Abstract
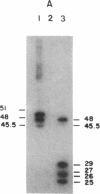
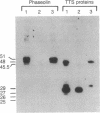
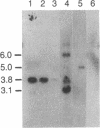
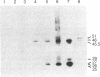
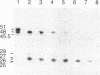
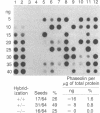
Recombinant phage λ177.4 contains a gene for β phaseolin, a major storage glycoprotein of French bean seed. A 3.8-kilobase Bgl II-BamHI fragment containing the entire 1700-base-pair coding region, together with 863 base pairs of 5′ and 1226 base pairs of 3′ flanking sequence, was inserted into the A66 Ti plasmid of Agrobacterium tumefaciens and used to transform tobacco. The level of phaseolin in the seeds of plants regenerated from cloned tissue was 1000-fold higher than in other tissues. The molecular weight of the phaseolin RNA transcript in tobacco seeds was identical to that found in bean seeds. The phaseolin protein in tobacco seed was glycosylated and appeared to undergo removal of the signal peptide. However, a large proportion of the phaseolin was cleaved into discrete peptides. These same peptides were formed as phaseolin was degraded during tobacco seed germination. The phaseolin gene appeared to be inserted as a single copy, and the proportion of phaseolin per genome copy in tobacco seeds (up to 3% of the total embryo proteins) resembled that in the bean seeds (40% of total seed protein, expressed from about 14 copies per diploid genome). Furthermore, the transplanted gene was turned on during tobacco seed development, and its protein product, phaseolin, was localized in the embryonic tissues. Finally, the phaseolin gene was inherited as a Mendelian dominant trait in tobacco.
Keywords: foreign gene expression, Mendelian segregation, electrophoretic immunoblot analysis
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton K. A., Binns A. N., Matzke A. J., Chilton M. D. Regeneration of intact tobacco plants containing full length copies of genetically engineered T-DNA, and transmission of T-DNA to R1 progeny. Cell. 1983 Apr;32(4):1033–1043. doi: 10.1016/0092-8674(83)90288-x. [DOI] [PubMed] [Google Scholar]
- Binns A. N., Sciaky D., Wood H. N. Variation in hormone autonomy and regenerative potential of cells transformed by strain A66 of Agrobacterium tumefaciens. Cell. 1982 Dec;31(3 Pt 2):605–612. doi: 10.1016/0092-8674(82)90316-6. [DOI] [PubMed] [Google Scholar]
- Braun A. C., Wood H. N. Suppression of the neoplastic state with the acquisition of specialized functions in cells, tissues, and organs of crown gall teratomas of tobacco. Proc Natl Acad Sci U S A. 1976 Feb;73(2):496–500. doi: 10.1073/pnas.73.2.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan A., Herrera-Estrella L., Inzé D., Van Haute E., Van Montagu M., Schell J., Zambryski P. Introduction of genetic material into plant cells. Science. 1983 Nov 18;222(4625):815–821. doi: 10.1126/science.222.4625.815. [DOI] [PubMed] [Google Scholar]
- De Block M., Herrera-Estrella L., Van Montagu M., Schell J., Zambryski P. Expression of foreign genes in regenerated plants and in their progeny. EMBO J. 1984 Aug;3(8):1681–1689. doi: 10.1002/j.1460-2075.1984.tb02032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraley R. T., Rogers S. G., Horsch R. B., Sanders P. R., Flick J. S., Adams S. P., Bittner M. L., Brand L. A., Fink C. L., Fry J. S. Expression of bacterial genes in plant cells. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4803–4807. doi: 10.1073/pnas.80.15.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Estrella L., Block M. D., Messens E., Hernalsteens J. P., Montagu M. V., Schell J. Chimeric genes as dominant selectable markers in plant cells. EMBO J. 1983;2(6):987–995. doi: 10.1002/j.1460-2075.1983.tb01532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsch R. B., Fraley R. T., Rogers S. G., Sanders P. R., Lloyd A., Hoffmann N. Inheritance of functional foreign genes in plants. Science. 1984 Feb 3;223(4635):496–498. doi: 10.1126/science.223.4635.496. [DOI] [PubMed] [Google Scholar]
- Jahn R., Schiebler W., Greengard P. A quantitative dot-immunobinding assay for proteins using nitrocellulose membrane filters. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1684–1687. doi: 10.1073/pnas.81.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Murai N., Kemp J. D., Sutton D. W., Murray M. G., Slightom J. L., Merlo D. J., Reichert N. A., Sengupta-Gopalan C., Stock C. A., Barker R. F., Hall T. C. Phaseolin gene from bean is expressed after transfer to sunflower via tumor-inducing plasmid vectors. Science. 1983 Nov 4;222(4623):476–482. doi: 10.1126/science.222.4623.476. [DOI] [PubMed] [Google Scholar]
- Murray M. G., Thompson W. F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980 Oct 10;8(19):4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S. S., Liener I. E. Degradation of the Major Storage Protein of Phaseolus vulgaris during Germination : Role of Endogenous Proteases and Protease Inhibitors. Plant Physiol. 1984 Mar;74(3):494–498. doi: 10.1104/pp.74.3.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Scholnick S. B., Morgan B. A., Hirsh J. The cloned dopa decarboxylase gene is developmentally regulated when reintegrated into the Drosophila genome. Cell. 1983 Aug;34(1):37–45. doi: 10.1016/0092-8674(83)90134-4. [DOI] [PubMed] [Google Scholar]
- Slightom J. L., Sun S. M., Hall T. C. Complete nucleotide sequence of a French bean storage protein gene: Phaseolin. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1897–1901. doi: 10.1073/pnas.80.7.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. The effect of chromosomal position on the expression of the Drosophila xanthine dehydrogenase gene. Cell. 1983 Aug;34(1):47–57. doi: 10.1016/0092-8674(83)90135-6. [DOI] [PubMed] [Google Scholar]
- Sun S. M., Mutschler M. A., Bliss F. A., Hall T. C. Protein Synthesis and Accumulation in Bean Cotyledons during Growth. Plant Physiol. 1978 Jun;61(6):918–923. doi: 10.1104/pp.61.6.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]