Summary
Mitochondria are autonomous cellular organelles that oversee a variety of functions such as metabolism, energy production, calcium buffering, and cell fate determination. Regulation of their morphology and diverse activities beyond energy production are being recognized as playing major roles in cellular health and dysfunction. This review is aimed at summarizing what is known regarding mitochondrial contributions to pathogenesis of lung diseases. Emphasis is given to understanding the importance of structural and functional aspects of mitochondria in both normal cellular function (based on knowledge from other cell types) and in development and modulation of lung diseases such as asthma, COPD, cystic fibrosis and cancer. Emerging techniques that allow examination of mitochondria, and potential strategies to target mitochondria in the treatment of lung diseases are also discussed.
Keywords: Mitochondria, fission, fusion, oxidative stress, reactive oxygen species, airway, proliferation, apoptosis, autophagy
1. Introduction
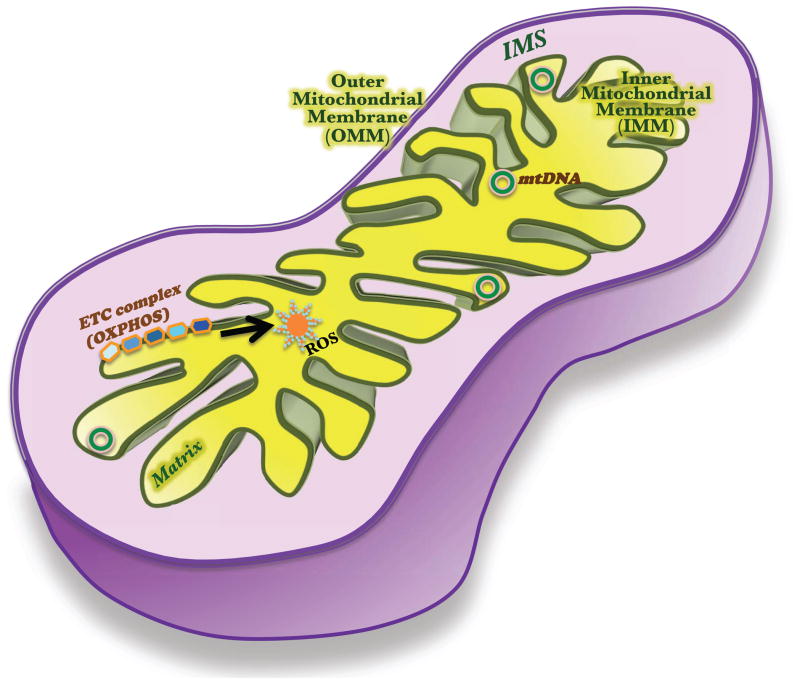
Mitochondria are cellular organelles with distinct features that belie their origins and unique functions. Originally derived from ancient aerobic bacteria [1], mitochondria are critical for meeting cellular energy demands by driving the synthesis of ATP. Possessing their own maternally-inherited genome and transcription/translational machineries has also conferred mitochondria with a certain level of functional autonomy [2]. The mitochondrion differs from the other organelles structurally as well: it is enveloped by a double-membrane assembly, with four distinct parts: outer membrane (OMM), inner membrane (IMM), intermembrane space (IMS), and matrix (Figure 1). The highly folded IMM (called cristae) harbors the oxidative phosphorylation (OXPHOS) enzyme complexes. Within the well-defined confines of the mitochondrion, carbohydrates and long chain fatty acids are metabolized, lipids and steroids are synthesized, ATP is made as a by-product, mitochondrial DNA (mtDNA) is replicated, and gene products are expressed.
Figure 1. The mitochondrion.
This semi-autonomous organelle is bounded by the outer mitochondrial membrane (OMM), which is separated from the second, inner mitochondrial membrane (IMM) by the intermembrane space (IMS). The mitochondrial DNA (mtDNA) codes mainly for electron transport chain (ETC) proteins, which via a series of oxidative phosphorylation reactions (OXPHOS), produce ATP (energy) and reactive oxygen species (ROS).
As part of their normal functioning, mitochondria generate reactive oxygen and nitrogen species (ROS and RNS) [3,4]. In turn, ROS and RNS are among the key regulators of mitochondrial function. While normal metabolic pathways such as glycolysis and β-oxidation of fatty acids are significant sources of these free radical species [5–7], cells also have defense measures in the form of scavengers: antioxidant enzymes such as superoxide dismutase, peroxidases and catalase [8–10], and small molecules such as melatonin [11] to curb the damaging effects of ROS and RNS, thus protecting the cell. The extensive roles that ROS and RNS play in the pathogenesis of several disease conditions, especially in the lung, will be discussed later in this review.
While such ROS and RNS can serve physiological functions [4,12], failure to clear excessive levels of these toxic moieties can be detrimental to the cell. In addition to affecting cell proliferation and apoptosis, ROS can cause mutations in the mitochondrial genome, thereby giving rise to nonfunctional OXPHOS enzymes and impacting cellular metabolism [13,14]. This, in turn, can adversely affect other organelles, and thus disrupt cellular homeostasis. Mitochondria further play a crucial role in cell fate determination by releasing certain molecules into the cytoplasm, triggering a self-destructive enzymatic cascade, leading to apoptotic cell death [15].
While mitochondria are now thought to be multifunctional in nature, emerging discoveries further suggest a dynamic aspect to mitochondrial location and structure [16–28]. In this regard, multiple regulatory mechanisms are thought to be involved in morphological changes of mitochondria, controlling the formation and maintenance of mitochondrial networks (fusion) and disruption of such networks (fission). Such changes result in spatial and temporal regulation of mitochondria. Accordingly, mitochondria and mitochondrially targeted proteins are thought to interact with organelles such as the plasma membrane, endoplasmic reticulum (ER; sarcoplasmic reticulum SR, in muscle cells), peroxisomes, lysosomes, and nucleus, thus enhancing intracellular communication and potentially affecting a wide range of cellular functions [18,29–34]. The relevance of such interactions lies in the broad effects that mitochondria can have on different organelles and processes in the context of disease, and furthermore the effect of mitochondrial dysfunction itself on cellular structure and function.
Overall, mitochondria appear to play an important role in different aspects of cell structure and function, and in the balance between processes that are detrimental vs. protective to the cell. These aspects become important in the cellular responses to a variety of genetic, epigenetic and environmental signals that lead to disease states. This is also important in the context of lung diseases where the idea that perturbations of mitochondrial structure and function are important to disease pathophysiology is gaining momentum. This review is aimed at summarizing and discussing the current state of knowledge regarding mitochondrial structure and function in the lung, how disease processes modulate these aspects, and in turn, how mitochondria can contribute to disease pathophysiology. Here, at the outset, it is important to emphasize that much of this data in terms of normal lung structure/function and lung disease is only emerging. Nonetheless, substantial important information can be gleaned from data in other non-lung cell systems and disease states, setting the stage for exploration of mitochondrial mechanisms in the lung. In this regard, what aspects of mitochondrial structure, function or dysfunction are important is not well-known. In this review, specific emphasis will be given to homeostatic aspects of mitochondrial biology: the fission-fusion balance of the mitochondrial reticulum, synthesis/clearance cycle of oxidants (ROS and RNS), and, proliferation vs. apoptosis or the cell fate determination step, all of which are important in cellular function relevant to the lung. By highlighting the significance of these processes, the review will facilitate better understanding and exploration of emerging concepts of targeting mitochondria to alleviate disease in general, and lung disease in particular.
2. Mitochondrial Fission and Fusion
2.2. Regulation of mitochondrial morphology
Although the origins of mitochondria are common across cell types, mitochondria assume remarkably different shapes, connectivities and morphological parameters in different cells, in different species and under different conditions [35–39]. Furthermore, even within a cell type or within individual cells, mitochondria are not static structures, but show continued changes in their network morphology and intracellular locations. The relevance of altered mitochondrial morphology lies in increasing evidence that regardless of cell types, such changes reflect (or go hand in hand with) mitochondrial function, especially in the setting of apoptotic cell death or oxidative stress [19,35,37,40]. Accordingly, understanding the processes that control mitochondrial morphology become important to determining the role of mitochondria in normal cellular function and mitochondrial dysfunction in the setting of disease. Here, it is important to stress that much of the information is derived from cellular expression systems and a variety of non-lung cell types.
Proteins that control mitochondrial fission vs. fusion are the primary regulators of mitochondrial morphology. Here, the mitofusins 1 and 2 (Mfn1 and Mfn2), and Optic Atrophy protein 1 (Opa1) are essential mediators of mitochondrial fusion [41] (Table 1). Genetic knockout studies have established that consistent with their localization patterns (Mfns on the OMM, and Opa1 on the IMM), these proteins are responsible for the fusion of mitochondrial OMM and IMM, respectively [41], allowing for formation of contiguous chains or networks of mitochondria within the cell. In contrast, fission requires dynamin related protein 1 (Drp1) to be recruited from the cytosol to the mitochondrial surface, a step that involves other proteins such as Fission 1 (Fis1) and Mitochondrial fission factor (Mff) [42]. Additional factors such as MiD49 and MiD51 are also important in the orchestration of fission where these proteins form foci/rings around mitochondria to assist in recruitment of Drp1 from the cytosol when fission is imminent [43].
Table 1.
Proteins involved in mitochondrial fission and fusion.
| Mammalian Protein | Synonyms/homologs | Function/specific step |
|---|---|---|
| Drp1 (Dynamin-related protein 1) Fis1 (Fission 1) Mff (Mitochondrial Fission Factor) Unknown |
Dnm1p (S.c) Fis1p (S.c) Unknown Mdv1 and Caf4 (S.c) |
OMM Fission Receptor for Drp1 on the OMM Alternate receptor for Drp1? Adaptor for Drp1-Fis1 |
| Mfn1/2 (Mitofusins 1 & 2) Opa1 (Optical Atrophy 1) GDAP1 Unknown |
Fzo1p (S.c) Mgm1p1 (S.c) Unknown Ugo1 (S.c) |
OMM Fusion IMM Fusion Linker for Fzo1 and Mgm1 |
| MARCH5 MAPL SENP3 SENP5 ULK1 & 2 |
Unknown Unknown Unknown Unknown Atg1 (S.c) |
Ubiquitin ligase for Mfn1/2, Drp1 and Fis1 SUMO ligase for Drp1 DeSUMOylating protease for Drp1 DeSUMOylating protease for Drp1 Autophagy |
A number of factors can modulate mitochondrial fission and fusion proteins. Indeed, intracellular Ca2+ can itself be a factor where mitochondrial fragmentation is activated during high Ca2+ conditions, and suppressed at resting [Ca2+]I. Such effects may serve to modulate mitochondrial localization for maximal energy utilization or other functions, and/or they may be outcomes of other changes induced by altered Ca2+. Changes in mitochondrial morphology are also linked to oxidative stress-mediated signaling and cell fate determination such that these events share several proteins [26,44–49]. For example, the Bcl2-family proteins (Bax and Bak), initially thought to regulate apoptosis by merely inducing cytochrome c release into the cytosol, have now been shown to also directly interact with the fission-fusion machinery [44,47,50,51]. By sequestering fusion protein Opa1, the Bcl2-family member Bnip3 promotes mitochondrial fission [47], and Bcl-xL, a related protein, has been shown to regulate both fission and fusion via binding to Drp1 [50]. In addition, data in human skin fibroblasts show that mitochondrial morphology is modulated by mitochondrial membrane potential (MMP) where dissipation of MMP causes fragmentation and inhibition of glycolysis worsens OXPHOS defect-induced morphological changes [45]. Apoptosis follows Bax-mediated Opa1 disassembly, cristae remodeling and CytC release [49].
Interactions between fission and fusion proteins can also regulate mitochondrial morphology. For example, Huang et al. found that the association between two heptad regions in Mfn2 renders this protein incapable of mediating fusion, and when Mfn2 is free to interact with Drp1 under these conditions, fission ensues [26].
Relevant to disease states, one mechanism by which mitochondrial morphology can be altered is via changes in the turnover of the fission and fusion proteins. In other words, in addition to activating signaling pathways that alter the transcription and translation of fission/fusion proteins, stimuli such as inflammatory mediators or environmental triggers also induce post-translational modifications like proteasome-mediated degradation [52]. This is best illustrated by data from familial Parkinson’s disease (PD); here, Pink1, a mitochondrially targeted Ser/Thr kinase, interacts directly with the fission protein Fis1, and regulates mitochondrial dynamics [53] by acting as a co-factor for Parkin, an E3 ubiquitin ligase, and targeting Mfn, helping to degrade damaged mitochondria [54]. Defects in Mfn2 protein turnover due to gene mutations are associated with CMT2A neuropathy [55]. The role of post-translational modification by ubiquitination or SUMOylation in controlling the degradation rates of other members of the fission-fusion machinery such as Opa1, Drp1, and Fis1 have also been reported [56–58]. Overall, these studies highlight the evolution of cellular quality control mechanisms directed towards protecting mitochondrial fission-fusion equilibrium, and their potential relevance to disease states. What is not known is the relative importance of fission and fusion machineries in different lung cell types, their roles in normal cell function, and the extent of their involvement in specific lung diseases.
2.2. Mitochondrial morphology and cell function
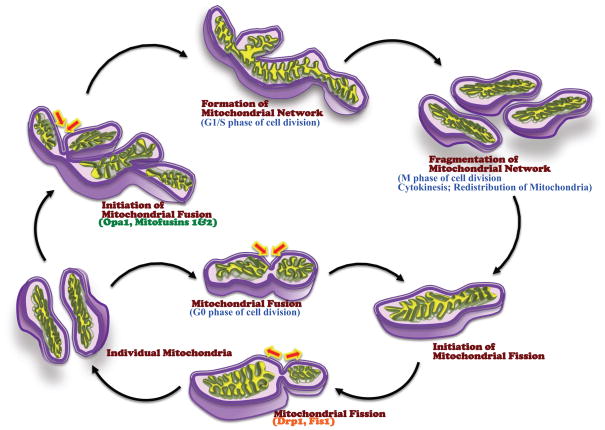
The relevance of mitochondrial fission/fusion processes is clear during the cell cycle. Mitochondria fragment and rejoin constitutively during the G0 phase (quiescent state) of the cell cycle. However, when the cell enters interphase, mitochondrial fusion is promoted, leading to extensive networking (Figure 2). As the mitotic and cytokinetic events occur during the M phase, mitochondria get fragmented, mixed and distributed equally among the progeny cells. At M phase exit, fusion resumes [27,59–62]. Furthermore, proteins involved in mitochondrial dynamics, such as Fis1 and GDAP1, have been reported to interact with microtubules, which are integral members in cell division [63]. These fission/fusion processes during the cell cycle become relevant to the disease state when factors such as inflammatory or infectious mediators, ROS/RNS and environmental stimuli alter these processes (e.g. in the airway or lung), influencing cell proliferation vs. stasis/apoptosis and overall cell numbers [64,65].
Figure 2. Mitochondrial fission-fusion cycle vs. the cell cycle.
During the quiescent (G0) phase, mitochondria fragment and rejoin, maintaining a constant mitochondrial number in the cell. When the cell enters mitosis (M phase), fusion is favored, and is driven by Opa1 and the mitofusins Mfn1 and Mfn2. As cell division comes to a close, the mitochondrial networks break apart and the mitochondria are redistributed between the daughter cells.
The dynamin GTPase Opa1 (regulator of mitochondrial inner membrane fusion and cristae remodeling) is thought to mediate adrenergic control of lipolysis by functioning as a cytosolic A-kinase anchoring protein (AKAP) [66,67]. Decreased Opa1 expression and increased fragmentation have been observed in failing hearts in both humans and rats [68]. In addition to its role in mitochondrial fusion, Mfn2 has been reported to be involved in oxidative metabolism, vascular cell proliferation, obesity, type 2 diabetes and regulation of the cytokines TNFα and IL-6 [69]. Mutations in Mfn2 gene have been linked to the autosomal dominant neurogenerative disease Charcot-Marie-Tooth 2A [70]; however, Mfn2 can also function as a protector of mtDNA integrity. Loss of Mfn2 results in accumulation of mutations in skeletal mtDNA in humans and mice [71]. It is evident from these emerging data not only that mitochondrial morphology and function are finely balanced domains, but also that their interdependency is an important factor in the role of mitochondria in disease development.
2.5. Mitochondrial fission/fusion and mitochondrial movement and distribution
Aspects other than mitochondrial morphology can also drive cellular functions. Here, mitochondrial localization to and interaction with other organelles are particularly important. In this regard, mitochondrial contacts with ER have been studied more extensively [23,24,29,30,72–75]. Acting as tether sites for specific proteins, mitochondria-ER junctions can aid in lipid synthesis, Ca2+ buffering and signaling, intracellular trafficking and mitochondrial biogenesis [24,75–78]. The fusion protein Mfn2 can physically harness ER to the mitochondrion via its association with Ras, thereby controlling mitochondrial uptake of Ca2+ from ER microdomains [23,72,74]. Regulation of cytosolic and ER luminal Ca2+ levels via such buffering is particularly important in tissues such as cardiac muscle, where ATP and ROS, as well as Ca2+, are rapidly and consistently exchanged between mitochondrion and SR. This bidirectional regulation of [Ca2+]i makes the mitochondria a major source of Ca2+ for contraction in addition to the SR [79]. The relevance of such mitochondria-ER interactions in the lung lies in the fact that several lung cell types including alveolar epithelium and airway smooth muscle use ER/SR Ca2+ release for contraction or other cellular functions, including protein regulation. Accordingly, mitochondrial modulation of plasma membrane vs. intracellular organelle function becomes important.
Mitochondria in proximity to the PM may be important in regulation of Ca2+ influx in different cell types. Mitochondrial buffering of the Ca2+ that enters may sustain or promote Ca2+ entry due to perceived lack of cytosolic Ca2+, or alternatively prevent Ca2+ entry through signaling interactions [80–85]. Whether mitochondrial fission or fusion proteins are involved in such modulation of Ca2+ influx remains to be determined. The importance of such a mechanism lies in the fact that several cell types, including those in the lung are highly dependent on Ca2+ influx.
Mitochondria may also indirectly facilitate ER-plasma membrane interactions. For example, in airway smooth muscle and other cell types, the ER/SR protein STIM1 senses SR Ca2+ release, and either translocates to or aggregates at the PM Ca2+ channel Orai1 [86–91], facilitating store-operated Ca2+ entry. It has recently been shown that STIM1 mobilization to ER-PM junctions also occurs upon mitochondrial release of Ca2+, and is an Mfn2-dependent step [78]. The relevance of these interactions lies in the fact inflammation has been shown to regulate mitochondrial Ca2+ in the airway [81], mitochondrial regulation of intracellular Ca2+ levels via the STIM1/Mfn2 pathway remains to be determined.
There is now strong evidence that mitochondrial movement and distribution within the cell is also important. Coordinated interactions between cytoskeletal elements (particularly actin and microtubules) and motors (such as kinesin and dynein) are thought to enable proper positioning of mitochondria. The mitochondrially-localized Miro protein seems to be a key regulator of mitochondrial motility, with its activity depending on its Ca2+- sensing and kinesin-binding abilities [92,93]. Interestingly, Miro suppresses Drp1-mediated fission, thus connecting mitochondrial fragmentation (activated during high Ca2+ conditions, and suppressed at resting [Ca2+]i), and fusion with mitochondrial movement [84]. While it has been known that mitochondria rely on the motor proteins kinesin and dynein for transport along microtubules [94,95], recent evidence suggest that dynein mediated mitochondrial transport may be essential for mitochondria-ER interactions during inflammation [96]. Similarly, mechanisms that regulate actin polymerization also seem to modulate mitochondrial fission [29]. Actin filaments that connect ER to mitochondria may initiate mitochondrial constriction, which then is completed by the fission protein Drp1. It is easy to envision mitochondrial movement being motivated by energy-demanding areas of a cell, i.e., mitochondria become less mobile and cluster at sites that require additional metabolic energy for function, signaled by factors such as [Ca2+]i or signaling intermediates. The relevance of mitochondrial movement and its modulation by fission/fusion in lung diseases lies in the potential disruption of these relationships by inflammation, ROS or other factors in different cell types. Such disruption may lead to a mismatch between energy demand and supply (particularly in bronchial or epithelial cells that undergo high turnover), availability of mitochondria for regulating other cellular organelles such as the ER/SR and PM, leading to dysregulating Ca2+ and contractility, cell proliferation/apoptosis etc.
3. The importance of ROS
3.1. Production and clearance of mitochondrial ROS
As mentioned above, mitochondria are the sites for metabolic processes and the electron transport chain, both of which give rise to significant amounts of ROS and RNS [97]. When production of these moieties overwhelms the innate scavenging mechanisms of the cell (via enzymes like SOD, catalase or peroxidase, to name a few), the resultant oxidative stress is deleterious to the cell at multiple levels [98]. In the lung, uncontrolled oxidant levels can contribute to pulmonary fibrosis and other diffuse lung diseases [12]. Several studies have shown that mitochondrial ROS subserve several functions, acting as signal transducers that trigger expression and/or release of proinflammatory cytokines and the relevant pathways [99,100], as well as modulators of transcription factors that are important in normal cellular redox homeostasis, cell proliferation/survival, and responses to inflammation [101]. These mediators play vital and multiple roles in the development and exacerbation of asthma and allergy, COPD and fibrosis by altering processes such as cell proliferation, extracellular matrix production, Ca2+ regulation [81,87,89,100,102–110]. Importantly, inflammatory mediators and ROS can in turn modulate mitochondrial morphology and function, thus linking mitochondria, ROS and inflammation in a functional cycle of consequence to disease pathophysiology. For example, IL-6 induces Bcl2, which interferes with mitochondrial Bak-Mfn2 interactions and blocks apoptosis [111]. Oxidative stress enhances Drp1 translocation from the cytosol, mitochondrial fragmentation, CytC release and apoptosis, while treatment with an antioxidant or overexpression of Mfn2 nullifies these effects [112].
In addition to oxidative stress occurring as a result of intracellular processes, the lung can be exposed to hyperoxia (e.g. in patients in the ICU, or those needing supplemental oxygen) as well as hypoxia (e.g. as in COPD, pulmonary fibrosis or pulmonary hypertension). We recently demonstrated that fetal airway smooth muscle cells exhibit an O2 dose-dependent response in terms of Ca2+ signaling, proliferation and mitochondrial morphology [113]. Here, we reported that both hypoxia and increasing levels of oxygen induce mitochondrial fragmentation and enhance expression of fission proteins (but blunt fusion protein levels). Hypoxia can also induce ROS and RNS release from IMM [4] which activates transcription factors such as HIF1 (Hypoxia inducible factor 1) [114] that plays a significant role in mediating hypoxia-induced effects on the mitochondria by hyperpolarizing OMM, increasing mitochondrial glycolytic activity and promoting cell proliferation [115,116].
In summary, mitochondria are not only the sites for releasing oxidative free radicals; they respond to the corresponding signals by altering their structural and functional features, and these are conveyed to downstream effectors as regulatory instructions for alterations in cellular behavior.
3.2. Mitochondrial Membrane Potential
Release of the mitochondrial IMS enzyme CytC into the cytoplasm is a marker for the onset of apoptosis since this event can occur only if OMM becomes permeable following mitochondrial depolarization. Mitochondrial membrane potential (MMP; ΔΨ) is thus an indicator of mitochondrial function, or dysfunction. Thus, in cancers where cell death is the intent, manipulation of MMP towards depolarization can boost sensitivity to chemotherapy agents [117,118]. Here, we recently showed a “protective” role for the mitochondrial protein MICU1 [119]. Conversely, a stable OMM barrier and inhibited apoptosis may be beneficial under other conditions [120]. Regulation of MMP appears to be linked to mitochondrial fission-fusion machinery but not fission/fusion itself. Inhibition of the fission protein Drp1 directly prevents CytC release and apoptosis by blocking mitochondrial depolarization [15,121]. The story is more complex as the fusion protein Mfn2 is also thought to predispose cells (cardiac myocytes) to apoptosis by expediting mitochondrial depolarization or fusion-triggered membrane destabilization and leakage [122,123]. However, it is likely that Mfn2 plays roles other than just mitochondrial union; in fact, Mfn2 has been shown to mediate autophagosome-lysosome fusion as well, and this process triggers apoptosis in cardiac cells [124]. Thus complex interactions between MMP, fusion/fission proteins and cell fate exist, and may color the overall effects of factors that induce mitochondrial depolarization.
4. Mitochondrial fission/fusion, apoptosis and proliferation
4.1. Mitochondria-dependent cell death
Several studies, including our own, have confirmed that mitochondria fragment following exposure to a wide range of toxic substances, signaling the onset of apoptosis [25,28,113,125,126]. Such a sequence is triggered by Drp1 translocation to mitochondria [109]. However, mitochondrial fission can occur, independent of apoptosis. For example, attenuation of mitochondrial fragmentation by Drp1 inhibition blunts CytC release into cytosol, but other mitochondria-derived cell death-related factors (caspases, adenylate kinase etc.) are not blocked [127,128]. Bax and Bak promote fragmentation and apoptosis [129,130], yet antagonizing these proteins inhibits CytC release but does not necessarily prevent fragmentation [131].
The connection of Drp1/fission to apoptosis and of Mfn/fusion to proliferation is not straightforward. Contrary to the idea that Drp1 is pro-apoptotic, recent studies show that Drp1 can induce a hyperproliferation phenotype in vascular smooth muscle cells relevant to pulmonary hypertension and in lung cancer [132,133]. On the other hand, several studies show that mitofusins (especially Mfn2) promote proliferation [48,134,135], with some reports of suppression [132,136,137]. Mfn2 expression is downregulated in atherosclerosis and other vascular disorders [138] and in hyperinsulinemia [139]. In airway smooth muscle cells, however, we find Drp1 to be anti-proliferative and Mfn2 to be anti-apoptotic (unpublished results, paper currently in review). Perhaps differences in cell type, stimuli, and growth conditions underlie such discrepancies, but the essential point is that mitochondrial morphology itself can drive cell survival vs. death. Accordingly, the role of mitochondrial morphology in the pathogenesis of lung diseases becomes important.
4.2. Autophagy/mitophagy
Autophagy is a cellular homeostatic process where organelles including mitochondria, proteins or bits of cytoplasm get enveloped by double-membraned vesicular structures (autophagosomes) that then fuse with lysosomes to get degraded and recycled [140]. Recycling of digested cellular components helps produce ATP during starvation [141]. Relevant to mitochondria (where the process is termed mitophagy), depolarized and damaged mitochondria, if left unchecked, will activate apoptotic signaling [142,143], and thus mitophagy plays a key role in cytoprotection, stress adaptation and cellular housekeeping [1]. The PINK1-parkin signaling system has been attributed a critical role in the pathogenesis in Parkinson’s disease (PD), as mutations in these genes cause defective mitophagy; lack of clearance or the accumulation of damaged mitochondria initiates apoptosis and thus to selective loss of dopaminergic neurons in substantia nigra [53,54,144]. There is now considerable interest in autophagy processes in lung diseases, particularly relevant to the effects of inflammation, especially because this phenomenon is no exception to the repetitive motif of balance and homeostasis in biological systems: emerging evidence suggests that autophagy can be either protective or deleterious in the lung in disease conditions such as pulmonary arterial hypertension, COPD, cystic fibrosis, and pneumonia [1,142,145–151]. What is less clear is which effect predominates whether autophagy should be inhibited or promoted in the context of therapy. The autophagy protein LC3B has a protective effect in PAH by regulating hypoxia-induced cell proliferation [149], but is detrimental in COPD and cigarette smoke-induced lung cell death [145,146]. Specific modifications of autophagy/mitophagy-associated proteins are essential for maintaining mitochondrial and cellular homeostasis: failure by AMPK (adenosine monophosphate-activated protein kinase), a cellular energy sensor, to phosphorylate autophagy proteins results in defective clearance of mitochondria [152]. How mitochondria in turn may play a role in modulating autophagy, and the link between autophagy, mitochondrial regulatory proteins and lung diseases are still under investigation.
5. Mitochondrial dysfunction in lung diseases
Most of the current knowledge on mitochondrial morphology and functions related to fission/fusion and other mechanisms is derived from neurons, cardiomyocytes and immune cells (summarized in Figure 3 and Table 2). While the fission-fusion machinery in these cells is fundamentally identical to that in the cells of the lung (smooth muscle, epithelium, fibroblasts, resident immune cells), dysregulation of mitochondrial morphology, localization or function (due to fission-fusion imbalance, for example) is very likely to be cell-type and context (disease)-specific. The airways and lung parenchyma are sensitive and responsive to numerous stimuli such as allergens, toxins, pollutants and microorganisms, all of which can enhance inflammation, oxidative and cellular stress and metabolism-induced signaling to different extents, resulting in different pathophysiological responses. Chronic exposure to such stimuli or the resulting cellular responses are thought to drive processes such as enhanced Ca2+ and contractility, greater cell proliferation with reduced apoptosis, mucus secretion, and overall structural and functional changes in the lung. As primary sources of ROS and as crucial modulators of a number of cellular processes relevant to lung disease, the importance of mitochondria is obvious. However, there is considerable paucity in our understanding of mechanisms by which mitochondrial structure and function regulate airway health and disease.
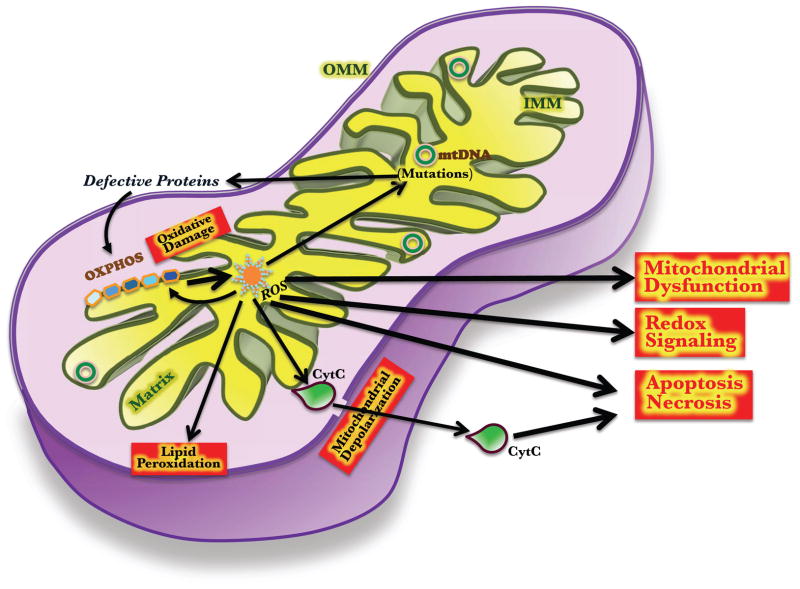
Figure 3. Mitochondrial regulation of cellular functions.
ROS produced by normal (and abnormal) glucose and fatty acid metabolism can induce mutations in mtDNA, which in turn can give rise to aberrant ETC proteins, and thus decrease the primary function of the mitochondria: energy production. Lipid peroxidation, and activation of cytoplasmic signals by ROS can lead the cell to apoptosis or senescence. Leakage of apoptosis mediators such as Cytochrome C (CytC), due to OMM depolarization can also initiate apoptosis.
Table 2.
Mitochondrial proteins that have been implicated in diseases
| Disease | Protein (specific function) |
|---|---|
| Cardiomyopathy Microencephaly, optic atrophy and hypoplasia |
Drp1 (Mitochondrial fission) |
| Charcot-Marie-Tooth (CMT2A) disease Optic Atrophy (ADOA) |
Mfn1 (Mitochondrial fusion) Mfn2 (Mitochondrial fusion) Opa1 (Mitochondrial fusion) |
| Pulmonary Hypertension (PAH) | MnSOD (ROS production) |
5.1. Airway hyperresponsiveness and asthma
As discussed above, the roles of mitochondria in ROS generation and processing, cell proliferation vs. apoptosis, regulation of Ca2+ and of other organelles are all relevant to inflammatory airway diseases such as asthma, which are characterized by hyperresponsiveness of the airway resulting from increased Ca2+ and contractility of airway smooth muscle, and by airway remodeling represented by epithelial and smooth muscle thickening, cell proliferation/migration and reduced apoptosis. Furthermore, polymorphisms or haplotype differences in mitochondrial genome have been found to be associated with asthma in humans [153]. However, there is limited but emerging data on how mitochondria may play a role in mediating or modulating the effects of inflammation in the airway.
Tumor necrosis factor-alpha (TNFα) is an early cytokine relevant to airway inflammation. TNFα has been shown to alter mitochondrial dynamics and OMM permeabilization, increasing ROS generation, and causing apoptosis in cultured adipocytes, and in murine lung microvascular endothelium [104,154–156]. Delmotte et al. recently showed that inflammatory cytokines such as TNFα and IL-13 have profound effects on the Ca2+ buffering activity of mitochondria of human airway smooth muscle [81], which may play a protective role in preventing Ca2+ overload. However, cytokines may also overwhelm the protective mechanisms of mitochondria, as we recently found in human airway smooth muscle where TNFα induced mitochondrial fission, increased Drp1 expression (with a concomitant decrease in Mfn2), and increased ROS production, particularly in cells derived from asthmatic patients [125]. Inhibiting ROS with antioxidants blocked these effects on mitochondrial morphology highlighting the link between ROS and mitochondrial fission/fusion in the airway. Interestingly, inhibition of Mfn2 increased ROS in airway cells, while inhibition of Drp1 suppressed it, suggesting that the fission/fusion machinery may play an active role in modulating oxidative stress in the inflamed airway.
TNFR1 has been implicated in TRAPS (an autoinflammatory disorder resulting from missense mutations in TNFR1 gene), possibly by causing mitochondrial dysfunction, increasing ROS production and cytokine levels [103]. Similarly, glucocorticoid receptor GRα and estrogen receptor ERβ, critical signaling pathway components in the cell, have been shown to localize to the mitochondria; their expression declines during allergic asthma [157]. It is possible that, at a molecular level, a combination of decreased apoptosis-promoting receptors and increased survival-enhancing receptors influences mitochondrial function and morphology, and eventually leading the airway cells towards proliferation and remodeling. In addition to the usual cytokine players, another group of inflammatory modulators is lipid metabolites and the enzymes involved in their production. For example, 13-S-hydroxyoctadecadienoic acid, a lipid metabolite derivative of linoleic acid, induces features of severe asthma and importantly mitochondrial dysfunction [158], while 12/15-lipoxygenase, an enzyme induced by the Th2 cytokine IL-13in can cause bronchial epithelial injury again involving mitochondria [159].
While exposure to environmental factors such as ozone, diesel exhaust particles, or cigarette smoke has been known to trigger and worsen asthma and allergy symptoms, the role of mitochondria in the airway is emerging. Here, the role of oxidative stress and ROS appears to be important. In general, it appears that levels of different antioxidants in serum, bronchoalveolar lavage (BAL) or tissues are lower in asthmatics (see [160] for review). For example, peripheral cells and tissues from asthmatic patients show lower antioxidant enzymes [161–163] such that serum SOD activity, but not manganese or copper- and zinc-containing superoxide dismutase, is lower, and correlates to the degree of airway constriction. In terms of ROS, exposure to either unfractionated cigarette smoke extract or its components generates excessive mitochondrial ROS in ASM and alveolar epithelial cells [125,164]. Similarly, exposure to allergens such as ragweed pollen enhances ROS generation, which in turn, damages components of the mitochondrial respiratory chain complex [165]. Interestingly, the innate metabolic and respiratory events, which are the primary source of ROS, have been noted to become more robust in some airway diseases; that is, oxygen consumption and mitochondrial biogenesis are elevated in asthmatic airway cells [166], suggesting an almost ‘suicidal’ mechanism of disease pathogenesis.
Overall, these limited but interesting data suggest that factors that contribute to asthma can influence mitochondrial ROS generation or handling in the face of reduced antioxidants, thus sustaining oxidative stress in the airways. Here, mitochondrial fission and fusion machinery may play a more active role in modulating oxidative stress in the inflamed airway. Accordingly, targeting of oxidative stress in general or aspects of the mechanisms that control mitochondrial morphology may be helpful in alleviating the effects of factors that cause asthma.
5. 2. Cystic Fibrosis (CF)
Cystic fibrosis is an autosomal recessive disease, most commonly due to a mutation ΔF508 in the CFTR gene, and characterized by abnormal transport of chloride and sodium ions across the epithelium, leading to viscous airway secretions. There is limited data showing that mitochondrial buffering is compromised in airway epithelial homozygous for ΔF508CFTR [167]. Cells with mutated CFTR also display extensive mitochondrial fragmentation, and OMM depolarization. In addition, the hypoxic pulmonary vasoconstriction (HPV) that occurs in CF is partly attributable to impaired mitochondrial OXPHOS in pulmonary arterial smooth muscle and endothelial cells under hypoxia, which alters ROS levels and AMPK activation [168]. Furthermore, enhanced ROS production and apoptosis in CF bronchial epithelium appear to be due to aberrant mitochondrial function [169]. These observations suggest that mitochondrial dysfunction can be a substantial contributor in CF.
5. 3. Chronic Obstructive Pulmonary Disease (COPD)
COPD involves a number of mechanisms including protease-antiprotease imbalance, oxidant-antioxidant imbalance, and ineffective repair mechanisms. Exposure to cigarette smoke is well known to be a major risk factor for developing COPD [145,170–177]. Given the importance of ROS in mediating cigarette smoke effects, oxidative stress is now recognized as a major factor in COPD pathogenesis [178], since antioxidant capacity is also reduced in COPD. Here, the role of mitochondria-derived ROS appears to be prominent [178]. Beyond ROS generation, the mechanisms by which mitochondria could contribute to COPD are still under investigation. For example, differential expression of the aryl hydrocarbon receptor in lung epithelial cells and fibroblasts, which renders some smokers more vulnerable to COPD, can induce mitochondrial dysfunction and apoptosis, and downregulate expression of antioxidant enzymes [179]. In COPD, the electron transport chain, mitochondrial membrane permeability and apoptotic signaling are all affected [180–182]. Aberrant apoptotic signaling may also reduce mitochondrial density and biogenesis [175]. A recent study includes sickle cell anemia as a risk factor for cigarette smoke-induced airway obstruction in children [183], bolstering the notion that O2 respiration, mitochondrial function, and COPD pathogenesis are linked.
5.4. Lung cancer
While a number of mechanisms can contribution to initiation and propagation of lung cancers, mitochondrial dysfunction during the initial stages of neoplastic transformation may result in superfluous ROS resulting in mtDNA damage and mutations [184,185]. Furthermore, mutations in traditional oncogenes and tumor suppressor genes such as Ras, Myc and p53 can induce mitochondrial dysfunction and uncontrolled production of ROS [186]. Anomalies in mitochondrial respiratory chain complexes and ROS generation mechanisms not only impair the normal autophagy-apoptosis stability, and thus initiate tumorigenesis, but can also render cancer cells less susceptible to chemotherapeutic agents [117,148].
The connection between mitochondrial morphology and lung cancer development is complex. Overexpression of Mfn2 may be pro-proliferative, but some studies show fission to be favored in lung cancer cells, where a simultaneous increase in Drp1 is noted (along with reduced Mfn2) such that Drp1 inhibition or Mfn2 overexpression increases apoptosis [133]. How these proteins regulate cell fate is not known and may also be context dependent. Regardless, the limited data suggest a potential novel target for lung cancers that may have pleiotropic effects.
6. Targeting mitochondria in therapy
Pharmacological agents that alter mitochondrial structure and/or function have been considered. Drugs such as glucocorticoids, antioxidants, phosphodiesterase inhibitors, leukotriene receptor antagonists and bronchodilators can regulate mitochondrial structure and function[106,187–190]. For example, lack of L-arginine bioavailability is thought to occur in asthma, leading to peroxynitrite accumulation and mitochondrial dysfunction [191,192]. Mabalirajan et al. found that in the bronchial epithelia of ovalbumin-sensitized/challenged mice, L-arginine administration restores mitochondrial ultrastructure (reduced fragmentation), and function (increased ATP levels and mitochondrial CytC activity), suggesting L-Arg as a promising asthma drug [191]. Potent antioxidants such as MitoE(2) and MitoQ(10) have been shown to increase mitochondrial matrix [Ca2+], which may favor Ca2+ overload, and alter the apoptosis-proliferation balance [193].Other antioxidants that have also shown promising results in preclinical studies are: baicalein [194,195], esculetin [196], and vitamin E [197,198]. For example, esculetin, a plant-derived coumarin has been reported to have potent bronchodilating properties, and to restore mitochondrial function in a mouse model of experimental asthma [196]. In neurons, glucocorticoids have been shown to induce glucocorticoid receptor interaction with Bcl2 and translocation to mitochondria [199], which may be beneficial in asthma. Here, it is interesting to note that steroid receptors (glucocorticoid, estrogen) are localized to mitochondria and participate in induction of apoptosis, but are downregulated in bronchial epithelium of allergic asthma patients [157]. Accordingly mechanisms to enhance such receptor expression may be helpful in inducing apoptosis and thus preventing airway remodeling in asthma. Here, it may be worth considering the idea that inducing mitochondrial dysfunction (rather attempting to normalize it) may actually be beneficial.
Non-traditional pharmacological approaches to targeting mitochondria have been tested. For example, a cinnamate compound enhances normal mitochondrial function, and reduces ROS production and mitochondrial fission, suggesting this drug as a good candidate for asthma treatment [200]. Similarly, Rd, a major ginsenoside component found in Panax ginseng, has been shown to ameliorate mitochondrial dysfunction in brain cells of a rat model for stroke [201], raising the possibility that this compound may have comparably beneficial effects on airway diseases.
In addition to pharmacological targeting of mitochondria, there are some non-traditional approaches that are yet to be applied to lung diseases but may hold potential. For example, CTVT, a cancerous cell line circulating in feral dogs, is thought to have acquired mitochondria from its host [202]. Based on this finding, a ‘mitochondria replacement therapy’ procedure to successfully abrogate symptoms of lipopolysaccharide-induced acute lung injury (ALI) in mice using mitochondria from bone marrow-derived stromal cells (BMSCs) has been recently reported [203]. Furthermore, transmitochondrial cybrid systems (cybrids) by fusing mitochondria from an aggressive tumor cell line with those from benign or less aggressive cancer cells have been shown to exhibit features of the non-cancerous (‘healthy’) mitochondria, and are capable of erasing the oncogenic properties of the aggressive tumor cells. Microarray analysis shows that oncogenic pathways are inhibited in cybrids with non-cancerous mitochondria, leading the idea that crosstalk between mitochondria and nucleus occurs, which helps repair mitochondrial dysfunction [204]. Another approach has been the targeting of microRNAs. For example, miR-210, which is overexpressed in late-stage non-small cell lung cancer, induces loss of OMM permeability and mitochondrial dysfunction, and regulates succinate dehydrogenase [205,206]. In the heart, miRs-499 and 484 are pro-proliferative by suppressing Drp1 and Fis1 [207]. Similar approaches in other lung diseases await investigation.
8. Expert Commentary
The above discussion clearly highlights the emerging recognition that mitochondria do much more than “just provide energy for cellular function”. Indeed, mitochondria are being increasingly seen as critical regulatory elements by virtue of their control of energy, ions such as Ca2+ and H+, and cellular processes such as proliferation, migration and apoptosis. In this regard, it appears that mitochondrial structure is a key aspect of mitochondrial function. Although a plethora of studies have provided us with the basics of mitochondrial morphology and function (Figure 4), the significance of this link and its relevance, as applicable to intracellular contacts and communications, are not completely elucidated in the lung. Certainly, a lot can be learned from data in other cell types. However, the emerging data in other aspects of cellular function clearly demonstrate the vast heterogeneity among cell types (even within an organ) that makes it difficult to simply extrapolate information to cells of the lung, particularly in the context of lung diseases. Accordingly, if we are to develop novel strategies for lung diseases that are based on mitochondrial structure and function, studies on human airways and lungs should focus on building upon what is known in other cell types and species (especially yeast where mitochondria have been studied most extensively), and identifying homologs and paralogs for the molecules that regulate mitochondrial fission, fusion, mobility, and interactions. Dissecting their expression patterns, mechanism(s) of action, both under normal and different disease conditions in cell types of interest will then improve the chances for developing more efficacious therapy regimens.
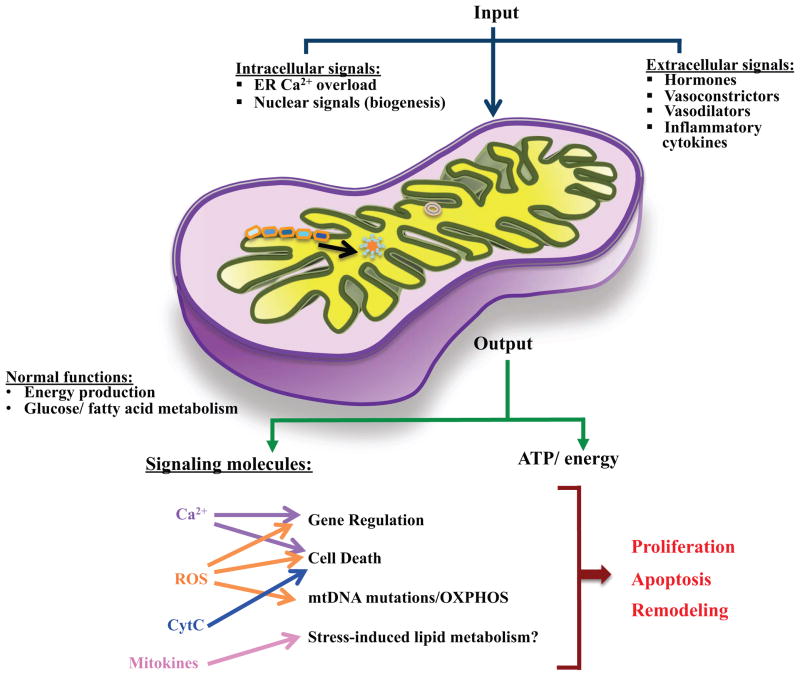
Figure 4. Mitochondrial contribution to lung diseases.
By receiving and processing intra- and extracellular signals (especially in chronic inflammation), mitochondria can be a major source for signaling and secondary messengers (ROS, Ca2+ etc). Unregulated production of these factors perturbs the proliferation-apoptosis balance of lung cells, and makes the environment conducive for altered cellular structure (proliferation greater than apoptosis) and other aspects of lung disease such as altered cellular protein production.
Five-year view
The authors foresee, based on the robust and multi-faceted advances in the molecular biology and imaging technologies, significant progress in the next few years, in the following: i) identification of various components of the mitochondria fission-fusion machinery (in both yeast and mammalian cells), regulators of mitochondria-ER interactions, and mitophagy. This should give us a clearer picture of the fundamental biology of mitochondria, as it applies to cellular physiology. ii) Evolution of the current imaging methods into better and more sensitive tools that can reliably quantify mitochondrial morphology, motility and interactions. iii) Extrapolation of studies done in neuronal, cardiac and immune cells, with regard to the central role of mitochondria in disease development and progression, to the lung and the airways. Such correlations will become relatively easier to make, as chronic inflammation, vasoconstriction and hyperreactivity are recognized as serious health conditions, and various mechanistic details about them come to light via clinical and basic research investigations. And finally, iv) looking at chronic airway diseases as mitochondrial dysfunctions. This will include recognizing the importance of mtDNA integrity and of mitochondrial ROS production as crucial determinants of lung health, developing novel methods to screening and diagnose detrimental changes in these parameters, and devising efficient therapeutic strategies (both conventional and unconventional) to combat them.
Key Issues.
Mitochondrial structure, dynamics and interactions with other organelles are tightly linked to their functional role in supplying cells with energy and signaling molecules, and in determining cellular fate.
Changes in mitochondrial morphology, manifested as fragmented or fused appearance, are not only indicators of mitochondrial stress, but they also correlate well with the metabolic efficiency of the mitochondrion and the resultant ROS production, integrity of mitochondrial membranes, and the subsequent choice of the cell to survive or to go into apoptosis.
Though considerable progress has been made with regard to the regulation of fission and fusion, mitochondrial movement and interactions, and ROS generation, the relevance of these phenomena to airway function and dysfunction/diseases is not as widely recognized as it is in other paradigms. However, available data suggest that mitochondria contribute significantly to chronic airway ailments (asthma, COPD, CF, PAH etc), and that manipulations that restore mitochondrial function and/or mitochondrially regulated cellular equilibriums ameliorate disease symptoms.
Integration of data from studies on non-airway tissues will be of paramount importance, as mitochondrial sensitivities, and responses to cellular environments in the airway are comparable to those in other organs. This is likely to not only provide us with mechanistic insights on balanced/normal mitochondrial function in the airway, but also help us improvise methodologies for further analyses, diagnoses and therapeutic interventions.
Recent emergence of state-of-the-art imaging and quantification techniques, in combination with cell and molecular biological tools, and bioinformatics, has enabled live studies on mitochondria. While a contiguous evolution of this process is to be expected, our current understanding of the genomic complement and the metabolic processes in the mitochondria, and how they relate to cellular and organismal physiology, is taking shape more vividly. Thus, factors like signaling pathway components, regulators of ROS production and scavenging, cell cycle/apoptosis participants are targeted for drug development.
Acknowledgments
Financial Disclosure/Acknowledgements
Supported by Young Clinical Scientist Award (Aravamudan) and Clinician Investigator Award (Pabelick) from the Flight Attendants Medical Research Institute (FAMRI) and grants from the National Institutes of Health (R01 HL088029, HL056470 (Prakash) and HL090595 (Pabelick)).
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science. 2011;333(6046):1109–1112. doi: 10.1126/science.1201940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev. 2008;88(2):611–638. doi: 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- 3.Pendyala S, Natarajan V. Redox regulation of Nox proteins. Respir Physiol Neurobiol. 2010;174(3):265–271. doi: 10.1016/j.resp.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poyton RO, Ball KA, Castello PR. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol Metab. 2009;20(7):332–340. doi: 10.1016/j.tem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Al Ghouleh I, Khoo NK, Knaus UG, et al. Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling. Free Radic Biol Med. 2011;51(7):1271–1288. doi: 10.1016/j.freeradbiomed.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bao L, Avshalumov MV, Patel JC, et al. Mitochondria are the source of hydrogen peroxide for dynamic brain-cell signaling. J Neurosci. 2009;29(28):9002–9010. doi: 10.1523/JNEUROSCI.1706-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doulias PT, Tenopoulou M, Greene JL, Raju K, Ischiropoulos H. Nitric oxide regulates mitochondrial fatty acid metabolism through reversible protein S-nitrosylation. Science signaling. 2013;6(256):rs1. doi: 10.1126/scisignal.2003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elchuri S, Oberley TD, Qi W, et al. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005;24(3):367–380. doi: 10.1038/sj.onc.1208207. [DOI] [PubMed] [Google Scholar]
- 9.Goth L. Catalase deficiency and type 2 diabetes. Diabetes care. 2008;31(12):e93. doi: 10.2337/dc08-1607. [DOI] [PubMed] [Google Scholar]
- 10.Muller FL, Song W, Liu Y, et al. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic Biol Med. 2006;40(11):1993–2004. doi: 10.1016/j.freeradbiomed.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 11.Paradies G, Petrosillo G, Paradies V, Reiter RJ, Ruggiero FM. Melatonin, cardiolipin and mitochondrial bioenergetics in health and disease. J Pineal Res. 2010;48(4):297–310. doi: 10.1111/j.1600-079X.2010.00759.x. [DOI] [PubMed] [Google Scholar]
- 12.Bargagli E, Olivieri C, Bennett D, Prasse A, Muller-Quernheim J, Rottoli P. Oxidative stress in the pathogenesis of diffuse lung diseases: a review. Respir Med. 2009;103(9):1245–1256. doi: 10.1016/j.rmed.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Crimi M, Rigolio R. The mitochondrial genome, a growing interest inside an organelle. International journal of nanomedicine. 2008;3(1):51–57. doi: 10.2147/ijn.s2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michelakis ED. Mitochondrial medicine: a new era in medicine opens new windows and brings new challenges. Circulation. 2008;117(19):2431–2434. doi: 10.1161/CIRCULATIONAHA.108.775163. [DOI] [PubMed] [Google Scholar]
- 15.Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11(9):621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 16.Anand R, Langer T, Baker MJ. Proteolytic control of mitochondrial function and morphogenesis. Biochim Biophys Acta. 2013;1833(1):195–204. doi: 10.1016/j.bbamcr.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 17.Benard G, Bellance N, James D, et al. Mitochondrial bioenergetics and structural network organization. J Cell Sci. 2007;120(Pt 5):838–848. doi: 10.1242/jcs.03381. [DOI] [PubMed] [Google Scholar]
- 18.Bravo-Sagua R, Rodriguez AE, Kuzmicic J, et al. Cell death and survival through the endoplasmic reticulum-mitochondrial axis. Current molecular medicine. 2013;13(2):317–329. doi: 10.2174/156652413804810781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campello S, Scorrano L. Mitochondrial shape changes: orchestrating cell pathophysiology. EMBO Rep. 2010;11(9):678–684. doi: 10.1038/embor.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castanier C, Garcin D, Vazquez A, Arnoult D. Mitochondrial dynamics regulate the RIG-I-like receptor antiviral pathway. EMBO Rep. 2010;11(2):133–138. doi: 10.1038/embor.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chalmers S, Saunter C, Wilson C, Coats P, Girkin JM, Mccarron JG. Mitochondrial motility and vascular smooth muscle proliferation. Arteriosclerosis, thrombosis, and vascular biology. 2012;32(12):3000–3011. doi: 10.1161/ATVBAHA.112.255174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H, Chan DC. Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Hum Mol Genet. 2009;18(R2):R169–176. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Brito OM, Scorrano L. Mitofusin-2 regulates mitochondrial and endoplasmic reticulum morphology and tethering: the role of Ras. Mitochondrion. 2009;9(3):222–226. doi: 10.1016/j.mito.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Friedman JR, Lackner LL, West M, Dibenedetto JR, Nunnari J, Voeltz GK. ER tubules mark sites of mitochondrial division. Science. 2011;334(6054):358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoppins S, Nunnari J. Cell Biology. Mitochondrial dynamics and apoptosis--the ER connection. Science. 2012;337(6098):1052–1054. doi: 10.1126/science.1224709. [DOI] [PubMed] [Google Scholar]
- 26.Huang P, Galloway CA, Yoon Y. Control of mitochondrial morphology through differential interactions of mitochondrial fusion and fission proteins. PLoS One. 2011;6(5):e20655. doi: 10.1371/journal.pone.0020655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hyde BB, Twig G, Shirihai OS. Organellar vs cellular control of mitochondrial dynamics. Semin Cell Dev Biol. 2010;21(6):575–581. doi: 10.1016/j.semcdb.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mabalirajan U, Dinda AK, Kumar S, et al. Mitochondrial structural changes and dysfunction are associated with experimental allergic asthma. J Immunol. 2008;181(5):3540–3548. doi: 10.4049/jimmunol.181.5.3540. [DOI] [PubMed] [Google Scholar]
- 29.Korobova F, Ramabhadran V, Higgs HN. An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2. Science. 2013;339(6118):464–467. doi: 10.1126/science.1228360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malhotra JD, Kaufman RJ. ER Stress and Its Functional Link to Mitochondria: Role in Cell Survival and Death. Cold Spring Harb Perspect Biol. 2011 doi: 10.1101/cshperspect.a004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mallilankaraman K, Cardenas C, Doonan PJ, et al. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat Cell Biol. 2012;14(12):1336–1343. doi: 10.1038/ncb2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neuspiel M, Schauss AC, Braschi E, et al. Cargo-selected transport from the mitochondria to peroxisomes is mediated by vesicular carriers. Current biology : CB. 2008;18(2):102–108. doi: 10.1016/j.cub.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 33.Shinde AV, Motiani RK, Zhang X, et al. STIM1 Controls Endothelial Barrier Function Independently of Orai1 and Ca2+ Entry. Science signaling. 2013;6(267):ra18. doi: 10.1126/scisignal.2003425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soubannier V, Mclelland GL, Zunino R, et al. A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Current biology : CB. 2012;22(2):135–141. doi: 10.1016/j.cub.2011.11.057. [DOI] [PubMed] [Google Scholar]
- 35.Ahmad T, Aggarwal K, Pattnaik B, et al. Computational classification of mitochondrial shapes reflects stress and redox state. Cell death & disease. 2013;4:e461. doi: 10.1038/cddis.2012.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36••.Koopman WJ, Visch HJ, Verkaart S, Van Den Heuvel LW, Smeitink JA, Willems PH. Mitochondrial network complexity and pathological decrease in complex I activity are tightly correlated in isolated human complex I deficiency. Am J Physiol Cell Physiol. 2005;289(4):C881–890. doi: 10.1152/ajpcell.00104.2005. Gives excellent insight into techniques to image and quantify mitochondrial morphology. [DOI] [PubMed] [Google Scholar]
- 37.Mannella CA. Structural diversity of mitochondria: functional implications. Ann N Y Acad Sci. 2008;1147:171–179. doi: 10.1196/annals.1427.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willems PH, Smeitink JA, Koopman WJ. Mitochondrial dynamics in human NADH:ubiquinone oxidoreductase deficiency. The international journal of biochemistry & cell biology. 2009;41(10):1773–1782. doi: 10.1016/j.biocel.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Willems PH, Swarts HG, Hink MA, Koopman WJ. Chapter 16 The use of fluorescence correlation spectroscopy to probe mitochondrial mobility and intramatrix protein diffusion. Methods in enzymology. 2009;456:287–302. doi: 10.1016/S0076-6879(08)04416-9. [DOI] [PubMed] [Google Scholar]
- 40•.Palmer CS, Osellame LD, Stojanovski D, Ryan MT. The regulation of mitochondrial morphology: intricate mechanisms and dynamic machinery. Cell Signal. 2011;23(10):1534–1545. doi: 10.1016/j.cellsig.2011.05.021. Concise summary of what is known about mitochondrial fission-fusion apparatus and how it is regulated. [DOI] [PubMed] [Google Scholar]
- 41.Song Z, Ghochani M, Mccaffery JM, Frey TG, Chan DC. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol Biol Cell. 2009;20(15):3525–3532. doi: 10.1091/mbc.E09-03-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otera H, Wang C, Cleland MM, et al. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J Cell Biol. 2010;191(6):1141–1158. doi: 10.1083/jcb.201007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Palmer CS, Osellame LD, Laine D, Koutsopoulos OS, Frazier AE, Ryan MT. MiD49 and MiD51, new components of the mitochondrial fission machinery. EMBO Rep. 2011;12(6):565–573. doi: 10.1038/embor.2011.54. Adds anchor proteins MiD49 and MiD51, involved in mitochondrial dynamics, to the list of factors that regulate mitochondrial morphology. Further studies on these may be useful for designing drugs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Autret A, Martin SJ. Emerging role for members of the Bcl-2 family in mitochondrial morphogenesis. Mol Cell. 2009;36(3):355–363. doi: 10.1016/j.molcel.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 45.Guillery O, Malka F, Frachon P, Milea D, Rojo M, Lombes A. Modulation of mitochondrial morphology by bioenergetics defects in primary human fibroblasts. Neuromuscul Disord. 2008;18(4):319–330. doi: 10.1016/j.nmd.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 46.Heath-Engel HM, Shore GC. Mitochondrial membrane dynamics, cristae remodelling and apoptosis. Biochim Biophys Acta. 2006;1763(5–6):549–560. doi: 10.1016/j.bbamcr.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Landes T, Emorine LJ, Courilleau D, Rojo M, Belenguer P, Arnaune-Pelloquin L. The BH3-only Bnip3 binds to the dynamin Opa1 to promote mitochondrial fragmentation and apoptosis by distinct mechanisms. EMBO Rep. 2010;11(6):459–465. doi: 10.1038/embor.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagaraj R, Gururaja-Rao S, Jones KT, et al. Control of mitochondrial structure and function by the Yorkie/YAP oncogenic pathway. Genes & development. 2012;26(18):2027–2037. doi: 10.1101/gad.183061.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamaguchi R, Lartigue L, Perkins G, et al. Opa1-mediated cristae opening is Bax/Bak and BH3 dependent, required for apoptosis, and independent of Bak oligomerization. Mol Cell. 2008;31(4):557–569. doi: 10.1016/j.molcel.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berman SB, Chen YB, Qi B, et al. Bcl-x L increases mitochondrial fission, fusion, and biomass in neurons. J Cell Biol. 2009;184(5):707–719. doi: 10.1083/jcb.200809060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rolland SG, Lu Y, David CN, Conradt B. The BCL-2-like protein CED-9 of C. elegans promotes FZO-1/Mfn1,2- and EAT-3/Opa1-dependent mitochondrial fusion. J Cell Biol. 2009;186(4):525–540. doi: 10.1083/jcb.200905070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neutzner A, Benard G, Youle RJ, Karbowski M. Role of the ubiquitin conjugation system in the maintenance of mitochondrial homeostasis. Ann N Y Acad Sci. 2008;1147:242–253. doi: 10.1196/annals.1427.012. [DOI] [PubMed] [Google Scholar]
- 53••.Yang Y, Ouyang Y, Yang L, et al. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci U S A. 2008;105(19):7070–7075. doi: 10.1073/pnas.0711845105. Connects mitochondrial morphology to proteasome degradation system, and thus gives a possible regulatory mechanism for fission-fusion homeostasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ziviani E, Tao RN, Whitworth AJ. Drosophila parkin requires PINK1 for mitochondrial translocation and ubiquitinates mitofusin. Proc Natl Acad Sci U S A. 2010;107(11):5018–5023. doi: 10.1073/pnas.0913485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amiott EA, Cohen MM, Saint-Georges Y, Weissman AM, Shaw JM. A mutation associated with CMT2A neuropathy causes defects in Fzo1 GTP hydrolysis, ubiquitylation, and protein turnover. Mol Biol Cell. 2009;20(23):5026–5035. doi: 10.1091/mbc.E09-07-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zungu M, Schisler J, Willis MS. All the little pieces. -Regulation of mitochondrial fusion and fission by ubiquitin and small ubiquitin-like modifer and their potential relevance in the heart. Circ J. 2011;75(11):2513–2521. doi: 10.1253/circj.cj-11-0967. [DOI] [PubMed] [Google Scholar]
- 57.Zunino R, Braschi E, Xu L, Mcbride HM. Translocation of SenP5 from the nucleoli to the mitochondria modulates DRP1-dependent fission during mitosis. J Biol Chem. 2009;284(26):17783–17795. doi: 10.1074/jbc.M901902200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zunino R, Schauss A, Rippstein P, Andrade-Navarro M, Mcbride HM. The SUMO protease SENP5 is required to maintain mitochondrial morphology and function. J Cell Sci. 2007;120(Pt 7):1178–1188. doi: 10.1242/jcs.03418. [DOI] [PubMed] [Google Scholar]
- 59.Finkel T, Hwang PM. The Krebs cycle meets the cell cycle: mitochondria and the G1-S transition. Proc Natl Acad Sci U S A. 2009;106(29):11825–11826. doi: 10.1073/pnas.0906430106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitra K, Wunder C, Roysam B, Lin G, Lippincott-Schwartz J. A hyperfused mitochondrial state achieved at G1-S regulates cyclin E buildup and entry into S phase. Proc Natl Acad Sci U S A. 2009;106(29):11960–11965. doi: 10.1073/pnas.0904875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schieke SM, Mccoy JP, Jr, Finkel T. Coordination of mitochondrial bioenergetics with G1 phase cell cycle progression. Cell Cycle. 2008;7(12):1782–1787. doi: 10.4161/cc.7.12.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Owusu-Ansah E, Yavari A, Mandal S, Banerjee U. Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nature genetics. 2008;40(3):356–361. doi: 10.1038/ng.2007.50. [DOI] [PubMed] [Google Scholar]
- 63.Estela A, Pla-Martin D, Sanchez-Piris M, Sesaki H, Palau F. Charcot-Marie-Tooth-related gene GDAP1 complements cell cycle delay at G2/M phase in Saccharomyces cerevisiae fis1 gene-defective cells. J Biol Chem. 2011;286(42):36777–36786. doi: 10.1074/jbc.M111.260042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ammit AJ, Panettieri RA., Jr Airway smooth muscle cell hyperplasia: a therapeutic target in airway remodeling in asthma? Progress in cell cycle research. 2003;5:49–57. [PubMed] [Google Scholar]
- 65.Ammit AJ, Panettieri RA., Jr Invited review: the circle of life: cell cycle regulation in airway smooth muscle. J Appl Physiol. 2001;91(3):1431–1437. doi: 10.1152/jappl.2001.91.3.1431. [DOI] [PubMed] [Google Scholar]
- 66.Belenguer P, Pellegrini L. The dynamin GTPase OPA1: More than mitochondria? Biochim Biophys Acta. 2013;1833(1):176–183. doi: 10.1016/j.bbamcr.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 67.Pidoux G, Witczak O, Jarnaess E, et al. Optic atrophy 1 is an A-kinase anchoring protein on lipid droplets that mediates adrenergic control of lipolysis. EMBO J. 2011;30(21):4371–4386. doi: 10.1038/emboj.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen L, Gong Q, Stice JP, Knowlton AA. Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc Res. 2009;84(1):91–99. doi: 10.1093/cvr/cvp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zorzano A, Liesa M, Sebastian D, Segales J, Palacin M. Mitochondrial fusion proteins: dual regulators of morphology and metabolism. Semin Cell Dev Biol. 2010;21(6):566–574. doi: 10.1016/j.semcdb.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 70.Zuchner S, Mersiyanova IV, Muglia M, et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nature genetics. 2004;36(5):449–451. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]
- 71.Chen H, Vermulst M, Wang YE, et al. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010;141(2):280–289. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456(7222):605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 73.Germain M, Mathai JP, Mcbride HM, Shore GC. Endoplasmic reticulum BIK initiates DRP1-regulated remodelling of mitochondrial cristae during apoptosis. EMBO J. 2005;24(8):1546–1556. doi: 10.1038/sj.emboj.7600592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Merkwirth C, Langer T. Mitofusin 2 builds a bridge between ER and mitochondria. Cell. 2008;135(7):1165–1167. doi: 10.1016/j.cell.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 75.Rizzuto R, Marchi S, Bonora M, et al. Ca(2+) transfer from the ER to mitochondria: when, how and why. Biochim Biophys Acta. 2009;1787(11):1342–1351. doi: 10.1016/j.bbabio.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kopach O, Kruglikov I, Pivneva T, Voitenko N, Fedirko N. Functional coupling between ryanodine receptors, mitochondria and Ca(2+) ATPases in rat submandibular acinar cells. Cell Calcium. 2008;43(5):469–481. doi: 10.1016/j.ceca.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 77.Rowland AA, Voeltz GK. Endoplasmic reticulum-mitochondria contacts: function of the junction. Nat Rev Mol Cell Biol. 2012;13(10):607–625. doi: 10.1038/nrm3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Singaravelu K, Nelson C, Bakowski D, et al. Mitofusin 2 regulates STIM1 migration from the Ca2+ store to the plasma membrane in cells with depolarized mitochondria. J Biol Chem. 2011;286(14):12189–12201. doi: 10.1074/jbc.M110.174029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ruiz-Meana M, Fernandez-Sanz C, Garcia-Dorado D. The SR-mitochondria interaction: a new player in cardiac pathophysiology. Cardiovasc Res. 2010;88(1):30–39. doi: 10.1093/cvr/cvq225. [DOI] [PubMed] [Google Scholar]
- 80.Celsi F, Pizzo P, Brini M, et al. Mitochondria, calcium and cell death: a deadly triad in neurodegeneration. Biochim Biophys Acta. 2009;1787(5):335–344. doi: 10.1016/j.bbabio.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Delmotte P, Yang B, Thompson MA, Pabelick CM, Prakash YS, Sieck GC. Inflammation alters regional mitochondrial Ca(2)+ in human airway smooth muscle cells. Am J Physiol Cell Physiol. 2012;303(3):C244–256. doi: 10.1152/ajpcell.00414.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Girodet PO, Ozier A, Bara I, Tunon De Lara JM, Marthan R, Berger P. Airway remodeling in asthma: new mechanisms and potential for pharmacological intervention. Pharmacology & therapeutics. 2011;130(3):325–337. doi: 10.1016/j.pharmthera.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 83.Santo-Domingo J, Demaurex N. Calcium uptake mechanisms of mitochondria. Biochim Biophys Acta. 2010;1797(6–7):907–912. doi: 10.1016/j.bbabio.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 84.Saotome M, Safiulina D, Szabadkai G, et al. Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc Natl Acad Sci U S A. 2008;105(52):20728–20733. doi: 10.1073/pnas.0808953105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Valsecchi F, Esseling JJ, Koopman WJ, Willems PH. Calcium and ATP handling in human NADH:ubiquinone oxidoreductase deficiency. Biochim Biophys Acta. 2009;1792(12):1130–1137. doi: 10.1016/j.bbadis.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 86.Delmotte P, Sanderson MJ. Effects of formoterol on contraction and Ca2+ signaling of mouse airway smooth muscle cells. Am J Respir Cell Mol Biol. 2010;42(3):373–381. doi: 10.1165/rcmb.2008-0403OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jia L, Delmotte P, Aravamudan B, Pabelick CM, Prakash YS, Sieck GC. The Effect of the Inflammatory Cytokines TNFalpha and IL-13 on STIM1 Aggregation in Human Airway Smooth Muscle [Ca] Regulation. Am J Respir Cell Mol Biol. 2013 doi: 10.1165/rcmb.2013-0040OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sathish V, Leblebici F, Kip SN, et al. Regulation of sarcoplasmic reticulum Ca2+ reuptake in porcine airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2008;294(4):L787–796. doi: 10.1152/ajplung.00461.2007. [DOI] [PubMed] [Google Scholar]
- 89.Sathish V, Thompson MA, Bailey JP, Pabelick CM, Prakash YS, Sieck GC. Effect of proinflammatory cytokines on regulation of sarcoplasmic reticulum Ca2+ reuptake in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2009;297(1):L26–34. doi: 10.1152/ajplung.00026.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang Y, Deng X, Mancarella S, et al. The calcium store sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science. 2010;330(6000):105–109. doi: 10.1126/science.1191086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Y, Deng X, Zhou Y, et al. STIM protein coupling in the activation of Orai channels. Proc Natl Acad Sci U S A. 2009;106(18):7391–7396. doi: 10.1073/pnas.0900293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saxton WM, Hollenbeck PJ. The axonal transport of mitochondria. J Cell Sci. 2012;125(Pt 9):2095–2104. doi: 10.1242/jcs.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schwarz TL. Mitochondrial trafficking in neurons. Cold Spring Harb Perspect Biol. 2013;5(6) doi: 10.1101/cshperspect.a011304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Howard J, Hyman AA. Dynamics and mechanics of the microtubule plus end. Nature. 2003;422(6933):753–758. doi: 10.1038/nature01600. [DOI] [PubMed] [Google Scholar]
- 95.Kardon JR, Vale RD. Regulators of the cytoplasmic dynein motor. Nat Rev Mol Cell Biol. 2009;10(12):854–865. doi: 10.1038/nrm2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Misawa T, Takahama M, Kozaki T, et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nature immunology. 2013;14(5):454–460. doi: 10.1038/ni.2550. [DOI] [PubMed] [Google Scholar]
- 97.Roberts RA, Laskin DL, Smith CV, et al. Nitrative and oxidative stress in toxicology and disease. Toxicol Sci. 2009;112(1):4–16. doi: 10.1093/toxsci/kfp179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. The World Allergy Organization journal. 2012;5(1):9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med. 2011;208(3):417–420. doi: 10.1084/jem.20110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reddy PH. Mitochondrial Dysfunction and Oxidative Stress in Asthma: Implications for Mitochondria-Targeted Antioxidant Therapeutics. Pharmaceuticals (Basel) 2011;4(3):429–456. doi: 10.3390/ph4030429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nat Rev Immunol. 2013;13(5):349–361. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aravamudan B, Thompson M, Pabelick C, Prakash YS. Brain-derived neurotrophic factor induces proliferation of human airway smooth muscle cells. J Cell Mol Med. 2012;16(4):812–823. doi: 10.1111/j.1582-4934.2011.01356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bulua AC, Simon A, Maddipati R, et al. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS) J Exp Med. 2011;208(3):519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dada LA, Sznajder JI. Mitochondrial Ca(2)+ and ROS take center stage to orchestrate TNF-alpha-mediated inflammatory responses. J Clin Invest. 2011;121(5):1683–1685. doi: 10.1172/JCI57748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ferecatu I, Borot MC, Bossard C, et al. Polycyclic aromatic hydrocarbon components contribute to the mitochondria-antiapoptotic effect of fine particulate matter on human bronchial epithelial cells via the aryl hydrocarbon receptor. Particle and fibre toxicology. 2010;7:18. doi: 10.1186/1743-8977-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hanania NA. Targeting airway inflammation in asthma: current and future therapies. Chest. 2008;133(4):989–998. doi: 10.1378/chest.07-0829. [DOI] [PubMed] [Google Scholar]
- 107.Kamp DW. Asbestos-induced lung diseases: an update. Translational research : the journal of laboratory and clinical medicine. 2009;153(4):143–152. doi: 10.1016/j.trsl.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kolliputi N, Waxman AB. IL-6 cytoprotection in hyperoxic acute lung injury occurs via suppressor of cytokine signaling-1-induced apoptosis signal-regulating kinase-1 degradation. Am J Respir Cell Mol Biol. 2009;40(3):314–324. doi: 10.1165/rcmb.2007-0287OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lin HY, Lai RH, Lin ST, et al. Suppressor of cytokine signaling 6 (SOCS6) promotes mitochondrial fission via regulating DRP1 translocation. Cell Death Differ. 2013;20(1):139–153. doi: 10.1038/cdd.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pelaia G, Renda T, Gallelli L, et al. Molecular mechanisms underlying airway smooth muscle contraction and proliferation: implications for asthma. Respir Med. 2008;102(8):1173–1181. doi: 10.1016/j.rmed.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 111.Waxman AB, Kolliputi N. IL-6 protects against hyperoxia-induced mitochondrial damage via Bcl-2-induced Bak interactions with mitofusins. Am J Respir Cell Mol Biol. 2009;41(4):385–396. doi: 10.1165/rcmb.2008-0302OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu S, Zhou F, Zhang Z, Xing D. Mitochondrial oxidative stress causes mitochondrial fragmentation via differential modulation of mitochondrial fission-fusion proteins. FEBS J. 2011;278(6):941–954. doi: 10.1111/j.1742-4658.2011.08010.x. [DOI] [PubMed] [Google Scholar]
- 113.Hartman WR, Smelter DF, Sathish V, et al. Oxygen dose responsiveness of human fetal airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2012;303(8):L711–719. doi: 10.1152/ajplung.00037.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schumacker PT. Lung cell hypoxia: role of mitochondrial reactive oxygen species signaling in triggering responses. Proc Am Thorac Soc. 2011;8(6):477–484. doi: 10.1513/pats.201103-032MW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol. 2008;294(2):H570–578. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- 116.Lambert CM, Roy M, Robitaille GA, Richard DE, Bonnet S. HIF-1 inhibition decreases systemic vascular remodelling diseases by promoting apoptosis through a hexokinase 2-dependent mechanism. Cardiovasc Res. 2010;88(1):196–204. doi: 10.1093/cvr/cvq152. [DOI] [PubMed] [Google Scholar]
- 117.Aichler M, Elsner M, Ludyga N, et al. Clinical response to chemotherapy in oesophageal adenocarcinoma patients is linked to defects in mitochondria. The Journal of pathology. 2013 doi: 10.1002/path.4199. [DOI] [PubMed] [Google Scholar]
- 118.Xue X, You S, Zhang Q, et al. Mitaplatin increases sensitivity of tumor cells to cisplatin by inducing mitochondrial dysfunction. Molecular pharmaceutics. 2012;9(3):634–644. doi: 10.1021/mp200571k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Arvizo RR, Moyano DF, Saha S, et al. Probing novel roles of the mitochondrial uniporter in ovarian cancer cells using nanoparticles. J Biol Chem. 2013;288(24):17610–17618. doi: 10.1074/jbc.M112.435206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bernardi P, Bonaldo P. Dysfunction of mitochondria and sarcoplasmic reticulum in the pathogenesis of collagen VI muscular dystrophies. Ann N Y Acad Sci. 2008;1147:303–311. doi: 10.1196/annals.1427.009. [DOI] [PubMed] [Google Scholar]
- 121.Cassidy-Stone A, Chipuk JE, Ingerman E, et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14(2):193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122•.Papanicolaou KN, Khairallah RJ, Ngoh GA, et al. Mitofusin-2 maintains mitochondrial structure and contributes to stress-induced permeability transition in cardiac myocytes. Mol Cell Biol. 2011;31(6):1309–1328. doi: 10.1128/MCB.00911-10. Proposes a novel idea that excessive fusion can actually damage mitochondrial membrane properties, and thus lead to leakage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Papanicolaou KN, Phillippo MM, Walsh K. Mitofusins and the mitochondrial permeability transition: the potential downside of mitochondrial fusion. Am J Physiol Heart Circ Physiol. 2012;303(3):H243–255. doi: 10.1152/ajpheart.00185.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhao T, Huang X, Han L, et al. Central role of mitofusin 2 in autophagosome-lysosome fusion in cardiomyocytes. J Biol Chem. 2012;287(28):23615–23625. doi: 10.1074/jbc.M112.379164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Aravamudan B, Kiel A, Thompson MA, Vassallo R, Sieck GC, Pabelick CM, Prakash YS. Mitochondrial Fragmentation and Dysfunction in Smoking Effects on Asthmatic Airways. 2013 Submitted. [Google Scholar]
- 126.Kuwano K. Epithelial cell apoptosis and lung remodeling. Cellular & molecular immunology. 2007;4(6):419–429. [PubMed] [Google Scholar]
- 127.Estaquier J, Arnoult D. Inhibiting Drp1-mediated mitochondrial fission selectively prevents the release of cytochrome c during apoptosis. Cell Death Differ. 2007;14(6):1086–1094. doi: 10.1038/sj.cdd.4402107. [DOI] [PubMed] [Google Scholar]
- 128.Ishihara N, Nomura M, Jofuku A, et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009;11(8):958–966. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- 129.Brooks C, Cho SG, Wang CY, Yang T, Dong Z. Fragmented mitochondria are sensitized to Bax insertion and activation during apoptosis. Am J Physiol Cell Physiol. 2011;300(3):C447–455. doi: 10.1152/ajpcell.00402.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cleland MM, Norris KL, Karbowski M, et al. Bcl-2 family interaction with the mitochondrial morphogenesis machinery. Cell Death Differ. 2011;18(2):235–247. doi: 10.1038/cdd.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sheridan C, Delivani P, Cullen SP, Martin SJ. Bax- or Bak-induced mitochondrial fission can be uncoupled from cytochrome C release. Mol Cell. 2008;31(4):570–585. doi: 10.1016/j.molcel.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 132.Marsboom G, Toth PT, Ryan JJ, et al. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ Res. 2012;110(11):1484–1497. doi: 10.1161/CIRCRESAHA.111.263848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133•.Rehman J, Zhang HJ, Toth PT, et al. Inhibition of mitochondrial fission prevents cell cycle progression in lung cancer. FASEB J. 2012;26(5):2175–2186. doi: 10.1096/fj.11-196543. References 132 and 133 argue not only in favor of Drp1 being a pro-proliferative factor but the argument is also made in a clinical context. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liesa M, Palacin M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009;89(3):799–845. doi: 10.1152/physrev.00030.2008. [DOI] [PubMed] [Google Scholar]
- 135.Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11(12):872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 136.Guo X, Chen KH, Guo Y, Liao H, Tang J, Xiao RP. Mitofusin 2 triggers vascular smooth muscle cell apoptosis via mitochondrial death pathway. Circ Res. 2007;101(11):1113–1122. doi: 10.1161/CIRCRESAHA.107.157644. [DOI] [PubMed] [Google Scholar]
- 137.Rehman J, Archer SL. A proposed mitochondrial-metabolic mechanism for initiation and maintenance of pulmonary arterial hypertension in fawn-hooded rats: the Warburg model of pulmonary arterial hypertension. Advances in experimental medicine and biology. 2010;661:171–185. doi: 10.1007/978-1-60761-500-2_11. [DOI] [PubMed] [Google Scholar]
- 138.Chen KH, Guo X, Ma D, et al. Dysregulation of HSG triggers vascular proliferative disorders. Nat Cell Biol. 2004;6(9):872–883. doi: 10.1038/ncb1161. [DOI] [PubMed] [Google Scholar]
- 139.Pawlikowska P, Gajkowska B, Orzechowski A. Mitofusin 2 (Mfn2): a key player in insulin-dependent myogenesis in vitro. Cell Tissue Res. 2007;327(3):571–581. doi: 10.1007/s00441-006-0320-3. [DOI] [PubMed] [Google Scholar]
- 140.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Grumati P, Coletto L, Sabatelli P, et al. Autophagy is defective in collagen VI muscular dystrophies, and its reactivation rescues myofiber degeneration. Nat Med. 2010;16(11):1313–1320. doi: 10.1038/nm.2247. [DOI] [PubMed] [Google Scholar]
- 143.Tolkovsky AM. Autophagy thwarts muscle disease. Nat Med. 2010;16(11):1188–1190. doi: 10.1038/nm1110-1188. [DOI] [PubMed] [Google Scholar]
- 144.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12(1):9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen ZH, Kim HP, Sciurba FC, et al. Egr-1 regulates autophagy in cigarette smoke-induced chronic obstructive pulmonary disease. PLoS One. 2008;3(10):e3316. doi: 10.1371/journal.pone.0003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen ZH, Lam HC, Jin Y, et al. Autophagy protein microtubule-associated protein 1 light chain-3B (LC3B) activates extrinsic apoptosis during cigarette smoke-induced emphysema. Proc Natl Acad Sci U S A. 2010;107(44):18880–18885. doi: 10.1073/pnas.1005574107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. The Journal of pathology. 2010;221(1):3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kongara S, Karantza V. The interplay between autophagy and ROS in tumorigenesis. Frontiers in oncology. 2012;2:171. doi: 10.3389/fonc.2012.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee SJ, Smith A, Guo L, et al. Autophagic protein LC3B confers resistance against hypoxia-induced pulmonary hypertension. Am J Respir Crit Care Med. 2011;183(5):649–658. doi: 10.1164/rccm.201005-0746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ryter SW, Nakahira K, Haspel JA, Choi AM. Autophagy in pulmonary diseases. Annu Rev Physiol. 2012;74:377–401. doi: 10.1146/annurev-physiol-020911-153348. [DOI] [PubMed] [Google Scholar]
- 151.Thomas KJ, Jacobson MR. Defects in mitochondrial fission protein dynamin-related protein 1 are linked to apoptotic resistance and autophagy in a lung cancer model. PLoS One. 2012;7(9):e45319. doi: 10.1371/journal.pone.0045319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Egan DF, Shackelford DB, Mihaylova MM, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331(6016):456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153•.Zifa E, Daniil Z, Skoumi E, et al. Mitochondrial genetic background plays a role in increasing risk to asthma. Molecular biology reports. 2012;39(4):4697–4708. doi: 10.1007/s11033-011-1262-8. Makes a case for asthma for being a genetic disease by bringing out the significance of inherited mutations in mitochondrial DNA. [DOI] [PubMed] [Google Scholar]
- 154.Chen XH, Zhao YP, Xue M, et al. TNF-alpha induces mitochondrial dysfunction in 3T3-L1 adipocytes. Mol Cell Endocrinol. 2010;328(1–2):63–69. doi: 10.1016/j.mce.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 155•.Irrinki KM, Mallilankaraman K, Thapa RJ, et al. Requirement of FADD, NEMO, and BAX/BAK for Aberrant Mitochondrial Function in Tumor Necrosis Factor Alpha-Induced Necrosis. Mol Cell Biol. 2011;31(18):3745–3758. doi: 10.1128/MCB.05303-11. Provides evidence for mitochondrial function being regulated by ubiquitination and pro-apoptotic signals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rowlands DJ, Islam MN, Das SR, et al. Activation of TNFR1 ectodomain shedding by mitochondrial Ca2+ determines the severity of inflammation in mouse lung microvessels. J Clin Invest. 2011;121(5):1986–1999. doi: 10.1172/JCI43839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157•.Simoes DC, Psarra AM, Mauad T, et al. Glucocorticoid and estrogen receptors are reduced in mitochondria of lung epithelial cells in asthma. PLoS One. 2012;7(6):e39183. doi: 10.1371/journal.pone.0039183. References 156 and 157 highlight the relevance of mitochondrial counterparts of plasma membrane receptors (TNFR1, ERs and GRa) on the pathogenesis of chronic airway inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mabalirajan U, Rehman R, Ahmad T, et al. Linoleic acid metabolite drives severe asthma by causing airway epithelial injury. Scientific reports. 2013;3:1349. doi: 10.1038/srep01349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mabalirajan U, Rehman R, Ahmad T, et al. 12/15-lipoxygenase expressed in non-epithelial cells causes airway epithelial injury in asthma. Scientific reports. 2013;3:1540. doi: 10.1038/srep01540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Calkins MJ, Manczak M, Mao P, Shirendeb U, Reddy PH. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer’s disease. Hum Mol Genet. 2011;20(23):4515–4529. doi: 10.1093/hmg/ddr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Comhair SA, Ricci KS, Arroliga M, et al. Correlation of systemic superoxide dismutase deficiency to airflow obstruction in asthma. Am J Respir Crit Care Med. 2005;172(3):306–313. doi: 10.1164/rccm.200502-180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162•.Michaeloudes C, Sukkar MB, Khorasani NM, Bhavsar PK, Chung KF. TGF-beta regulates Nox4, MnSOD and catalase expression, and IL-6 release in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2011;300(2):L295–304. doi: 10.1152/ajplung.00134.2010. Portrays growth factor/cytokine effects as deficiencies of mitochondrial ROS scavenging and thus sugegsts this mechanism as a target for tretaing airway inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sahawneh MA, Ricart KC, Roberts BR, et al. Cu,Zn-superoxide dismutase increases toxicity of mutant and zinc-deficient superoxide dismutase by enhancing protein stability. J Biol Chem. 2010;285(44):33885–33897. doi: 10.1074/jbc.M110.118901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Van Der Toorn M, Rezayat D, Kauffman HF, et al. Lipid-soluble components in cigarette smoke induce mitochondrial production of reactive oxygen species in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;297(1):L109–114. doi: 10.1152/ajplung.90461.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Aguilera-Aguirre L, Bacsi A, Saavedra-Molina A, Kurosky A, Sur S, Boldogh I. Mitochondrial dysfunction increases allergic airway inflammation. J Immunol. 2009;183(8):5379–5387. doi: 10.4049/jimmunol.0900228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Trian T, Benard G, Begueret H, et al. Bronchial smooth muscle remodeling involves calcium-dependent enhanced mitochondrial biogenesis in asthma. J Exp Med. 2007;204(13):3173–3181. doi: 10.1084/jem.20070956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Antigny F, Girardin N, Raveau D, Frieden M, Becq F, Vandebrouck C. Dysfunction of mitochondria Ca2+ uptake in cystic fibrosis airway epithelial cells. Mitochondrion. 2009;9(4):232–241. doi: 10.1016/j.mito.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 168.Evans AM, Hardie DG, Peers C, Mahmoud A. Hypoxic pulmonary vasoconstriction: mechanisms of oxygen-sensing. Current opinion in anaesthesiology. 2011;24(1):13–20. doi: 10.1097/ACO.0b013e3283421201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kamdar O, Le W, Zhang J, Ghio AJ, Rosen GD, Upadhyay D. Air pollution induces enhanced mitochondrial oxidative stress in cystic fibrosis airway epithelium. FEBS Lett. 2008;582(25–26):3601–3606. doi: 10.1016/j.febslet.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bartal M. COPD and tobacco smoke. Monaldi Arch Chest Dis. 2005;63(4):213–225. doi: 10.4081/monaldi.2005.623. [DOI] [PubMed] [Google Scholar]
- 171.Chiba Y, Murata M, Ushikubo H, et al. Effect of cigarette smoke exposure in vivo on bronchial smooth muscle contractility in vitro in rats. Am J Respir Cell Mol Biol. 2005;33(6):574–581. doi: 10.1165/rcmb.2005-0177OC. [DOI] [PubMed] [Google Scholar]
- 172.Ejiofor S, Turner AM. Pharmacotherapies for COPD. Clinical medicine insights. Circulatory, respiratory and pulmonary medicine. 2013;7:17–34. doi: 10.4137/CCRPM.S7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Feinson JA, Chidekel AS. Adult smoking and environmental tobacco smoke: a persistent health threat to children. Del Med J. 2006;78(6):213–218. [PubMed] [Google Scholar]
- 174.Mathis C, Poussin C, Weisensee D, et al. Human bronchial epithelial cells exposed in vitro to cigarette smoke at the air-liquid interface resemble bronchial epithelium from human smokers. Am J Physiol Lung Cell Mol Physiol. 2013;304(7):L489–503. doi: 10.1152/ajplung.00181.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Meyer A, Zoll J, Charles AL, et al. Skeletal muscle mitochondrial dysfunction during chronic obstructive pulmonary disease: central actor and therapeutic target. Experimental physiology. 2013;98(6):1063–1078. doi: 10.1113/expphysiol.2012.069468. [DOI] [PubMed] [Google Scholar]
- 176.Soulitzis N, Neofytou E, Psarrou M, et al. Downregulation of lung mitochondrial prohibitin in COPD. Respir Med. 2012;106(7):954–961. doi: 10.1016/j.rmed.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 177.Van Rijt SH, Keller IE, John G, et al. Acute cigarette smoke exposure impairs proteasome function in the lung. Am J Physiol Lung Cell Mol Physiol. 2012;303(9):L814–823. doi: 10.1152/ajplung.00128.2012. [DOI] [PubMed] [Google Scholar]
- 178.Kirkham PA, Barnes PJ. Oxidative Stress in COPD. Chest. 2013;144(1):266–273. doi: 10.1378/chest.12-2664. [DOI] [PubMed] [Google Scholar]
- 179.Rico De Souza A, Zago M, Pollock SJ, Sime PJ, Phipps RP, Baglole CJ. Genetic ablation of the aryl hydrocarbon receptor causes cigarette smoke-induced mitochondrial dysfunction and apoptosis. J Biol Chem. 2011;286(50):43214–43228. doi: 10.1074/jbc.M111.258764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Puente-Maestu L, Perez-Parra J, Godoy R, et al. Abnormal mitochondrial function in locomotor and respiratory muscles of COPD patients. Eur Respir J. 2009;33(5):1045–1052. doi: 10.1183/09031936.00112408. [DOI] [PubMed] [Google Scholar]
- 181.Puente-Maestu L, Perez-Parra J, Godoy R, et al. Abnormal transition pore kinetics and cytochrome C release in muscle mitochondria of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2009;40(6):746–750. doi: 10.1165/rcmb.2008-0289OC. [DOI] [PubMed] [Google Scholar]
- 182.Puente-Maestu L, Tejedor A, Lazaro A, et al. Site of mitochondrial reactive oxygen species production in skeletal muscle of chronic obstructive pulmonary disease and its relationship with exercise oxidative stress. Am J Respir Cell Mol Biol. 2012;47(3):358–362. doi: 10.1165/rcmb.2011-0382OC. [DOI] [PubMed] [Google Scholar]
- 183.Cohen RT, Strunk RC, Field JJ, et al. Environmental tobacco smoke and airway obstruction in children with sickle cell anemia. Chest. 2013 doi: 10.1378/chest.12-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mazzei F, Guarrera S, Allione A, et al. 8-Oxoguanine DNA-glycosylase repair activity and expression: a comparison between cryopreserved isolated lymphocytes and EBV-derived lymphoblastoid cell lines. Mutation research. 2011;718(1–2):62–67. doi: 10.1016/j.mrgentox.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 185.Paz-Elizur T, Sevilya Z, Leitner-Dagan Y, Elinger D, Roisman LC, Livneh Z. DNA repair of oxidative DNA damage in human carcinogenesis: potential application for cancer risk assessment and prevention. Cancer letters. 2008;266(1):60–72. doi: 10.1016/j.canlet.2008.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kamp DW, Shacter E, Weitzman SA. Chronic inflammation and cancer: the role of the mitochondria. Oncology (Williston Park) 2011;25(5):400–410. 413. [PubMed] [Google Scholar]
- 187.Giembycz MA. Can the anti-inflammatory potential of PDE4 inhibitors be realized: guarded optimism or wishful thinking? Br J Pharmacol. 2008;155(3):288–290. doi: 10.1038/bjp.2008.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Giembycz MA, Kaur M, Leigh R, Newton R. A Holy Grail of asthma management: toward understanding how long-acting beta(2)-adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids. Br J Pharmacol. 2008;153(6):1090–1104. doi: 10.1038/sj.bjp.0707627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Montuschi P. Leukotrienes, antileukotrienes and asthma. Mini reviews in medicinal chemistry. 2008;8(7):647–656. doi: 10.2174/138955708784567395. [DOI] [PubMed] [Google Scholar]
- 190.Prenner BM. Asthma 2008: targeting immunoglobulin E to achieve disease control. The Journal of asthma : official journal of the Association for the Care of Asthma. 2008;45(6):429–436. doi: 10.1080/02770900802085485. [DOI] [PubMed] [Google Scholar]
- 191.Mabalirajan U, Ahmad T, Leishangthem GD, Dinda AK, Agrawal A, Ghosh B. L-arginine reduces mitochondrial dysfunction and airway injury in murine allergic airway inflammation. International immunopharmacology. 2010;10(12):1514–1519. doi: 10.1016/j.intimp.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 192.Robin E, Derichard A, Vallet B, Hassoun SM, Neviere R. Nitric oxide scavenging modulates mitochondrial dysfunction induced by hypoxia/reoxygenation. Pharmacol Rep. 2011;63(5):1189–1194. doi: 10.1016/s1734-1140(11)70638-7. [DOI] [PubMed] [Google Scholar]
- 193.Leo S, Szabadkai G, Rizzuto R. The mitochondrial antioxidants MitoE(2) and MitoQ(10) increase mitochondrial Ca(2+) load upon cell stimulation by inhibiting Ca(2+) efflux from the organelle. Ann N Y Acad Sci. 2008;1147:264–274. doi: 10.1196/annals.1427.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lee IK, Kang KA, Zhang R, Kim BJ, Kang SS, Hyun JW. Mitochondria protection of baicalein against oxidative damage via induction of manganese superoxide dismutase. Environmental toxicology and pharmacology. 2011;31(1):233–241. doi: 10.1016/j.etap.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 195.Naveenkumar C, Raghunandhakumar S, Asokkumar S, Devaki T. Baicalein abrogates reactive oxygen species (ROS)-mediated mitochondrial dysfunction during experimental pulmonary carcinogenesis in vivo. Basic & clinical pharmacology & toxicology. 2013;112(4):270–281. doi: 10.1111/bcpt.12025. [DOI] [PubMed] [Google Scholar]
- 196.Mabalirajan U, Dinda AK, Sharma SK, Ghosh B. Esculetin restores mitochondrial dysfunction and reduces allergic asthma features in experimental murine model. J Immunol. 2009;183(3):2059–2067. doi: 10.4049/jimmunol.0900342. [DOI] [PubMed] [Google Scholar]
- 197.Gille L, Staniek K, Rosenau T, Duvigneau JC, Kozlov AV. Tocopheryl quinones and mitochondria. Molecular nutrition & food research. 2010;54(5):601–615. doi: 10.1002/mnfr.200900386. [DOI] [PubMed] [Google Scholar]
- 198.Majima HJ, Indo HP, Suenaga S, Matsui H, Yen HC, Ozawa T. Mitochondria as possible pharmaceutical targets for the effects of vitamin E and its homologues in oxidative stress-related diseases. Current pharmaceutical design. 2011;17(21):2190–2195. doi: 10.2174/138161211796957490. [DOI] [PubMed] [Google Scholar]
- 199.Du J, Wang Y, Hunter R, et al. Dynamic regulation of mitochondrial function by glucocorticoids. Proc Natl Acad Sci U S A. 2009;106(9):3543–3548. doi: 10.1073/pnas.0812671106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200•.Kumar S, Mabalirajan U, Rehman R, et al. A novel cinnamate derivative attenuates asthma features and reduces bronchial epithelial injury in mouse model. International immunopharmacology. 2013;15(1):150–159. doi: 10.1016/j.intimp.2012.10.024. Offers an alternative therapeutic strategy (herbal compound-based) targeting the mitochondria to treat asthma. [DOI] [PubMed] [Google Scholar]
- 201.Ye R, Zhang X, Kong X, et al. Ginsenoside Rd attenuates mitochondrial dysfunction and sequential apoptosis after transient focal ischemia. Neuroscience. 2011;178:169–180. doi: 10.1016/j.neuroscience.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 202.Rebbeck CA, Leroi AM, Burt A. Mitochondrial capture by a transmissible cancer. Science. 2011;331(6015):303. doi: 10.1126/science.1197696. [DOI] [PubMed] [Google Scholar]
- 203.Islam MN, Das SR, Emin MT, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204••.Kaipparettu BA, Ma Y, Park JH, et al. Crosstalk from non-cancerous mitochondria can inhibit tumor properties of metastatic cells by suppressing oncogenic pathways. PLoS One. 2013;8(5):e61747. doi: 10.1371/journal.pone.0061747. References 203 and 204 both describe elegantly the relatively new (and potentially very effective) technique of mitochondrial transfer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chan YC, Banerjee J, Choi SY, Sen CK. miR-210: the master hypoxamir. Microcirculation. 2012;19(3):215–223. doi: 10.1111/j.1549-8719.2011.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Puissegur MP, Mazure NM, Bertero T, et al. miR-210 is overexpressed in late stages of lung cancer and mediates mitochondrial alterations associated with modulation of HIF-1 activity. Cell Death Differ. 2011;18(3):465–478. doi: 10.1038/cdd.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wang K, Long B, Jiao JQ, et al. miR-484 regulates mitochondrial network through targeting Fis1. Nature communications. 2012;3:781. doi: 10.1038/ncomms1770. [DOI] [PubMed] [Google Scholar]