1983 Jan;16(1):65–76. [PubMed] [Google Scholar]
- Fagg B. Is erythropoietin the only factor which regulates late erythroid differentiation? Nature. 1981 Jan 15;289(5794):184–186. doi: 10.1038/289184a0. [DOI] [PubMed] [Google Scholar]
- Gallicchio V. S., Murphy M. J., Jr In vitro erythropoiesis. II. Cytochemical enumeration of erythroid stem cells (CFU-e and BFU-e) from normal mouse and human hematopoietic tissues. Exp Hematol. 1979 May;7(5):219–224. [PubMed] [Google Scholar]
- Garcia J. F., Ebbe S. N., Hollander L., Cutting H. O., Miller M. E., Cronkite E. P. Radioimmunoassay of erythropoietin: circulating levels in normal and polycythemic human beings. J Lab Clin Med. 1982 May;99(5):624–635. [PubMed] [Google Scholar]
- Garland J. M., Crompton S. A preliminary report: preparations containing Interleukin-3 (IL-3) promote proliferation of multipotential stem cells (CFUs) in the mouse. Exp Hematol. 1983 Sep;11(8):757–761. [PubMed] [Google Scholar]
- Graber S. E., Krantz S. B. Erythropoietin and the control of red cell production. Annu Rev Med. 1978;29:51–66. doi: 10.1146/annurev.me.29.020178.000411. [DOI] [PubMed] [Google Scholar]
- Greenberger J. S., Davisson P. B., Gans P. J., Moloney W. C. In vitro induction of continuous acute promyelocytic leukemia cell lines by Friend or Abelson murine leukemia virus. Blood. 1979 May;53(5):987–1001. [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Henderson L., Klein F., Palaszynski E. Procedures for the purification of interleukin 3 to homogeneity. J Immunol. 1982 Dec;129(6):2431–2436. [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Oroszlan S., Henderson L. E., Copeland T. D., Fitch F., Prystowsky M. B., Goldwasser E., Schrader J. W., Palaszynski E. Biologic properties of homogeneous interleukin 3. I. Demonstration of WEHI-3 growth factor activity, mast cell growth factor activity, p cell-stimulating factor activity, colony-stimulating factor activity, and histamine-producing cell-stimulating factor activity. J Immunol. 1983 Jul;131(1):282–287. [PubMed] [Google Scholar]
- Ihle J. N., Pepersack L., Rebar L. Regulation of T cell differentiation: in vitro induction of 20 alpha-hydroxysteroid dehydrogenase in splenic lymphocytes from athymic mice by a unique lymphokine. J Immunol. 1981 Jun;126(6):2184–2189. [PubMed] [Google Scholar]
- Johnson G. R. Colony formation in agar by adult bone marrow multipotential hemopoietic cells. J Cell Physiol. 1980 Jun;103(3):371–383. doi: 10.1002/jcp.1041030302. [DOI] [PubMed] [Google Scholar]
- Johnson G. R., Metcalf D. Pure and mixed erythroid colony formation in vitro stimulated by spleen conditioned medium with no detectable erythropoietin. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3879–3882. doi: 10.1073/pnas.74.9.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattime E. C., Pecoraro G. A., Stutman O. The activity of natural cytotoxic cells is augmented by interleukin 2 and interleukin 3. J Exp Med. 1983 Mar 1;157(3):1070–1075. doi: 10.1084/jem.157.3.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod D. L., Shreeve M. M., Axelrad A. A. Chromosome marker evidence for the bipotentiality of BFU-E. Blood. 1980 Aug;56(2):318–322. [PubMed] [Google Scholar]
- Nabel G., Galli S. J., Dvorak A. M., Dvorak H. F., Cantor H. Inducer T lymphocytes synthesize a factor that stimulates proliferation of cloned mast cells. Nature. 1981 May 28;291(5813):332–334. doi: 10.1038/291332a0. [DOI] [PubMed] [Google Scholar]
- Nagao K., Yokoro K., Aaronson S. A. Continuous lines of basophil/mast cells derived from normal mouse bone marrow. Science. 1981 Apr 17;212(4492):333–335. doi: 10.1126/science.7209531. [DOI] [PubMed] [Google Scholar]
- Porter P. N., Ogawa M., Leary A. G. Enhancement of the growth of human early erythroid progenitors by bone marrow conditioned media. Exp Hematol. 1980 Jan;8(1):83–88. [PubMed] [Google Scholar]
- Razin E., Cordon-Cardo C., Good R. A. Growth of a pure population of mouse mast cells in vitro with conditioned medium derived from concanavalin A-stimulated splenocytes. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2559–2561. doi: 10.1073/pnas.78.4.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich I. N., Heit W., Kubanek B. Extrarenal erythropoietin production by macrophages. Blood. 1982 Oct;60(4):1007–1018. [PubMed] [Google Scholar]
- Schrader J. W. Bone marrow differentiation in vitro. Crit Rev Immunol. 1983;4(3):197–277. [PubMed] [Google Scholar]
- Schrader J. W., Clark-Lewis I. A T cell-derived factor stimulating multipotential hemopoietic stem cells: molecular weight and distinction from T cell growth factor and T cell-derived granulocyte-macrophage colony-stimulating factor. J Immunol. 1982 Jul;129(1):30–35. [PubMed] [Google Scholar]
- Schrader J. W., Lewis S. J., Clark-Lewis I., Culvenor J. G. The persisting (P) cell: histamine content, regulation by a T cell-derived factor, origin from a bone marrow precursor, and relationship to mast cells. Proc Natl Acad Sci U S A. 1981 Jan;78(1):323–327. doi: 10.1073/pnas.78.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S., Zhu B., Axelrad A. A "serum-free" medium for the production of erythropoietic bursts by murine bone marrow cells. Exp Hematol. 1984 Jun;12(5):309–318. [PubMed] [Google Scholar]
- Tsang R. W., Aye M. T. Evidence for proliferation of erythroid progenitor cells in the absence of added erythropoietin. Exp Hematol. 1979 Aug;7(7):383–388. [PubMed] [Google Scholar]
- Weinstein Y. 20alpha-hydroxysteroid dehydrogenase: a T lymphocyte-associated enzyme. J Immunol. 1977 Oct;119(4):1223–1229. [PubMed] [Google Scholar]
- Yung Y. P., Eger R., Tertian G., Moore M. A. Long-term in vitro culture of murine mast cells. II. Purification of a mast cell growth factor and its dissociation from TCGF. J Immunol. 1981 Aug;127(2):794–799. [PubMed] [Google Scholar]


