Abstract
Early brain development is regulated by the coordinated actions of multiple signaling centers at key boundaries between compartments. Three telencephalic midline structures are in a position to play such roles in forebrain patterning: The cortical hem, the septum, and the thalamic eminence at the diencephalic–telencephalic boundary. These structures express unique complements of signaling molecules, and they also produce distinct populations of Cajal–Retzius cells, which are thought to act as “mobile patterning units,” migrating tangentially to cover the telencephalic surface. We show that these 3 structures require the transcription factor Lhx2 to delimit their extent. In the absence of Lhx2 function, all 3 structures are greatly expanded, and the Cajal–Retzius cell population is dramatically increased. We propose that the hem, septum, and thalamic eminence together form a “forebrain hem system” that defines and regulates the formation of the telencephalic midline. Disruptions in the forebrain hem system may be implicated in severe brain malformations such as holoprosencephaly. Lhx2 functions as a central regulator of this system's development. Since all components of the forebrain hem system have been identified across several vertebrate species, the mechanisms that regulate them may have played a fundamental role in driving key aspects of forebrain evolution.
Keywords: Cajal–Retzius cells, human, Lhx2, midline, mouse, patterning, thalamic eminence
Introduction
The midline of the central nervous system arises as a result of complex morphogenetic and inductive interactions. In the spinal cord, the roof plate is formed dorsally by the fusion of the 2 lateral margins of the neural plate, whereas the floor plate is induced ventrally by signals from the underlying notochord. The roof plate and the floor plate, in turn, function as secondary organizers, to regulate dorso-ventral identity along the entire length of the spinal cord (Yamada et al. 1991; Briscoe and Ericson 1999; Liem et al. 1997). In the forebrain, the dorsal midline is more complicated as a result of the prosencephalic vesicle giving rise to paired telencephalic evaginations and a central diencephalic vesicle. The choroid plexus has a central position in the forebrain: It forms at the medial edges of the telencephalic neuroepithelium (the telencephalic choroid plexus) and also at the roof of the diencephalon. An additional site of choroid plexus formation is the roof of the hindbrain. In this study, we examine 3 key forebrain structures connected with the telencephalic choroid plexus.
The telencephalic choroid plexus is flanked rostrally by the septum, on its telencephalic side by the cortical hem, and on its diencephalic side by the thalamic eminence.
Though the septum, hem, and thalamic eminence have been studied in literature as independent structures, they have certain fundamental similarities with respect to anatomy and function. Anatomically, these 3 structures surround the telencephalic choroid plexus, being physically connected with it (Figs 1 and 2). They are each positioned at strategic boundaries in the forebrain and have known or suggested roles as secondary organizers. Finally, each of these structures produce Cajal–Retzius cells, which are the sources of Reelin that is necessary for cortical development (D'Arcangelo et al. 1995; Ogawa et al. 1995).
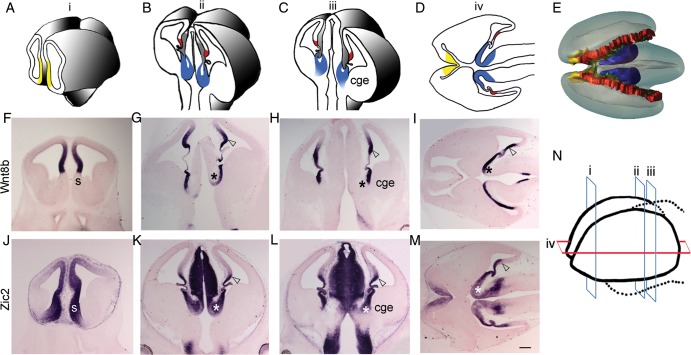
Figure 1.
Medial forebrain structures. (A–C) Schematics of 3 coronal levels of sectioning in the E12.5 mouse brain, corresponding to “i,” “ii,” and “iii” in N, respectively. “i” is at the level of septum (yellow); “ii” and “iii” show the cortical hem (red) and the thalamic eminence (TE; blue). TE is contiguous with the CGE as seen in level “iii.” (D) Schematic depicting the positions of septum, cortical hem, and TE in a horizontal section, corresponding to “iv” in N. The 3 rostro-caudal coronal levels (i, ii, and iii) and 1 horizontal level (iv) of sectioning in the E12.5 whole forebrain are schematized in N. (E) Dorsal view of a 3-D model of E12.5 brain showing the locations of septum (yellow), hem (red), and TE (blue), encircling the choroid plexus (green). Wnt8b is expressed in the medial neuroepithelium of the dorsal telencephalon, including the pallial septum (F), cortical hem (open arrowhead), and also in the ventricular zone of TE (asterisk; G–I). (J–M) Zic2 is expressed in the entire medial forebrain, including the forebrain hem system. CGE, caudal ganglionic eminence; h, hem; s, septum; TE, thalamic eminence. Scale bar: 300 µm.
Figure 2.
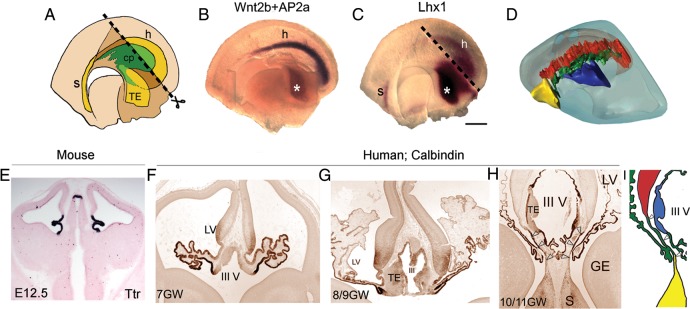
Connection of the choroid plexus with components of the forebrain hem system. (A) Schematic of the medial view of an E11.5 hemi-forebrain showing the position of the forebrain hem system (yellow), encircling the choroid plexus (cp; green). The dashed line (A and C) corresponds to the diencephalic midline that was bisected for the hemi-forebrain preparation (B). Whole mount in situ hybridization of an E12.5 hemi-forebrain reveals Wnt2b expression, specific to the cortical hem (purple), and AP2α expression, specific to the thalamic eminence (asterisk; brown). (C) Lhx1 is expressed in the E11.5 septum and thalamic eminence (asterisk) and also weakly in the cortical hem. (D) Lateral view of a single hemisphere in 3-D model of E12.5 brain showing the location of the septum (yellow), hem (red), and thalamic eminence (blue), encircling the choroid plexus (green). (E) E12.5 mouse brain sections processed for marker Ttr show the presence of the choroid plexus adjacent to both the lateral ventricles and the third ventricle. (F–H) Human sections immunostained by calbindin antibody show the development of choroid plexus from 7 to 10/11 GWs. (H) An oblique section of 10/11-GW human brain that shows the connection of choroid plexus with both the septum and cortical hem (open arrowheads), as also depicted in I. cp, choroid plexus; h, hem; s, septum; TE, thalamic eminence; III V, third ventricle. Scale bar: 200 µm (B and C).
The cortical hem is a Wnt and bone morphogenetic protein (Bmp) rich signaling centre that is connected to the choroid plexus on one side and the cortical neuroepithelium on the other (Grove et al. 1998). The hem was recently found to act as a secondary organizer that induces the formation of the hippocampus in the adjacent cortical neuroepithelium (Mangale et al. 2008), drawing a remarkable parallel with the roof plate of the spinal cord. At the rostral end of the telencephalon, the cortical hem is contiguous with another midline structure, the septum, a site that is enriched in Fgf family signaling molecules (Hoshikawa et al. 1998) and known to function as an organizer for cortical areal patterning (Fukuchi-Shimogori and Grove 2001). The thalamic eminence is a transient medial structure at the diencephalic–telencephalic boundary (DTB) of the forebrain. Based on its position and expression of signaling molecules, the thalamic eminence has been hypothesized to also serve as an organizer or a putative signaling centre (Abbott and Jacobowitz 1999; Kim et al. 2001; Fotaki et al. 2008). Though the septum continues into maturity, the hem and thalamic eminence are not discernible by late embryonic life: The hem gives way to the fimbria, while the thalamic eminence gets subsumed in diencephalic derivatives (Grove et al. 1998; Abbott and Jacobowitz 1999).
A distinctive feature of the septum, hem, and thalamic eminence is that they each produce a unique population of Cajal–Retzius cells, which are among the earliest born neurons of the developing forebrain (Meyer et al. 2002; Takiguchi-Hayashi et al. 2004; Bielle et al. 2005; Yoshida et al. 2006; Cabrera-Socorro et al. 2007; Abellan and Medina 2009; Abellan et al. 2010; Tissir et al. 2009; Meyer 2010). Though the Cajal–Retzius cells arising from these different origins are molecularly distinct, all secrete Reelin, a glycoprotein known to be critical for cortical lamination (D'Arcangelo et al. 1995; Ogawa et al. 1995). The expression of Reelin has been examined in extant vertebrates and appears to have been amplified in mammals, consistent with a role in the evolution of migratory mechanisms that led to the formation of laminated neocortex (Bar et al. 2000; Tissir et al. 2002). In the developing human brain, which has a protracted migratory period, Cajal–Retzius cells display complex morphologies and extend a distinctive Reelin-positive axonal plexus at the interface of both the marginal zone and the cortical plate (Meyer and Goffinet 1998). Furthermore, human Cajal–Retzius cells specifically express the Human accelerated region gene, HARF1, indicating human-specific traits of this cell type, further supporting an important role for these cells in neocortical evolution (Pollard et al. 2006). Finally, experiments using the combinations of transgenic and knockout mice have suggested that Cajal–Retzius cells may act as “mobile patterning units” providing regionalization cues to the developing telencephalon (Griveau et al. 2010). The septum, hem, and thalamic eminence, being the sites of the origin of Cajal–Retzius cells, are therefore important not only from a developmental, but also an evolutionary perspective.
In earlier work, we showed that the cortical hem expands in the absence of LIM-homeodomain transcription factor Lhx2 (Bulchand et al. 2001; Mangale et al. 2008). Here, we show that loss of Lhx2 also results in the expansion of septum and thalamic eminence. Concomitantly, there is a dramatic overproduction of Cajal–Retzius cells in the Lhx2 mutant. Thus Lhx2, a critical regulator of early forebrain development, controls the extent of the septum, hem, and thalamic eminence, and as a consequence, also the number of Cajal–Retzius cells produced. Since these 3 midline structures are developmentally and functionally linked, we propose that they together form a “forebrain hem system” that serves as a multicomponent patterning and organizing center for the medial forebrain.
Defective formation of the forebrain midline can give rise to severe malformations such as holoprosencephaly, one of the most common defects in brain development. Here, we compare these structures in the embryonic mouse and human forebrain. Our interpretation of the septum, hem, and thalamic eminence as a unified forebrain hem system offers a new framework in which such defects may be interpreted, leading to a better understanding of midline pathologies.
Materials and Methods
Animals and Sample Preparation
Mice
All procedures followed Institute Animal Ethics Committee guidelines and National Institutes of Health guidelines for the care and use of animals (mice). Timed pregnant Lhx2+/− mice (gift of F.D. Porter, NIH) were used. Pregnant dams were obtained from the Tata Institute animal breeding facility. A tamoxifen-inducible CreER line (strain name: B6; 129-Gt(ROSA)26Sortm1(cre/ERT)Nat/J; stock number: 004847) was obtained from the Jackson Laboratory. The floxed Lhx2 line (Lhx2lox/lox) was obtained from E.S. Monuki's lab. For in situ hybridization, the mouse embryos were harvested in phosphate buffer saline at E11, E11.5, and E12.5, fixed in 4% paraformaldehyde (PFA), equilibrated in 30% sucrose made in 4% PFA, and sectioned at 30 μm on a freezing microtome.
Human
Seven human embryonic and fetal brains (from 6 to 10/11 gestational weeks, GW) were examined. Embryonic brains were obtained and staged after legal abortions, following national guidelines in Spain and Belgium and in accordance with the institutional medical ethics committee guidelines (Meyer et al. 2000). Fetal brains were obtained after spontaneous abortions, under the same ethical guidelines. The whole heads were fixed immediately upon collection, in Bouin's fixative, embedded in paraffin, and cut in series of 10 μm sections. They were immunostained with antibodies to calbindin and Tbr1, as well as other antibodies, as described in Meyer et al. (2000) and re-examined for the present study.
Histochemical Procedures
In situ hybridization was performed as described previously in Bulchand et al. (2003). Briefly, the hybridization was performed overnight at 70°C in 4× sodium chloride-sodium citrate-citric acid buffer (SSC), 50% formamide, and 10% sodium dodecyl sulphate. Post hybridization washes were at 70°C in 2× SSC and 50% formamide. These were followed by washes in 2× SSC, 0.2× SSC, and then Tris-buffered saline with 1% Tween-20 (TBST). Anti-digoxigenin Fab fragments (Roche) were used at 1:5000 in TBST, and the color reaction using 4-nitro blue tetrazolium chloride + 5-bromo-4-chloro-3-indolyl phosphate (Roche) was performed according to the manufacturer's instructions. Immunohistochemistry were performed as described in Subramanian et al. (2011). Primary antibodies used are mouse anti-5-bromo-2′deoxyuridine (anti-BrdU; Roche; 1:100) and rabbit polyclonal anti-Ki67 (Abcam; 1:50). Immunohistochemistry on human embryonic and fetal sections was performed as described in Meyer et al. (2000) using a rabbit polyclonal calbindin antibody (Swant, Bellinzona, Switzerland) and Tbr1 antibody (kind gift from R. Hevner).
Quantitative Analysis
For area measurement studies, data were collected from 3 contiguous sections taken from 3 mouse brains at E12.5. The area was calculated using ImageJ software (NIH). For labeling index and quit fraction studies, confocal microscopy was used. Individual cells in the sections were imaged at a magnification of 40× using Zeiss LSM 510 Meta. Image stacks were generated by scanning at intervals of 0.44 μm using filters of the appropriate wavelengths. The cells were quantified using ImageJ software. The proliferating pool of cells in the telencephalon is known to include not only neuroepithelial stem cells, but also microglia and endothelial cells. However, the microglia have only just begun to invade the cortex at E10.5–E11.5 (Swinnen et al. 2012). Consistent with the description of microglial morphology (Kettenmann et al. 2011), cells with compact 4′,6-diamidino-2-phenylindole (DAPI)-positive nuclei surrounded by DAPI-negative cytoplasm were found in our sections, mostly near the pial surface. Neural progenitors in contrast had large DAPI-positive nuclei that occupied most of the volume of the cell. Endothelial cells enter the cortex from capillaries, and at the early stages we examined would be expected to be in close association with them. We did not use regions with capillaries in our counts. Even though both microglia and endothelial cells peak in number only later, it is possible that our cell counts included some cells other than neuroepithelial cells. Sections from 3 brains were scored. For each brain, 600–1000 cells were counted for the labeling index and 500–700 cells for the quit fraction. Statistical analysis was done using a 2-tailed Student's t-test in Microsoft Excel.
3-D Modeling and Video
The 3-dimensional (3-D) model of the forebrain hem system was developed by using the software BioViz3D version 3.0. 42 serial sections (25 µm each) of an E12.5 wild-type brain were imaged and arranged in the rostrocaudal order. Contours were drawn in each section, based on morphology for the choroid plexus, and based on the expression of specific markers for the cortical hem, septum, and thalamic eminence. The 3-D reconstruction was created using these contours for the entire stack. The movie was made using the movie maker software Hypercam2.
Results
The Morphological Relationships of Medial Forebrain Structures
The septum, hem, and thalamic eminence share the expression of several transcription factors and signaling molecules. In E12.5 coronal sections, the septum appears at rostral levels, whereas the hem and thalamic eminence appear at mid and caudal levels. In rostral sections, Wnt8b expression is seen in the medial telencephalon extending into the pallial septum, but excludes the subpallial septum (Fig. 1F). Wnt8b is also expressed in the thalamic eminence and hem (Fig. 1G,H). At caudal levels, the thalamic eminence is contiguous with the caudal ganglionic eminence (CGE) in the telencephalon (Fig. 1C,H,L). Horizontal sections at E12.5 are useful to examine all 3 structures simultaneously. In such sections, Wnt8b expression on either side of the choroid plexus suggests that the thalamic eminence may be considered to be a “diencephalic hem,” a counterpart to the telencephalic cortical hem (Fig. 1I). The expression of transcription factor Zic2 is seen in a broad region of the medial forebrain, including the septum, hem, and thalamic eminence (Fig. 1J–M; Okada et al. 2008). Zic2 is a major candidate gene implicated in holoprosencephaly, and alterations in Zic2 function may affect the entire forebrain hem system.
A rostrocaudal series of E12.5 coronal sections were collated into a 3-D model, and animated into a movie (M1; still images in Figs 1E and 2D) that begins with the choroid plexus and illustrates how the septum, hem, and thalamic eminence collectively encircle it. These 3 structures at key forebrain boundaries mark the transition from the telencephalic choroid plexus to the rest of the forebrain: The septum and hem connecting the choroid plexus to the telencephalon, and the thalamic eminence connecting it to the diencephalon.
To examine the forebrain system in intact preparations, we prepared whole-mount “hemi forebrains” at E11.5–E12.5, by making a cut along the diencephalic midline, such that each telencephalic hemisphere remains attached to a hemi-diencephalon. In such preparations, in situ hybridization reveals Wnt2b expression in the cortical hem, and AP2α in the thalamic eminence; Lhx1 expression labels both these structures and the septum (Fig. 2B,C).
The relative position of these structures is revealed in the sections of human embryonic and early fetal brains, with an E12.5 mouse section alongside for comparison, in which the overall stage of development appears similar to that seen in a 7-GW human embryo at the preplate stage (Fig. 2E,F). A definitive marker of the choroid plexus is transthyretin (Ttr; Herbert et al. 1986; Grove et al. 1998), which identifies this structure in the lateral ventricles and in the third ventricle of an E12.5 mouse section (Fig. 2E). In the human brain, the entire hem system and choroid plexus express calbindin (Meyer 2010). From 7 to 11 GW, the human choroid plexus extensively expands, maintaining its connection with the hem, septum, and thalamic eminence (Fig. 2F–H). This connection is schematized in Figure 2I. The choroid plexuses of lateral and third ventricles also appear fused to each other in the human, seen in a horizontal section of a 6-GW embryo, published in Cabrera-Socorro et al. (2007).
In prior work, we reported that Lhx2 acts to suppress cortical hem fate and is required to restrict the cortical hem to its medial location (Bulchand et al. 2001; Mangale et al. 2008). Furthermore, the telencephalic choroid plexus, which arises from the cortical hem (Louvi et al. 2007), is greatly increased in the Lhx2 mutant mice (Monuki et al. 2001). This motivated an examination of the other derivative of the cortical hem, the Cajal–Retzius cells. Furthermore, since the septum and thalamic eminence also produce Cajal–Retzius cells (Meyer et al. 2002; Bielle et al. 2005; Tissir et al. 2009), we examined the role of Lhx2 in the development of these medial structures as well. Lhx2 expression is seen in the septum, thalamic eminence, and hem at E11.0, but expression declines to weak or undetectable levels by E12.5 (Supplementary Fig. S1).
The Septum Expands in the Absence of Lhx2
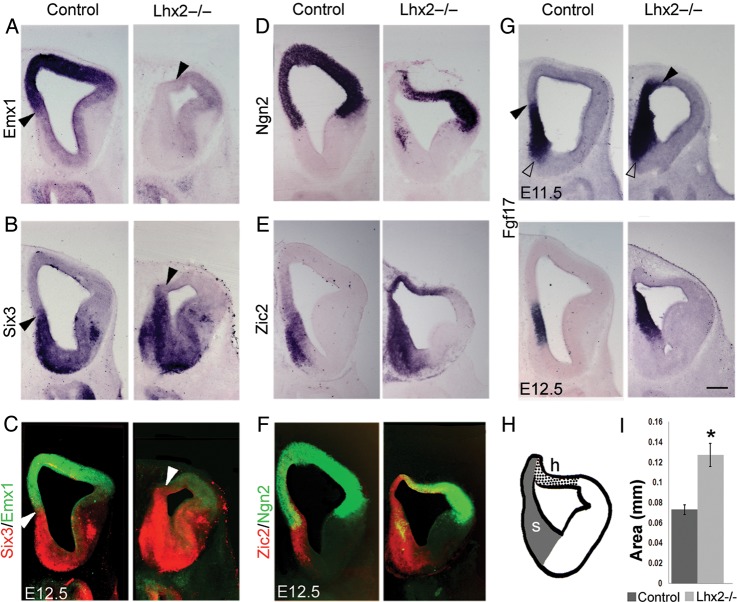
The septum functions as a rostral signaling center in the telencephalon, expressing multiple members of the Fgf family of signaling molecules and their targets (Hoshikawa et al. 1998; Fukuchi-Shimogori and Grove 2001; Fukuchi-Shimogori and Grove 2003; Okada et al. 2008). Transcription factors, Six3 and Zic2, are also expressed in the septum, with Six3 being restricted to its subpallial component (Oliver et al. 1995; Inoue et al. 2007; Fig. 3B,E). Fgf17 expression is specific to the entire septum (Fig. 3G). Emx1 and Ngn2, definitive markers of the pallial neuroepithelium, are expressed in the pallial component of the septum and the adjacent medial pallium. In the Lhx2 mutant, expression of these genes is not seen in the medial pallium at rostral levels, suggesting a loss of pallial identity. Instead, Zic2, Six3, and Fgf17 expression extend into the territory from which Emx1 and Ngn2 have receded (Fig. 3A–F). The expansion of Six3, in particular, indicates that, at rostral levels, the medial pallium has acquired an identity corresponding to the subpallial septum. We quantitated the septal area and found it to be significantly increased in the Lhx2 mutant at E12.5 (P < 0.01; Fig. 3I).
Figure 3.
The septum expands in the Lhx2 mutant. (A and D) In control brains at E12.5, Emx1 (A) and Ngn2 (D) are expressed in the pallium (dorsal telencephalon), their expression extending medially to include the pallial septum. (B) Six3 is expressed in the subpallial component of the septum and also in the subpallium (E). Zic2 is expressed in both components of the septum and also adjacent medial pallial regions. In Lhx2 mutant brains, Emx1 and Ngn2 expression recedes from the medial telencephalon, whereas Zic2 and Six3 expression expand to fill this territory (A, B, D, E), as seen in false-color overlays of adjacent sections showing Emx1/Six3 (C) and Ngn2/Zic2 (F) expression. Ngn2 and Zic2 expressions overlap in the cortical hem (F and H). Arrowheads in A, B, and C mark the dorsal limit of the septum and the beginning of the Emx1-expressing domain (A) in control and Lhx2 mutant sections. (G) Fgf17 is expressed in both pallial and subpallial components of control septum shown at E11.5 and E12.5. In Lhx2 mutant, Fgf17 expression expands dorsally (arrowhead), while its ventral limit remains normally positioned (open arrowhead). (H) Schematic of Lhx2 mutant section at level “i” of Figure 1F, showing the extent of the expanded septum (s) and hem (h). (I) Area occupied by the septum is significantly increased in Lhx2 mutant brains compared with that of control brains (*P < 0.01). Scale bar: 300 µm.
In our previous studies, we reported of the expansion of the hem into territory that is normally occupied by the cortical neuroepithelium (Bulchand et al. 2001; Mangale et al. 2008). This occurs throughout the telencephalon except at extreme rostral levels, where the neuroepithelium takes the identity of the septum medially and hem dorsally, as shown in Figure 3H. The hem expresses both Zic2 and Ngn2, which accounts for their overlap in Figure 3F.
Cajal–Retzius Cells are Increased in the Lhx2 Mutant
Cajal–Retzius cells are early-born neurons that arise from multiple origins and are thought to control radial migration of cortical neurons via the reelin-Dab1 signaling pathway (Howell et al. 1999; Tissir and Goffinet 2003). These cells arise from the cortical hem, septum, and thalamic eminence (Meyer et al. 2002; Takiguchi-Hayashi et al. 2004; Bielle et al. 2005; Cabrera-Socorro et al. 2007; Abellan and Medina 2009; Abellan et al. 2010; Tissir et al. 2009; Meyer 2010).
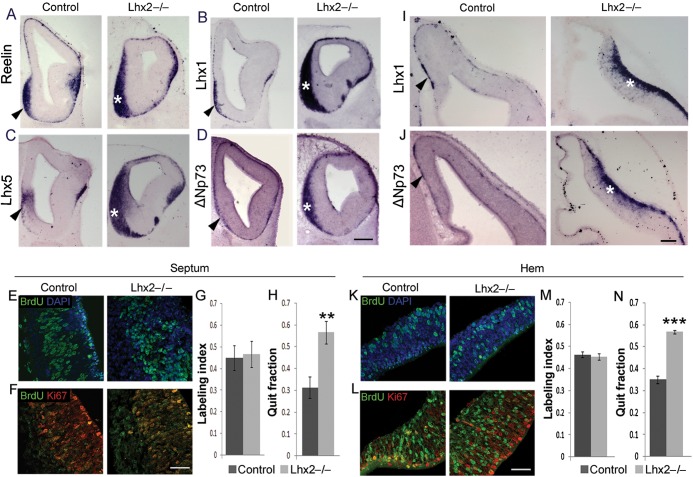
In E12.5 Lhx2 mutant forebrain, there is an overall increase in Cajal–Retzius cells as seen by expression of Reelin, Lhx1, Lhx5, and ΔNp73, a definitive marker of Cajal–Retzius cells arising from the forebrain hem system (Fig. 4A–D,I,J). Examination of labeled cells after a 2-h pulse of BrdU administered at E11.5 reveals that the labeling index in control and mutant septum (45% and 47%, respectively) and hem (46% and 45%, respectively) is similar. This indicates that cell division rates are unaffected in the absence of Lhx2. However, when BrdU injection at E11.5 is followed by BrdU/Ki67 double labeling in brains harvested 1 day later, at E12.5, the quit fraction of the mutant septum (57%) and hem (57%) is significantly increased when compared with the control septum (31%) and hem (35%; Fig. 4E–H,K–N). These data indicate that, in the Lhx2 mutant septum and hem, cells exit the cell cycle prematurely, leading to an overproduction of postmitotic cells.
Figure 4.
Loss of Lhx2 results in an increase in Cajal–Retzius cells. (A–D) Expression of Reelin (A), Lhx1 (B), Lhx5 (C), and ΔNp73 (D) at E12.5 reveals a considerable increase in the Cajal–Retzius cells accumulated at the septum of Lhx2 mutants (asterisks) compared with control brains (arrowheads). (I and J) Similarly, the mantle of the mutant hem (asterisks) also shows increased Cajal–Retzius cell marker expression compared with controls (arrowheads). (E–H) corresponds to sections and cell counts for the septum and (K–N), for the hem. (E and K) Sections of E11.5 control and Lhx2 mutant brains, after a 2-h BrdU pulse, processed for BrdU and DAPI staining. Labeling indices (BrdU+ cells/total DAPI+ cells) at E11.5 are similar for control and Lhx2 mutant septum (45% and 47%, respectively; E and G) and hem (46% and 45%, respectively; K and M). (F and L) Sections of E12.5 Lhx2 control and mutant brains harvested 1 day after BrdU administration at E11.5 and processed for BrdU–Ki67 double immunohistochemistry. The quit fraction (BrdU+; Ki67− cells/total BrdU+ cells) is significantly greater in the Lhx2 mutant septum (57%; **P < 0.005) and hem (57%; ***P < 0.0005) than in the control septum (31%) and hem (35%). Scale bars: 300 µm (A–D); 50 µm (E, F, K, L); 100 µm (I, J).
The Thalamic Eminence Expands in the Lhx2 Mutant
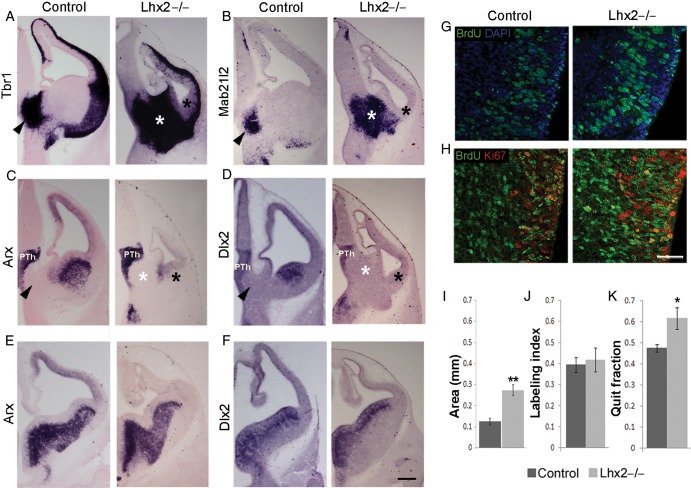
The thalamic eminence is seen in coronal sections as the neuroepithelium connecting the telencephalic choroid plexus to the diencephalon. Postmitotic cells of the thalamic eminence express a battery of transcription factors including Tbr1, Lhx1, Lhx5, and Lhx9 (Bulfone et al. 1995; Sheng et al 1997; Retaux et al. 1999; Yamazaki et al. 2004; Abellan et al. 2010; Fig. 5A; Supplementary Fig. S2). Whereas these markers are also expressed elsewhere in the forebrain, transcription factor, Mab21l2, expression uniquely marks the thalamic eminence (Wong et al. 1999; Yamada et al. 2004; Fig. 5B). In the absence of Lhx2, each of these markers reveals the thalamic eminence to be dramatically expanded. We compared the area of the thalamic eminence in control and Lhx2 mutant brains and found it to be significantly greater in the absence of Lhx2 (P < 0.005; Fig. 5I).
Figure 5.
The thalamic eminence expands in the Lhx2 mutant. (A and B) Tbr1 and Mab21l2 expression in the mantle of the thalamic eminence in E12.5 control brains (arrowheads) reveal a profound expansion of this territory in the Lhx2 mutant (white asterisk). (A–D) The Lhx2 mutant CGE at E12.5 appears morphologically reduced and lacks Arx expression in its mantle (black asterisk, C) and Dlx2 expression in its ventricular zone and mantle (black asterisk, D). (E and F) Arx and Dlx2 expression appear normal in the LGE and MGE (E and F) and prethalamus (PTh; C and D). Arrowheads in control sections and white asterisks in Lhx2 mutant sections in A–D indicate the thalamic eminence. (G and J) Sections of E11.5 control and Lhx2 mutant brains, after a 2-h BrdU pulse, processed for BrdU and DAPI staining. Labeling indices (BrdU+ cells/total DAPI+ cells) at E11.5 are similar in the thalamic eminence of control (39%) and Lhx2 mutant (42%) brains. (H and K) Sections of E12.5 Lhx2 control and mutant brains harvested 1 day after BrdU administration at E11.5; and processed for BrdU–Ki67 double immunohistochemistry. The quit fraction (BrdU+; Ki67− cells/total BrdU+ cells) is significantly greater in the Lhx2 mutant thalamic eminence (62%; P < 0.05) when compared with control brains (48%). (I) Area occupied by the thalamic eminence is significantly increased in Lhx2 mutant brains compared with that of controls (**P < 0.005). Scale bars: 300 µm (A–F) and 50 µm (G and H).
We examined whether the increase in the area of the thalamic eminence was at the expense of the surrounding tissue as is the case with the cortical hem and septum. These 2 structures expand at the expense of the adjacent cortical neuroepithelium (Fig. 3; Bulchand et al. 2001). Therefore, we analyzed diencephalic and telencephalic domains adjacent to the thalamic eminence in the Lhx2 mutant. The loss of Lhx2 does not appear to affect the position, or the extent of the prethalamus, or the thin Lhx1/5-expressing domain adjacent to the zona limitans intrathalamica (Fig. 5C,D; Supplementary Fig. S2). However, in the Lhx2 mutant, the CGE, which is normally located adjacent to the thalamic eminence, is undetectable in terms of the expression of Dlx2 and its downstream target Arx. While both these genes display normal expression in the mutant lateral and medial ganglionic eminences (LGE and MGE; Fig. 5E,F) as well as in the prethalamus, what remains in the location of the CGE is a highly shrunken Dlx2- and Arx-negative structure (black asterisk, Fig. 5A–D). Thus, the expansion of the thalamic eminence is accompanied by shrinkage of the adjacent CGE, suggesting a parallel with the expansion of the cortical hem and septum.
An examination of labeling after a 2-h BrdU pulse administered at E11.5 reveals a similar labeling index in control (39%) and mutant brains (42%; Fig. 5J), indicating that cell division rates are unaffected in the absence of Lhx2. However, when BrdU injection at E11.5 is followed by BrdU/Ki67 double labeling in brains harvested 1 day later, at E12.5, the mutant thalamic eminence is seen to have a significantly increased quit fraction (62%) when compared with the control thalamic eminence (48%, Fig. 5K). This indicates that similar to the septum and hem, cells exit the cell cycle prematurely in the Lhx2 mutant thalamic eminence as well. Interestingly, the expression of Reelin and ΔNp73 (Supplementary Fig. S3) is only modestly increased in the Lhx2 mutant thalamic eminence compared with that of Tbr1 or Mab21l2 (Fig. 5A,B). Therefore, it appears that, in the absence of Lhx2, other postmitotic derivatives of the thalamic eminence are produced together with Reelin and p73-expressing Cajal–Retzius cells.
A Critical Period for Lhx2 Function in Restricting the Forebrain Hem System
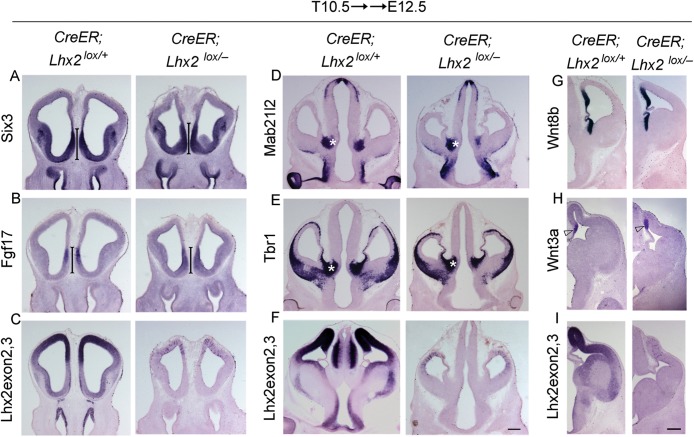
Lhx2 expression in the septum, hem, and thalamic eminence is diminished by E11.0 and undetectable by E12.5 (Supplementary Fig. S1). In an earlier study, we used a conditional knockout line to demonstrate that deletion of Lhx2 after E10.5 did not result in an expansion of the cortical hem (Mangale et al. 2008). We used the same conditional knockout line crossed to CreER and administered tamoxifen at E10.5. We examined these conditional knockout embryos at E12.5 and find that neither the septum nor the thalamic eminence is expanded (Fig. 6). Therefore, the role of Lhx2 in restricting the extent of the forebrain hem system ends by E10.5, which is the stage at which the first neurons of the cortex are born, and the septum, hem, and thalamic eminence can be identified by marker gene expression in the mouse (Grove et al. 1998; Zembrzycki et al. 2007; Fotaki et al. 2008; Supplementary Fig. S4).
Figure 6.
Critical period for Lhx2 to regulate the forebrain hem system ends by E10.5. Conditional deletion of Lhx2 using a tamoxifen-inducible CreER driver. Tamoxifen administration at E10.5 (T10.5) does not interfere with forebrain development in control (CreER;Lhx2lox/+) embryos (A–I). A probe against Lhx2 exon 2,3 indicates that Lhx2 deletion has occurred throughout the brain of the floxed (CreER;Lhx2lox/−) embryos (C, F, I). (A–C) The septum in the floxed embryos appears normal as evident from Six3 and Fgf17 expression (bars; A and B). (D and E) Thalamic eminence expressing Mab21l2 and Tbr1 appear similar in the floxed embryos as that in controls (asterisk). Similarly, expression of Wnt8b in the cortical hem, medial pallium, and thalamic eminence as well as of Wnt3a in the cortical hem is comparable between floxed and control embryos (G and H). Scale bar: 300 µm.
Forebrain Hem System in Extant Vertebrates
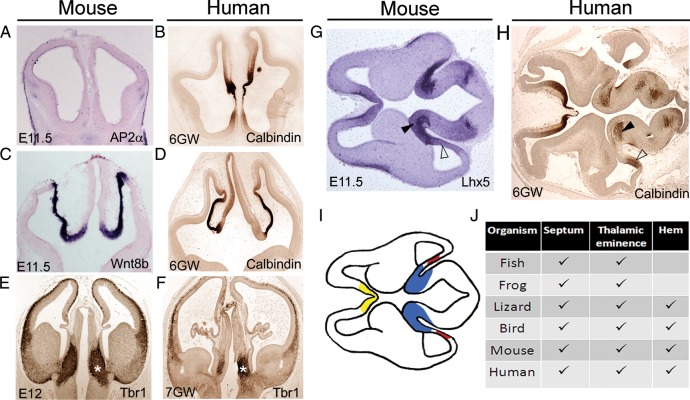
The thalamic eminence and septum are thought to be evolutionarily ancient structures, having been described in fish (Wullimann and Rink 2002; Wullimann and Mueller 2004), amphibians (Brox et al. 2002, 2003), reptiles (Cabrera-Socorro et al. 2007), birds (Puelles et al. 2000; Cobos et al. 2001), and mammals (Abbott and Jacobowitz 1999; Puelles et al. 2000). If these indeed play a fundamental role in development, then they would be expected to be conserved in humans as well. We examined human brains at 6 and 7 GWs and made morphological comparisons of the components of the forebrain hem system between human and mouse (Fig. 7). The cortical hem and thalamic eminence flank the telencephalic choroid plexus in the human embryo just as they do in the mouse (Fig. 7E–H). In addition to calbindin, we present a comparison of 7-GW human and E12 mouse embryos using Tbr1 immunostaining, Tbr1 being expressed in the postmitotic neurons of the entire forebrain hem system in both species (Fig. 7E,F). A horizontal section of an E11.5 mouse brain with a morphology similar to that of the human section is shown alongside for the comparison of the location of the thalamic eminence and hem in the 2 species (Fig. 7G,H). The components of the forebrain hem system reported in extant vertebrates are summarized in Figure 7J. The cortical hem appears to be an evolutionarily newer structure, reported only in reptiles and mammals and being particularly prominent in the human brain (Grove et al. 1998; Meyer et al. 2002; Cabrera-Socorro et al. 2007; Meyer 2010).
Figure 7.
Presence of the forebrain hem system components in extant vertebrates. (A–F) Morphological comparisons between stage-matched mouse and human embryonic sections at rostral (A and B), middle (C and D), and caudal (E and F) levels. Tbr1 immunostaining marks the thalamic eminence in both E12 mouse and 7-GW human fetus. (G and H) A horizontal section of an E11.5 mouse brain appears morphologically comparable with a 6-GW human fetal brain section. Both species reveal the hem in the caudal telencephalon (open arrowhead) and the thalamic eminence (arrowhead) at the transition of the caudal telencephalon to the diencephalon. (I) Schematic of the mouse brain section in A, depicting the positions of the septum (yellow), cortical hem (red), and thalamic eminence (blue). (J) Tabulation of the presence of the 3 medial sources of Cajal–Retzius cells in the developing forebrain of different vertebrates (Grove et al. 1998; Abbott and Jacobowitz 1999; Puelles et al. 2000; Cobos et al. 2001; Brox et al. 2002, 2003; Meyer et al. 2002; Wullimann and Rink 2002; Wullimann and Mueller 2004; Cabrera-Socorro et al. 2007).
Discussion
In this study, we examine 3 structures located at important morphological boundaries in the medial forebrain, septum, hem, and thalamic eminence and find that they are unified by significant common features. It was already known that these boundary structures express specific complements of signaling molecules (Fgfs, Wnts, and Bmps) (Furuta et al. 1997; Grove et al. 1998; Hoshikawa et al. 1998; Kimura et al. 2005). These structures were also previously known or proposed to play major roles in forebrain patterning events, and to produce Cajal–Retzius cells, which are implicated in aspects of patterning and cell migration in the forebrain (Meyer et al. 2002; Takiguchi-Hayashi et al. 2004; Bielle et al. 2005; Yoshida et al. 2006; Cabrera-Socorro et al. 2007; Abellan and Medina 2009; Abellan et al. 2010; Tissir et al. 2009; Meyer 2010). We discovered a key feature that unifies these structures, namely that transcription factor Lhx2 acts to limit their extent. We observe all 3 structures to expand in the Lhx2 mutant. The major patterning disruptions occur in the Lhx2 mutant involving the expansion of the septum, hem, and thalamic eminence into adjacent territories (Bulchand et al. 2001; Mangale et al. 2008; this study). This expansion appears to be due to a fate change of the adjacent neuroepithelium: The rostral cortex in the case of the septum; the mid level cortex in the case of the hem; and the CGE in the case of the thalamic eminence. These expanded neuroepithelial domains also display an enhanced quit fraction, resulting in a great excess of postmitotic cells produced from the septum, hem, and thalamic eminence. We propose that these structures function as a multicomponent medial organizing system, the forebrain hem system, which regulates the development of the forebrain.
Our forebrain hem system model builds upon and extends the idea of the cortical hem and the septum as medial organizers in the telencephalon. It is well established that the Wnt-rich cortical hem acts as an organizer for the adjacent cortical neuroepithelium to become hippocampus. In mosaic embryos consisting of Lhx2 null and wild-type cells, multiple hems are formed within the cortical neuroepithelium, and ectopic hippocampi are induced adjacent to each hem (Mangale et al. 2008). Fgf8, expressed in the septum, is critical in regulating cortical arealization. An ectopic source of Fgf8 in the caudal telencephalon is sufficient to produce a mirror-image duplication of the cortical area map (Fukuchi-Shimogori and Grove 2001). The thalamic eminence is known to secrete a composite set of signaling molecules (Kimura et al. 2005) and is suggested to be a putative signaling centre at the DTB (Abbott and Jacobowitz 1999; Kim et al. 2001). The thalamic eminence is also the site through which the thalamocortical axons cross the diencephalon to enter the telencephalon. In the Lhx2 mutant, the thalamic eminence expands at the expense of the CGE, which is an important source of interneurons for the caudal cerebral cortex and hippocampus (Nery et al. 2002; Yozu et al. 2005). We suggest that this structure may function as a diencephalic hem, to regulate key aspects of forebrain patterning and development.
Our new perspective unifies 3 key forebrain midline structures and may provide a way of understanding holoprosencephaly, the most common congenital malformation of the human forebrain with a prevalence of 1 in 250 conceptions and 1 in 16 000 live births (Oliver et al. 1995; Brown et al. 1998; Wallis and Muenke 1999; Arauz et al. 2010; Paulussen et al. 2010; Solomon et al. 2010). Key genes that are implicated in holoprosencephaly are transcription factors Zic2, Six3, and signaling molecules Fgf8 and Bmps, which are expressed in part or all of the forebrain hem system, and Shh, which is expressed in adjacent ventral regions. Six3, expressed in the subpallial septum and other ventral structures, is a major locus affected in human holoprosencephaly (Oliver et al. 1995; Wallis and Muenke 1999). Shh is a critical ventral signaling molecule and its absence results in holoprosencephaly with malformation/reduction of only ventral midline structures; in contrast, the Bmpr1a/b double mutant displays dorsal holoprosencephaly (midline interhemispheric holoprosencephaly), including a severe reduction of the hem, absence of the choroid plexus, and partial fusion of the hemispheres (Fernandes et al. 2007). The absence of Zic2 results in both dorsal and ventral midline defects (Brown et al. 1998). Zic2 levels appear to regulate fundamental morphogenetic events such as the timing of neurulation and roof plate formation (Nagai et al. 2000). Mechanistically, Zic2 is thought to act in part via the regulation of β-catenin activity, which is a major downstream mediator of Wnt signaling (Pourebrahim et al. 2011). Our results show that Zic2 is expressed in the entire forebrain hem system and provides a new framework in which phenotypes associated with its deficiency can be interpreted. Broadly, the defects associated with holoprosencephaly are due to deficiencies in the specification of, or signaling from key midline structures. In contrast, the absence of Lhx2 results in an *expansion* of the same midline structures. We propose that our interpretation of the forebrain hem system as an entity may lead to different approaches to analyzing holoprosencephalies.
Our findings position Lhx2 in a fundamental role in regulating forebrain development by controlling the formation of this organizer system, acting not only to delimit its extent, but also to maintain its neuroepithelial stem cells in a proliferative mode. In Mangale et al. (2008), we examined mosaic embryos that contained Lhx2 mutant and wild-type patches of neuroepithelium and showed that the expansion of the hem is due to a cell autonomous role of Lhx2. We expect a similar cell-autonomous function for Lhx2 in both the septum and thalamic eminence as well, with a critical period that ends at E10.5 by which time these structures are identifiable (Fig. 6). Supporting this interpretation, Lhx2 expression in the forebrain hem system drops significantly by this stage, consistent with a scenario in which Lhx2 must be downregulated in order for the structures to gain their identity.
An interesting feature of the hem and septum is that they appear to cross-regulate each other, after they are formed. This regulation is mutually repressive in which BMPs from the hem curtail the extent of the septum, and Fgf8 from the septum curtails the extent of the Wnt expression domain, that is, the hem (Shimogori et al. 2004). But since the role of Lhx2 is complete by E10.5, it is unlikely that these cross-repressive interactions might mediate the function of Lhx2. Rather, they appear to act to maintain internal boundaries within the forebrain hem system. How Lhx2 suppresses a range of alternative fates to preserve the extent of the cortex, or the forebrain at large is a compelling question for future studies.
An important property of the forebrain hem system is that all its components are sources of the Cajal–Retzius cells which, in turn, are the main source of Reelin signal in the developing telencephalon. Each component of the forebrain hem system is also connected to the choroid plexus, and at least one component, the hem, is thought to produce the choroid plexus as well (Louvi et al. 2007). A mechanistic link between Cajal–Retzius cells and the choroid plexus has been discovered in the mouse, in which Ngn2 and Hes genes antagonistically regulate the specification of these 2 fates, respectively (Imayoshi et al. 2008). Both have critical roles in cortical development: Cajal–Retzius cells being sources of Reelin (D'Arcangelo et al. 1995; Ogawa et al. 1995) and choroid plexus being the source of the cerebrospinal fluid, which contains a complex cocktail of proteins that nourishes the brain (Thouvenot et al. 2006; Marques et al. 2011). The forebrain hem system is therefore positioned to regulate the development of the telencephalon, and potentially also its expansion in evolution. As summarized in Figure 7J, components of this system have been identified in several vertebrate species. Both the septum and thalamic eminence have been identified in all vertebrate phyla including fish (Fig. 7J) and may represent the beginnings of the evolution of a comprehensive medial organizing cluster, the forebrain hem system. The hem appears to have arisen most recently in evolution, possibly in the common ancestor of reptiles and mammals, since it has been reported in both phyla. The hem may be particularly important for human brain development since it provides the Cajal–Retzius cells for the greatly expanded neocortex; furthermore, the organizing activities of the forebrain hem system may culminate in determining the extent of the human neocortex.
Author contributions
A.R. performed experiments for the mouse components of the data. M.G.-G. and G.M. performed experiments for the human components. A.P. contributed reagents. A.R., A.P., G.M., and S.T. analyzed the data. A.R., G.M., and S.T. wrote the paper. G.M. and S.T. conceived the project and are co-senior authors of the paper.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by Spanish Ministry of Science and Innovation (G.M.), a grant from the DBT (Department of Biotechnology, Govt. of India), and intramural funding from the Tata Institute of Fundamental Research to S.T. Funding to pay the Open Access publication charges for this article was provided by a grant from the DBT (Department of Biotechnology, Govt. of India).
Supplementary Material
Notes
We thank F.D. Porter for Lhx2+/− breeding pairs and Edwin S. Monuki for Lhx2 floxed breeding pairs of mice; the TIFR Animal house staff for breeding and maintaining the colonies; C. Cepko, E. Grove, R. McInnes; Y. Nakagawa; T. Okada; F.D. Porter, C. Ragsdale, J. Rubenstein, and Y. Zhao for DNA reagents. Conflict of Interest: None declared.
References
- Abbott LC, Jacobowitz DM. Developmental expression of calretinin-immunoreactivity in the thalamic eminence of the fetal mouse. Int J Dev Neurosci. 1999;17:331–345. doi: 10.1016/s0736-5748(99)00037-4. [DOI] [PubMed] [Google Scholar]
- Abellan A, Medina L. Subdivisions and derivatives of the chicken subpallium based on expression of LIM and other regulatory genes and markers of neuron subpopulations during development. J Comp Neurol. 2009;515:465–501. doi: 10.1002/cne.22083. [DOI] [PubMed] [Google Scholar]
- Abellan A, Menuet A, Dehay C, Medina L, Retaux S. Differential expression of LIM-homeodomain factors in Cajal-Retzius cells of primates, rodents, and birds. Cereb Cortex. 2010;20:1788–1798. doi: 10.1093/cercor/bhp242. [DOI] [PubMed] [Google Scholar]
- Arauz RF, Solomon BD, Pineda-Alvarez DE, Gropman AL, Parsons JA, Roessler E, Muenke MA. Hypomorphic allele in the FGF8 gene contributes to holoprosencephaly and is allelic to gonadotropin-releasing hormone deficiency in humans. Mol Syndromol. 2010;1:59–66. doi: 10.1159/000302285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar I, Lambert de Rouvroit C, Goffinet AM. The evolution of cortical development. An hypothesis based on the role of the Reelin signaling pathway. Trends Neurosci. 2000;23:633–638. doi: 10.1016/s0166-2236(00)01675-1. [DOI] [PubMed] [Google Scholar]
- Bielle F, Griveau A, Narboux-Neme N, Vigneau S, Sigrist M, Arber S, Wassef M, Pierani A. Multiple origins of Cajal-Retzius cells at the borders of the developing pallium. Nat Neurosci. 2005;8:1002–1012. doi: 10.1038/nn1511. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Ericson J. The specification of neuronal identity by graded Sonic Hedgehog signalling. Semin Cell Dev Biol. 1999;10:353–362. doi: 10.1006/scdb.1999.0295. [DOI] [PubMed] [Google Scholar]
- Brown SA, Warburton D, Brown LY, Yu CY, Roeder ER, Stengel-Rutkowski S, Hennekam RC, Muenke M. Holoprosencephaly due to mutations in ZIC2, a homologue of Drosophila odd-paired. Nat Genet. 1998;20:180–183. doi: 10.1038/2484. [DOI] [PubMed] [Google Scholar]
- Brox A, Ferreiro B, Puelles L, Medina L. The telencephalon of the frog Xenopus based on calretinin immunostaining and gene expression patterns. Brain Res Bull. 2002;57:381–384. doi: 10.1016/s0361-9230(01)00709-2. [DOI] [PubMed] [Google Scholar]
- Brox A, Puelles L, Ferreiro B, Medina L. Expression of the genes GAD67 and Distal-less-4 in the forebrain of Xenopus laevis confirms a common pattern in tetrapods. J Comp Neurol. 2003;461:370–393. doi: 10.1002/cne.10688. [DOI] [PubMed] [Google Scholar]
- Bulchand S, Grove EA, Porter FD, Tole S. LIM-homeodomain gene Lhx2 regulates the formation of the cortical hem. Mech Dev. 2001;100:165–175. doi: 10.1016/s0925-4773(00)00515-3. [DOI] [PubMed] [Google Scholar]
- Bulchand S, Subramanian L, Tole S. Dynamic spatiotemporal expression of LIM genes and cofactors in the embryonic and postnatal cerebral cortex. Dev Dyn. 2003;226:460–469. doi: 10.1002/dvdy.10235. [DOI] [PubMed] [Google Scholar]
- Bulfone A, Smiga SM, Shimamura K, Peterson A, Puelles L, Rubenstein JL. T-brain-1: a homolog of Brachyury whose expression defines molecularly distinct domains within the cerebral cortex. Neuron. 1995;15:63–78. doi: 10.1016/0896-6273(95)90065-9. [DOI] [PubMed] [Google Scholar]
- Cabrera-Socorro A, Hernandez-Acosta NC, Gonzalez-Gomez M, Meyer G. Comparative aspects of p73 and Reelin expression in Cajal-Retzius cells and the cortical hem in lizard, mouse and human. Brain Res. 2007;1132:59–70. doi: 10.1016/j.brainres.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Cobos I, Shimamura K, Rubenstein JL, Martinez S, Puelles L. Fate map of the avian anterior forebrain at the four-somite stage, based on the analysis of quail-chick chimeras. Dev Biol. 2001;239:46–67. doi: 10.1006/dbio.2001.0423. [DOI] [PubMed] [Google Scholar]
- D'Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- Fernandes M, Gutin G, Alcorn H, McConnell SK, Hebert JM. Mutations in the BMP pathway in mice support the existence of two molecular classes of holoprosencephaly. Development. 2007;134:3789–3794. doi: 10.1242/dev.004325. [DOI] [PubMed] [Google Scholar]
- Fotaki V, Price DJ, Mason JO. Newly identified patterns of Pax2 expression in the developing mouse forebrain. BMC Dev Biol. 2008;8:79. doi: 10.1186/1471-213X-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA. Emx2 patterns the neocortex by regulating FGF positional signaling. Nat Neurosci. 2003;6:825–831. doi: 10.1038/nn1093. [DOI] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA. Neocortex patterning by the secreted signaling molecule FGF8. Science. 2001;294:1071–1074. doi: 10.1126/science.1064252. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Piston DW, Hogan BL. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development. 1997;124:2203–2212. doi: 10.1242/dev.124.11.2203. [DOI] [PubMed] [Google Scholar]
- Herbert J, Wilcox JN, Pham KT, Fremeau RT, Jr, Zeviani M, Dwork A, Soprano DR, Makover A, Goodman DS, Zimmerman EA, et al. Transthyretin: a choroid plexus-specific transport protein in human brain. The 1986 S. Weir Mitchell award. Neurology. 1986;36:900–911. doi: 10.1212/wnl.36.7.900. [DOI] [PubMed] [Google Scholar]
- Griveau A, Borello U, Causeret F, Tissir F, Boggetto N, Karaz S, Pierani A. A novel role for Dbx1-derived Cajal-Retzius cells in early regionalization of the cerebral cortical neuroepithelium. PLoS Biol. 2010;8:e1000440. doi: 10.1371/journal.pbio.1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove EA, Tole S, Limon J, Yip L, Ragsdale CW. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development. 1998;125:2315–2325. doi: 10.1242/dev.125.12.2315. [DOI] [PubMed] [Google Scholar]
- Hoshikawa M, Ohbayashi N, Yonamine A, Konishi M, Ozaki K, Fukui S, Itoh N. Structure and expression of a novel fibroblast growth factor, FGF-17, preferentially expressed in the embryonic brain. Biochem Biophys Res Commun. 1998;244:187–191. doi: 10.1006/bbrc.1998.8239. [DOI] [PubMed] [Google Scholar]
- Howell BW, Herrick TM, Cooper JA. Reelin-induced tyrosine [corrected] phosphorylation of disabled 1 during neuronal positioning. Genes Dev. 1999;13:643–648. doi: 10.1101/gad.13.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I, Shimogori T, Ohtsuka T, Kageyama R. Hes genes and neurogenin regulate non-neural versus neural fate specification in the dorsal telencephalic midline. Development. 2008;135:2531–2541. doi: 10.1242/dev.021535. [DOI] [PubMed] [Google Scholar]
- Inoue T, Ota M, Ogawa M, Mikoshiba K, Aruga J. Zic1 and Zic3 regulate medial forebrain development through expansion of neuronal progenitors. J Neurosci. 2007;27:5461–5473. doi: 10.1523/JNEUROSCI.4046-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Kim AS, Anderson SA, Rubenstein JL, Lowenstein DH, Pleasure SJ. Pax-6 regulates expression of SFRP-2 and Wnt-7b in the developing CNS. J Neurosci. 2001;21:RC132. doi: 10.1523/JNEUROSCI.21-05-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J, Suda Y, Kurokawa D, Hossain ZM, Nakamura M, Takahashi M, Hara A, Aizawa S. Emx2 and Pax6 function in cooperation with Otx2 and Otx1 to develop caudal forebrain primordium that includes future archipallium. J Neurosci. 2005;25:5097–5108. doi: 10.1523/JNEUROSCI.0239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem KF, Jr, Tremml G, Jessell TM. A role for the roof plate and its resident TGFbeta-related proteins in neuronal patterning in the dorsal spinal cord. Cell. 1997;91:127–138. doi: 10.1016/s0092-8674(01)80015-5. [DOI] [PubMed] [Google Scholar]
- Louvi A, Yoshida M, Grove EA. The derivatives of the Wnt3a lineage in the central nervous system. J Comp Neurol. 2007;504:550–569. doi: 10.1002/cne.21461. [DOI] [PubMed] [Google Scholar]
- Mangale VS, Hirokawa KE, Satyaki PR, Gokulchandran N, Chikbire S, Subramanian L, Shetty AS, Martynoga B, Paul J, Mai MV, et al. Lhx2 selector activity specifies cortical identity and suppresses hippocampal organizer fate. Science. 2008;319:304–309. doi: 10.1126/science.1151695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques F, Sousa JC, Coppola G, Gao F, Puga R, Brentani H, Geschwind DH, Sousa N, Correia-Neves M, Palha JA. Transcriptome signature of the adult mouse choroid plexus. Fluids Barriers CNS. 2011;8:10. doi: 10.1186/2045-8118-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G. Building a human cortex: the evolutionary differentiation of Cajal-Retzius cells and the cortical hem. J Anat. 2010;217:334–343. doi: 10.1111/j.1469-7580.2010.01266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G, Goffinet AM. Prenatal development of reelin-immunoreactive neurons in the human neocortex. J Comp Neurol. 1998;397:29–40. [PubMed] [Google Scholar]
- Meyer G, Perez-Garcia CG, Abraham H, Caput D. Expression of p73 and Reelin in the developing human cortex. J Neurosci. 2002;22:4973–4986. doi: 10.1523/JNEUROSCI.22-12-04973.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G, Schaaps JP, Moreau L, Goffinet AM. Embryonic and early fetal development of the human neocortex. J Neurosci. 2000;20:1858–1868. doi: 10.1523/JNEUROSCI.20-05-01858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monuki ES, Porter FD, Walsh CA. Patterning of the dorsal telencephalon and cerebral cortex by a roof plate-Lhx2 pathway. Neuron. 2001;32:591–604. doi: 10.1016/s0896-6273(01)00504-9. [DOI] [PubMed] [Google Scholar]
- Nagai T, Aruga J, Minowa O, Sugimoto T, Ohno Y, Noda T, Mikoshiba K. Zic2 regulates the kinetics of neurulation. Proc Natl Acad Sci USA. 2000;97:1618–1623. doi: 10.1073/pnas.97.4.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci. 2002;5:1279–1287. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Miyata T, Nakajima K, Yagyu K, Seike M, Ikenaka K, Yamamoto H, Mikoshiba K. The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron. 1995;14:899–912. doi: 10.1016/0896-6273(95)90329-1. [DOI] [PubMed] [Google Scholar]
- Okada T, Okumura Y, Motoyama J, Ogawa M. FGF8 signaling patterns the telencephalic midline by regulating putative key factors of midline development. Dev Biol. 2008;320:92–101. doi: 10.1016/j.ydbio.2008.04.034. [DOI] [PubMed] [Google Scholar]
- Oliver G, Mailhos A, Wehr R, Copeland NG, Jenkins NA, Gruss P. Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development. 1995;121:4045–4055. doi: 10.1242/dev.121.12.4045. [DOI] [PubMed] [Google Scholar]
- Paulussen AD, Schrander-Stumpel CT, Tserpelis DC, Spee MK, Stegmann AP, Mancini GM, Brooks AS, Collee M, Maat-Kievit A, Simon ME, et al. The unfolding clinical spectrum of holoprosencephaly due to mutations in SHH, ZIC2, SIX3 and TGIF genes. Eur J Hum Genet. 2010;18:999–1005. doi: 10.1038/ejhg.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard KS, Salama SR, Lambert N, Lambot MA, Coppens S, Pedersen JS, Katzman S, King B, Onodera C, Siepel A, et al. An RNA gene expressed during cortical development evolved rapidly in humans. Nature. 2006;443:167–172. doi: 10.1038/nature05113. [DOI] [PubMed] [Google Scholar]
- Pourebrahim R, Houtmeyers R, Ghogomu S, Janssens S, Thelie A, Tran HT, Langenberg T, Vleminckx K, Bellefroid E, Cassiman JJ, et al. Transcription factor Zic2 inhibits Wnt/beta-catenin protein signaling. J Biol Chem. 2011;286:37732–37740. doi: 10.1074/jbc.M111.242826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles L, Kuwana E, Puelles E, Bulfone A, Shimamura K, Keleher J, Smiga S, Rubenstein JL. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J Comp Neurol. 2000;424:409–438. doi: 10.1002/1096-9861(20000828)424:3<409::aid-cne3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Retaux S, Rogard M, Bach I, Failli V, Besson MJ. Lhx9: a novel LIM-homeodomain gene expressed in the developing forebrain. J Neurosci. 1999;19:783–793. doi: 10.1523/JNEUROSCI.19-02-00783.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng HZ, Bertuzzi S, Chiang C, Shawlot W, Taira M, Dawid I, Westphal H. Expression of murine Lhx5 suggests a role in specifying the forebrain. Dev Dyn. 1997;208:266–277. doi: 10.1002/(SICI)1097-0177(199702)208:2<266::AID-AJA13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Shimogori T, Banuchi V, Ng HY, Strauss JB, Grove EA. Embryonic signaling centers expressing BMP, WNT and FGF proteins interact to pattern the cerebral cortex. Development. 2004;131:5639–5647. doi: 10.1242/dev.01428. [DOI] [PubMed] [Google Scholar]
- Solomon BD, Lacbawan F, Mercier S, Clegg NJ, Delgado MR, Rosenbaum K, Dubourg C, David V, Olney AH, Wehner LE, et al. Mutations in ZIC2 in human holoprosencephaly: description of a novel ZIC2 specific phenotype and comprehensive analysis of 157 individuals. J Med Genet. 2010;47:513–524. doi: 10.1136/jmg.2009.073049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian L, Sarkar A, Shetty AS, Muralidharan B, Padmanabhan H, Piper M, Monuki ES, Bach I, Gronostajski RM, Richards LJ, et al. Transcription factor Lhx2 is necessary and sufficient to suppress astrogliogenesis and promote neurogenesis in the developing hippocampus. Proc Natl Acad Sci USA. 2011;108:E265–274. doi: 10.1073/pnas.1101109108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen N, Smolders S, Avila A, Notelaers K, Paesen R, Ameloot M, Brone B, Legendre P, Rigo JM. Complex invasion pattern of the cerebral cortex by microglial cells during development of the mouse embryo. Glia. 2012 doi: 10.1002/glia.22421. doi:10.1002/glia.22421. [DOI] [PubMed] [Google Scholar]
- Takiguchi-Hayashi K, Sekiguchi M, Ashigaki S, Takamatsu M, Hasegawa H, Suzuki-Migishima R, Yokoyama M, Nakanishi S, Tanabe Y. Generation of reelin-positive marginal zone cells from the caudomedial wall of telencephalic vesicles. J Neurosci. 2004;24:2286–2295. doi: 10.1523/JNEUROSCI.4671-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thouvenot E, Lafon-Cazal M, Demettre E, Jouin P, Bockaert J, Marin P. The proteomic analysis of mouse choroid plexus secretome reveals a high protein secretion capacity of choroidal epithelial cells. Proteomics. 2006;6:5941–5952. doi: 10.1002/pmic.200600096. [DOI] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM. Reelin and brain development. Nat Rev Neurosci. 2003;4:496–505. doi: 10.1038/nrn1113. [DOI] [PubMed] [Google Scholar]
- Tissir F, Lambert de Rouvroit C, Goffinet AM. The role of reelin in the development and evolution of the cerebral cortex. Braz J Med Biol Res. 2002;35:1473–1484. doi: 10.1590/s0100-879x2002001200007. [DOI] [PubMed] [Google Scholar]
- Tissir F, Ravni A, Achouri Y, Riethmacher D, Meyer G, Goffinet AM. DeltaNp73 regulates neuronal survival in vivo. Proc Natl Acad Sci USA. 2009;106:16871–16876. doi: 10.1073/pnas.0903191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis DE, Muenke M. Molecular mechanisms of holoprosencephaly. Mol Genet Metab. 1999;68:126–138. doi: 10.1006/mgme.1999.2895. [DOI] [PubMed] [Google Scholar]
- Wong RL, Chan KK, Chow KL. Developmental expression of Mab21l2 during mouse embryogenesis. Mech Dev. 1999;87:185–188. doi: 10.1016/s0925-4773(99)00127-6. [DOI] [PubMed] [Google Scholar]
- Wullimann MF, Mueller T. Identification and morphogenesis of the eminentia thalami in the zebrafish. J Comp Neurol. 2004;471:37–48. doi: 10.1002/cne.20011. [DOI] [PubMed] [Google Scholar]
- Wullimann MF, Rink E. The teleostean forebrain: a comparative and developmental view based on early proliferation, Pax6 activity and catecholaminergic organization. Brain Res Bull. 2002;57:363–370. doi: 10.1016/s0361-9230(01)00666-9. [DOI] [PubMed] [Google Scholar]
- Yamada R, Mizutani-Koseki Y, Koseki H, Takahashi N. Requirement for Mab21l2 during development of murine retina and ventral body wall. Dev Biol. 2004;274:295–307. doi: 10.1016/j.ydbio.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Yamada T, Placzek M, Tanaka H, Dodd J, Jessell TM. Control of cell pattern in the developing nervous system: polarizing activity of the floor plate and notochord. Cell. 1991;64:635–647. doi: 10.1016/0092-8674(91)90247-v. [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Sekiguchi M, Takamatsu M, Tanabe Y, Nakanishi S. Distinct ontogenic and regional expressions of newly identified Cajal-Retzius cell-specific genes during neocorticogenesis. Proc Natl Acad Sci USA. 2004;101:14509–14514. doi: 10.1073/pnas.0406295101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Assimacopoulos S, Jones KR, Grove EA. Massive loss of Cajal-Retzius cells does not disrupt neocortical layer order. Development. 2006;133:537–545. doi: 10.1242/dev.02209. [DOI] [PubMed] [Google Scholar]
- Yozu M, Tabata H, Nakajima K. The caudal migratory stream: a novel migratory stream of interneurons derived from the caudal ganglionic eminence in the developing mouse forebrain. J Neurosci. 2005;25:7268–7277. doi: 10.1523/JNEUROSCI.2072-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zembrzycki A, Griesel G, Stoykova A, Mansouri A. Genetic interplay between the transcription factors Sp8 and Emx2 in the patterning of the forebrain. Neural Dev. 2007;2:8. doi: 10.1186/1749-8104-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.