Abstract
The mammalian immune system must specifically recognize and eliminate foreign invaders but refrain from damaging the host. This task is accomplished in part by the production of a large number of T lymphocytes, each bearing a different antigen receptor to match the enormous variety of antigens present in the microbial world. However, because antigen receptor diversity is generated by a random mechanism, the immune system must tolerate the function of T lymphocytes that by chance express a self-reactive antigen receptor. Therefore, during early development, T cells that are specific for antigens expressed in the thymus are physically deleted. The population of T cells that leaves the thymus and seeds the secondary lymphoid organs contains helpful cells that are specific for antigens from microbes but also potentially dangerous T cells that are specific for innocuous extrathymic self antigens. The outcome of an encounter by a peripheral T cell with these two types of antigens is to a great extent determined by the inability of naive T cells to enter nonlymphoid tissues or to be productively activated in the absence of inflammation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson G., Jenkinson E. J., Moore N. C., Owen J. J. MHC class II-positive epithelium and mesenchyme cells are both required for T-cell development in the thymus. Nature. 1993 Mar 4;362(6415):70–73. doi: 10.1038/362070a0. [DOI] [PubMed] [Google Scholar]
- Arbonés M. L., Ord D. C., Ley K., Ratech H., Maynard-Curry C., Otten G., Capon D. J., Tedder T. F. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994 Jul;1(4):247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Arnold B., Schönrich G., Hämmerling G. J. Multiple levels of peripheral tolerance. Immunol Today. 1993 Jan;14(1):12–14. doi: 10.1016/0167-5699(93)90317-E. [DOI] [PubMed] [Google Scholar]
- Ashton-Rickardt P. G., Van Kaer L., Schumacher T. N., Ploegh H. L., Tonegawa S. Peptide contributes to the specificity of positive selection of CD8+ T cells in the thymus. Cell. 1993 Jun 4;73(5):1041–1049. doi: 10.1016/0092-8674(93)90281-t. [DOI] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Bellgrau D., Gold D., Selawry H., Moore J., Franzusoff A., Duke R. C. A role for CD95 ligand in preventing graft rejection. Nature. 1995 Oct 19;377(6550):630–632. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
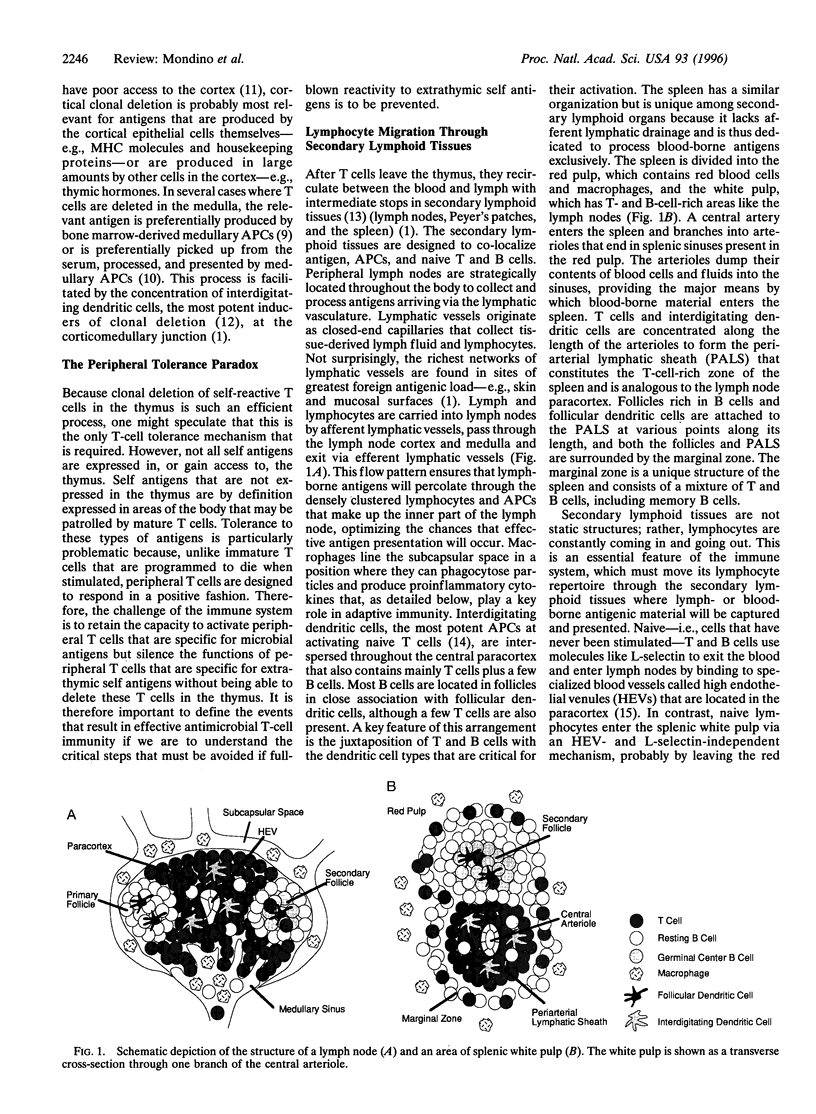
- Berek C., Berger A., Apel M. Maturation of the immune response in germinal centers. Cell. 1991 Dec 20;67(6):1121–1129. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- Bernard C. C., Carnegie P. R. Experimental autoimmune encephalomyelitis in mice: immunologic response to mouse spinal cord and myelin basic proteins. J Immunol. 1975 May;114(5):1537–1540. [PubMed] [Google Scholar]
- Bogen B., Dembic Z., Weiss S. Clonal deletion of specific thymocytes by an immunoglobulin idiotype. EMBO J. 1993 Jan;12(1):357–363. doi: 10.1002/j.1460-2075.1993.tb05664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce M. G., Ferguson A. The influence of intestinal processing on the immunogenicity and molecular size of absorbed, circulating ovalbumin in mice. Immunology. 1986 Oct;59(2):295–300. [PMC free article] [PubMed] [Google Scholar]
- Buhlmann J. E., Foy T. M., Aruffo A., Crassi K. M., Ledbetter J. A., Green W. R., Xu J. C., Shultz L. D., Roopesian D., Flavell R. A. In the absence of a CD40 signal, B cells are tolerogenic. Immunity. 1995 Jun;2(6):645–653. doi: 10.1016/1074-7613(95)90009-8. [DOI] [PubMed] [Google Scholar]
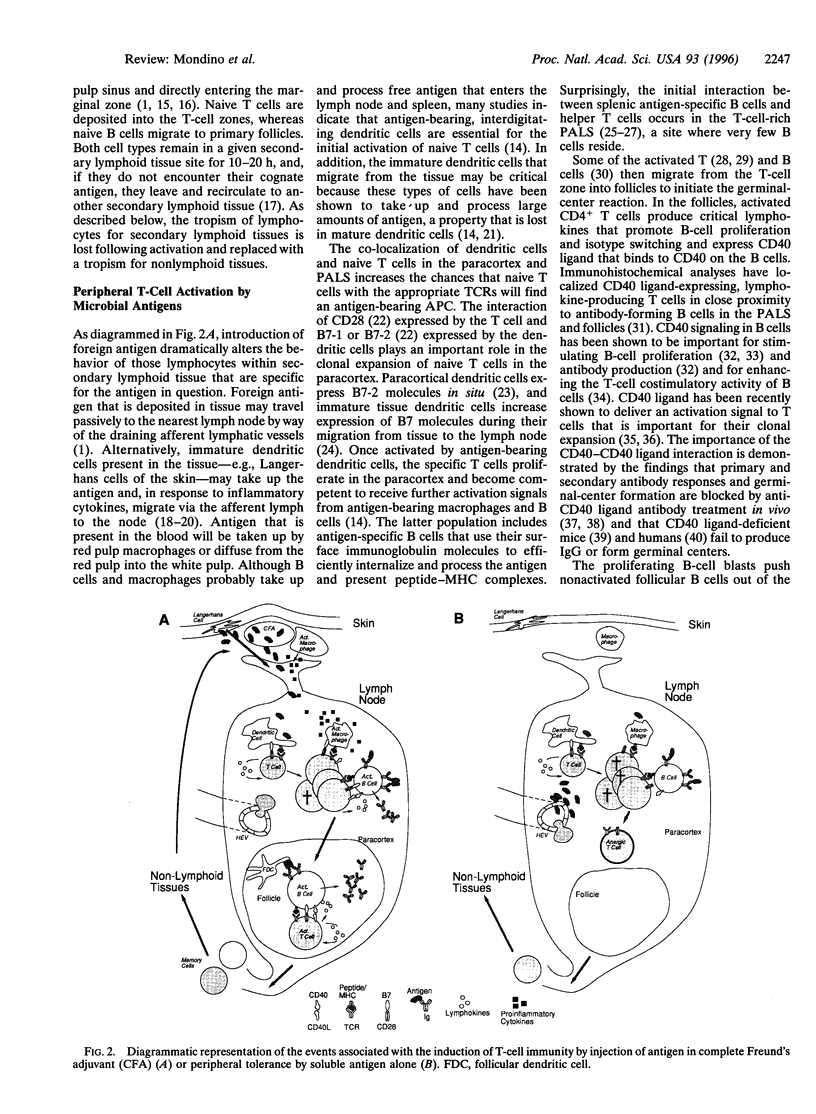
- Chen Y., Kuchroo V. K., Inobe J., Hafler D. A., Weiner H. L. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994 Aug 26;265(5176):1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- Chiller J. M., Habicht G. S., Weigle W. O. Kinetic differences in unresponsiveness of thymus and bone marrow cells. Science. 1971 Feb 26;171(3973):813–815. doi: 10.1126/science.171.3973.813. [DOI] [PubMed] [Google Scholar]
- Cooke M. P., Heath A. W., Shokat K. M., Zeng Y., Finkelman F. D., Linsley P. S., Howard M., Goodnow C. C. Immunoglobulin signal transduction guides the specificity of B cell-T cell interactions and is blocked in tolerant self-reactive B cells. J Exp Med. 1994 Feb 1;179(2):425–438. doi: 10.1084/jem.179.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell R., Walker W. A., Isselbacher K. J. Small intestinal absorption of horseradish peroxidase. A cytochemical study. Lab Invest. 1971 Jul;25(1):42–48. [PubMed] [Google Scholar]
- Cosgrove D., Gray D., Dierich A., Kaufman J., Lemeur M., Benoist C., Mathis D. Mice lacking MHC class II molecules. Cell. 1991 Sep 6;66(5):1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- Cumberbatch M., Kimber I. Tumour necrosis factor-alpha is required for accumulation of dendritic cells in draining lymph nodes and for optimal contact sensitization. Immunology. 1995 Jan;84(1):31–35. [PMC free article] [PubMed] [Google Scholar]
- Cyster J. G., Hartley S. B., Goodnow C. C. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 1994 Sep 29;371(6496):389–395. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- DRESSER D. W. Effectiveness of lipid and lipidophilic substances as adjuvants. Nature. 1961 Sep 16;191:1169–1171. doi: 10.1038/1911169a0. [DOI] [PubMed] [Google Scholar]
- DRESSER D. W. Specific inhibition of antibody production. II. Paralysis induced in adult mice by small quantities of protein antigen. Immunology. 1962 May;5:378–388. [PMC free article] [PubMed] [Google Scholar]
- Eynon E. E., Parker D. C. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J Exp Med. 1992 Jan 1;175(1):131–138. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy T. M., Laman J. D., Ledbetter J. A., Aruffo A., Claassen E., Noelle R. J. gp39-CD40 interactions are essential for germinal center formation and the development of B cell memory. J Exp Med. 1994 Jul 1;180(1):157–163. doi: 10.1084/jem.180.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy T. M., Shepherd D. M., Durie F. H., Aruffo A., Ledbetter J. A., Noelle R. J. In vivo CD40-gp39 interactions are essential for thymus-dependent humoral immunity. II. Prolonged suppression of the humoral immune response by an antibody to the ligand for CD40, gp39. J Exp Med. 1993 Nov 1;178(5):1567–1575. doi: 10.1084/jem.178.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. J., Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992 Nov 13;258(5085):1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- Fuller K. A., Kanagawa O., Nahm M. H. T cells within germinal centers are specific for the immunizing antigen. J Immunol. 1993 Nov 1;151(9):4505–4512. [PubMed] [Google Scholar]
- Girard J. P., Springer T. A. High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol Today. 1995 Sep;16(9):449–457. doi: 10.1016/0167-5699(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Goodnow C. C., Crosbie J., Jorgensen H., Brink R. A., Basten A. Induction of self-tolerance in mature peripheral B lymphocytes. Nature. 1989 Nov 23;342(6248):385–391. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- Gray D. Recruitment of virgin B cells into an immune response is restricted to activation outside lymphoid follicles. Immunology. 1988 Sep;65(1):73–79. [PMC free article] [PubMed] [Google Scholar]
- Greenwood J., Howes R., Lightman S. The blood-retinal barrier in experimental autoimmune uveoretinitis. Leukocyte interactions and functional damage. Lab Invest. 1994 Jan;70(1):39–52. [PubMed] [Google Scholar]
- Grewal I. S., Xu J., Flavell R. A. Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature. 1995 Dec 7;378(6557):617–620. doi: 10.1038/378617a0. [DOI] [PubMed] [Google Scholar]
- Griffith T. S., Brunner T., Fletcher S. M., Green D. R., Ferguson T. A. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995 Nov 17;270(5239):1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- Guerder S., Meyerhoff J., Flavell R. The role of the T cell costimulator B7-1 in autoimmunity and the induction and maintenance of tolerance to peripheral antigen. Immunity. 1994 May;1(2):155–166. doi: 10.1016/1074-7613(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Guerder S., Picarella D. E., Linsley P. S., Flavell R. A. Costimulator B7-1 confers antigen-presenting-cell function to parenchymal tissue and in conjunction with tumor necrosis factor alpha leads to autoimmunity in transgenic mice. Proc Natl Acad Sci U S A. 1994 May 24;91(11):5138–5142. doi: 10.1073/pnas.91.11.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Zheng B., Dal Porto J., Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. IV. Affinity-dependent, antigen-driven B cell apoptosis in germinal centers as a mechanism for maintaining self-tolerance. J Exp Med. 1995 Dec 1;182(6):1635–1644. doi: 10.1084/jem.182.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan D. M., Hengartner H., Huang M. L., Kang Y. H., Abe R., Moreadith R. W., Pircher H., Gray G. S., Ohashi P. S., Freeman G. J. Mice expressing both B7-1 and viral glycoprotein on pancreatic beta cells along with glycoprotein-specific transgenic T cells develop diabetes due to a breakdown of T-lymphocyte unresponsiveness. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3137–3141. doi: 10.1073/pnas.91.8.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermiston M. L., Gordon J. I. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995 Nov 17;270(5239):1203–1207. doi: 10.1126/science.270.5239.1203. [DOI] [PubMed] [Google Scholar]
- Hogquist K. A., Gavin M. A., Bevan M. J. Positive selection of CD8+ T cells induced by major histocompatibility complex binding peptides in fetal thymic organ culture. J Exp Med. 1993 May 1;177(5):1469–1473. doi: 10.1084/jem.177.5.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämmerling G. J., Schönrich G., Momburg F., Auphan N., Malissen M., Malissen B., Schmitt-Verhulst A. M., Arnold B. Non-deletional mechanisms of peripheral and central tolerance: studies with transgenic mice with tissue-specific expression of a foreign MHC class I antigen. Immunol Rev. 1991 Aug;122:47–67. doi: 10.1111/j.1600-065x.1991.tb00596.x. [DOI] [PubMed] [Google Scholar]
- Hörnquist E., Lycke N. Cholera toxin adjuvant greatly promotes antigen priming of T cells. Eur J Immunol. 1993 Sep;23(9):2136–2143. doi: 10.1002/eji.1830230914. [DOI] [PubMed] [Google Scholar]
- Imhof B. A., Dunon D. Leukocyte migration and adhesion. Adv Immunol. 1995;58:345–416. doi: 10.1016/s0065-2776(08)60623-9. [DOI] [PubMed] [Google Scholar]
- Inaba K., Witmer-Pack M., Inaba M., Hathcock K. S., Sakuta H., Azuma M., Yagita H., Okumura K., Linsley P. S., Ikehara S. The tissue distribution of the B7-2 costimulator in mice: abundant expression on dendritic cells in situ and during maturation in vitro. J Exp Med. 1994 Nov 1;180(5):1849–1860. doi: 10.1084/jem.180.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J., Kassir R., Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J Exp Med. 1991 May 1;173(5):1165–1175. doi: 10.1084/jem.173.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J., Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. II. A common clonal origin for periarteriolar lymphoid sheath-associated foci and germinal centers. J Exp Med. 1992 Sep 1;176(3):679–687. doi: 10.1084/jem.176.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C. A., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- June C. H., Bluestone J. A., Nadler L. M., Thompson C. B. The B7 and CD28 receptor families. Immunol Today. 1994 Jul;15(7):321–331. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Kearney E. R., Pape K. A., Loh D. Y., Jenkins M. K. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994 Jul;1(4):327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Kearney E. R., Walunas T. L., Karr R. W., Morton P. A., Loh D. Y., Bluestone J. A., Jenkins M. K. Antigen-dependent clonal expansion of a trace population of antigen-specific CD4+ T cells in vivo is dependent on CD28 costimulation and inhibited by CTLA-4. J Immunol. 1995 Aug 1;155(3):1032–1036. [PubMed] [Google Scholar]
- Keren D. F. Antigen processing in the mucosal immune system. Semin Immunol. 1992 Aug;4(4):217–226. [PubMed] [Google Scholar]
- Kisielow P., Blüthmann H., Staerz U. D., Steinmetz M., von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988 Jun 23;333(6175):742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Korthäuer U., Graf D., Mages H. W., Brière F., Padayachee M., Malcolm S., Ugazio A. G., Notarangelo L. D., Levinsky R. J., Kroczek R. A. Defective expression of T-cell CD40 ligand causes X-linked immunodeficiency with hyper-IgM. Nature. 1993 Feb 11;361(6412):539–541. doi: 10.1038/361539a0. [DOI] [PubMed] [Google Scholar]
- Ksander B. R., Streilein J. W. Regulation of the immune response within privileged sites. Chem Immunol. 1994;58:117–145. [PubMed] [Google Scholar]
- Kulkarni A. B., Huh C. G., Becker D., Geiser A., Lyght M., Flanders K. C., Roberts A. B., Sporn M. B., Ward J. M., Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyburz D., Aichele P., Speiser D. E., Hengartner H., Zinkernagel R. M., Pircher H. T cell immunity after a viral infection versus T cell tolerance induced by soluble viral peptides. Eur J Immunol. 1993 Aug;23(8):1956–1962. doi: 10.1002/eji.1830230834. [DOI] [PubMed] [Google Scholar]
- Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993 Oct 22;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Larsen C. P., Ritchie S. C., Pearson T. C., Linsley P. S., Lowry R. P. Functional expression of the costimulatory molecule, B7/BB1, on murine dendritic cell populations. J Exp Med. 1992 Oct 1;176(4):1215–1220. doi: 10.1084/jem.176.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C. P., Steinman R. M., Witmer-Pack M., Hankins D. F., Morris P. J., Austyn J. M. Migration and maturation of Langerhans cells in skin transplants and explants. J Exp Med. 1990 Nov 1;172(5):1483–1493. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenschow D. J., Zeng Y., Thistlethwaite J. R., Montag A., Brady W., Gibson M. G., Linsley P. S., Bluestone J. A. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992 Aug 7;257(5071):789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- Lin H., Bolling S. F., Linsley P. S., Wei R. Q., Gordon D., Thompson C. B., Turka L. A. Long-term acceptance of major histocompatibility complex mismatched cardiac allografts induced by CTLA4Ig plus donor-specific transfusion. J Exp Med. 1993 Nov 1;178(5):1801–1806. doi: 10.1084/jem.178.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Wallace P. M., Johnson J., Gibson M. G., Greene J. L., Ledbetter J. A., Singh C., Tepper M. A. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992 Aug 7;257(5071):792–795. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- Linthicum D. S., Frelinger J. A. Acute autoimmune encephalomyelitis in mice. II. Susceptibility is controlled by the combination of H-2 and histamine sensitization genes. J Exp Med. 1982 Jul 1;156(1):31–40. doi: 10.1084/jem.156.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linthicum D. S., Munoz J. J., Blaskett A. Acute experimental autoimmune encephalomyelitis in mice. I. Adjuvant action of Bordetella pertussis is due to vasoactive amine sensitization and increased vascular permeability of the central nervous system. Cell Immunol. 1982 Nov 1;73(2):299–310. doi: 10.1016/0008-8749(82)90457-9. [DOI] [PubMed] [Google Scholar]
- Liu Y. J., Joshua D. E., Williams G. T., Smith C. A., Gordon J., MacLennan I. C. Mechanism of antigen-driven selection in germinal centres. Nature. 1989 Dec 21;342(6252):929–931. doi: 10.1038/342929a0. [DOI] [PubMed] [Google Scholar]
- Liu Y. J., Zhang J., Lane P. J., Chan E. Y., MacLennan I. C. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol. 1991 Dec;21(12):2951–2962. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- Liu Y., Janeway C. A., Jr Microbial induction of co-stimulatory activity for CD4 T-cell growth. Int Immunol. 1991 Apr;3(4):323–332. doi: 10.1093/intimm/3.4.323. [DOI] [PubMed] [Google Scholar]
- MacLennan I. C. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- Macatonia S. E., Knight S. C., Edwards A. J., Griffiths S., Fryer P. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. Functional and morphological studies. J Exp Med. 1987 Dec 1;166(6):1654–1667. doi: 10.1084/jem.166.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay C. R., Marston W. L., Dudler L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med. 1990 Mar 1;171(3):801–817. doi: 10.1084/jem.171.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Basaraba L., Peacock S. C., Gall D. G. Binding of Yersinia enterocolitica to rabbit intestinal brush border membranes, mucus, and mucin. Infect Immun. 1989 Nov;57(11):3292–3299. doi: 10.1128/iai.57.11.3292-3299.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger P., Guerder S. Does T-cell tolerance require a dedicated antigen-presenting cell? Nature. 1989 Mar 2;338(6210):74–76. doi: 10.1038/338074a0. [DOI] [PubMed] [Google Scholar]
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- Nasir A., Ferbel B., Salminen W., Barth R. K., Gaspari A. A. Exaggerated and persistent cutaneous delayed-type hypersensitivity in transgenic mice whose epidermal keratinocytes constitutively express B7-1 antigen. J Clin Invest. 1994 Aug;94(2):892–898. doi: 10.1172/JCI117411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis P., Stet R. J., Wagenaar J. P., Wubbena A. S., Kampinga J., Karrenbeld A. The transcapsular route: a new way for (self-) antigens to by-pass the blood-thymus barrier? Immunol Today. 1988 Dec;9(12):372–375. doi: 10.1016/0167-5699(88)91236-4. [DOI] [PubMed] [Google Scholar]
- Noelle R. J., Roy M., Shepherd D. M., Stamenkovic I., Ledbetter J. A., Aruffo A. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6550–6554. doi: 10.1073/pnas.89.14.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi P. S., Oehen S., Buerki K., Pircher H., Ohashi C. T., Odermatt B., Malissen B., Zinkernagel R. M., Hengartner H. Ablation of "tolerance" and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991 Apr 19;65(2):305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- Parker D. C., Greiner D. L., Phillips N. E., Appel M. C., Steele A. W., Durie F. H., Noelle R. J., Mordes J. P., Rossini A. A. Survival of mouse pancreatic islet allografts in recipients treated with allogeneic small lymphocytes and antibody to CD40 ligand. Proc Natl Acad Sci U S A. 1995 Oct 10;92(21):9560–9564. doi: 10.1073/pnas.92.21.9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F. T cells in inflammatory bowel disease: protective and pathogenic roles. Immunity. 1995 Aug;3(2):171–174. doi: 10.1016/1074-7613(95)90086-1. [DOI] [PubMed] [Google Scholar]
- Pulendran B., Kannourakis G., Nouri S., Smith K. G., Nossal G. J. Soluble antigen can cause enhanced apoptosis of germinal-centre B cells. Nature. 1995 May 25;375(6529):331–334. doi: 10.1038/375331a0. [DOI] [PubMed] [Google Scholar]
- Ranheim E. A., Kipps T. J. Activated T cells induce expression of B7/BB1 on normal or leukemic B cells through a CD40-dependent signal. J Exp Med. 1993 Apr 1;177(4):925–935. doi: 10.1084/jem.177.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha B., von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991 Mar 8;251(4998):1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- Sallusto F., Cella M., Danieli C., Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995 Aug 1;182(2):389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosshauer B. The blood-brain barrier: morphology, molecules, and neurothelin. Bioessays. 1993 May;15(5):341–346. doi: 10.1002/bies.950150508. [DOI] [PubMed] [Google Scholar]
- Schönrich G., Momburg F., Malissen M., Schmitt-Verhulst A. M., Malissen B., Hämmerling G. J., Arnold B. Distinct mechanisms of extrathymic T cell tolerance due to differential expression of self antigen. Int Immunol. 1992 May;4(5):581–590. doi: 10.1093/intimm/4.5.581. [DOI] [PubMed] [Google Scholar]
- Shokat K. M., Goodnow C. C. Antigen-induced B-cell death and elimination during germinal-centre immune responses. Nature. 1995 May 25;375(6529):334–338. doi: 10.1038/375334a0. [DOI] [PubMed] [Google Scholar]
- Shull M. M., Ormsby I., Kier A. B., Pawlowski S., Diebold R. J., Yin M., Allen R., Sidman C., Proetzel G., Calvin D. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992 Oct 22;359(6397):693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer G. G., Abbas A. K. The fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994 Aug;1(5):365–371. doi: 10.1016/1074-7613(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Smith M. E., Ford W. L. The recirculating lymphocyte pool of the rat: a systematic description of the migratory behaviour of recirculating lymphocytes. Immunology. 1983 May;49(1):83–94. [PMC free article] [PubMed] [Google Scholar]
- Sprent J. T and B memory cells. Cell. 1994 Jan 28;76(2):315–322. doi: 10.1016/0092-8674(94)90338-7. [DOI] [PubMed] [Google Scholar]
- Sprent J., Webb S. R. Intrathymic and extrathymic clonal deletion of T cells. Curr Opin Immunol. 1995 Apr;7(2):196–205. doi: 10.1016/0952-7915(95)80004-2. [DOI] [PubMed] [Google Scholar]
- Steinman R. M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Streilein J. W. Unraveling immune privilege. Science. 1995 Nov 17;270(5239):1158–1159. doi: 10.1126/science.270.5239.1158. [DOI] [PubMed] [Google Scholar]
- Surh C. D., Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994 Nov 3;372(6501):100–103. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- Szakal A. K., Kosco M. H., Tew J. G. Microanatomy of lymphoid tissue during humoral immune responses: structure function relationships. Annu Rev Immunol. 1989;7:91–109. doi: 10.1146/annurev.iy.07.040189.000515. [DOI] [PubMed] [Google Scholar]
- Tafuri A., Alferink J., Möller P., Hämmerling G. J., Arnold B. T cell awareness of paternal alloantigens during pregnancy. Science. 1995 Oct 27;270(5236):630–633. doi: 10.1126/science.270.5236.630. [DOI] [PubMed] [Google Scholar]
- Tuohy V. K., Sobel R. A., Lees M. B. Myelin proteolipid protein-induced experimental allergic encephalomyelitis. Variations of disease expression in different strains of mice. J Immunol. 1988 Mar 15;140(6):1868–1873. [PubMed] [Google Scholar]
- Van den Eertwegh A. J., Noelle R. J., Roy M., Shepherd D. M., Aruffo A., Ledbetter J. A., Boersma W. J., Claassen E. In vivo CD40-gp39 interactions are essential for thymus-dependent humoral immunity. I. In vivo expression of CD40 ligand, cytokines, and antibody production delineates sites of cognate T-B cell interactions. J Exp Med. 1993 Nov 1;178(5):1555–1565. doi: 10.1084/jem.178.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella A. T., McCormack J. E., Linsley P. S., Kappler J. W., Marrack P. Lipopolysaccharide interferes with the induction of peripheral T cell death. Immunity. 1995 Mar;2(3):261–270. doi: 10.1016/1074-7613(95)90050-0. [DOI] [PubMed] [Google Scholar]
- Wallace P. M., Rodgers J. N., Leytze G. M., Johnson J. S., Linsley P. S. Induction and reversal of long-lived specific unresponsiveness to a T-dependent antigen following CTLA4Ig treatment. J Immunol. 1995 Jun 1;154(11):5885–5895. [PubMed] [Google Scholar]
- Warren H. S., Vogel F. R., Chedid L. A. Current status of immunological adjuvants. Annu Rev Immunol. 1986;4:369–388. doi: 10.1146/annurev.iy.04.040186.002101. [DOI] [PubMed] [Google Scholar]
- Warshaw A. L., Walker W. A., Cornell R., Isselbacher K. J. Small intestinal permeability to macromolecules. Transmission of horseradish peroxidase into mesenteric lymph and portal blood. Lab Invest. 1971 Dec;25(6):675–684. [PubMed] [Google Scholar]
- Webb S., Morris C., Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990 Dec 21;63(6):1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- Wilbanks G. A., Streilein J. W. Characterization of suppressor cells in anterior chamber-associated immune deviation (ACAID) induced by soluble antigen. Evidence of two functionally and phenotypically distinct T-suppressor cell populations. Immunology. 1990 Nov;71(3):383–389. [PMC free article] [PubMed] [Google Scholar]
- Williams I. R., Ort R. J., Kupper T. S. Keratinocyte expression of B7-1 in transgenic mice amplifies the primary immune response to cutaneous antigens. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12780–12784. doi: 10.1073/pnas.91.26.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A. D., Bailey M., Williams N. A., Stokes C. R. The in vitro production of cytokines by mucosal lymphocytes immunized by oral administration of keyhole limpet hemocyanin using cholera toxin as an adjuvant. Eur J Immunol. 1991 Oct;21(10):2333–2339. doi: 10.1002/eji.1830211007. [DOI] [PubMed] [Google Scholar]
- Xu-Amano J., Kiyono H., Jackson R. J., Staats H. F., Fujihashi K., Burrows P. D., Elson C. O., Pillai S., McGhee J. R. Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J Exp Med. 1993 Oct 1;178(4):1309–1320. doi: 10.1084/jem.178.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Foy T. M., Laman J. D., Elliott E. A., Dunn J. J., Waldschmidt T. J., Elsemore J., Noelle R. J., Flavell R. A. Mice deficient for the CD40 ligand. Immunity. 1994 Aug;1(5):423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Yong T., Bebo B. F., Jr, Sapatino B. V., Welsh C. J., Orr E. L., Linthicum D. S. Histamine-induced microvascular leakage in pial venules: differences between the SJL/J and BALB/c inbred strains of mice. J Neurotrauma. 1994 Apr;11(2):161–171. doi: 10.1089/neu.1994.11.161. [DOI] [PubMed] [Google Scholar]
- Zal T., Volkmann A., Stockinger B. Mechanisms of tolerance induction in major histocompatibility complex class II-restricted T cells specific for a blood-borne self-antigen. J Exp Med. 1994 Dec 1;180(6):2089–2099. doi: 10.1084/jem.180.6.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamvil S. S., Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Moskophidis D., Kündig T., Oehen S., Pircher H., Hengartner H. Effector T-cell induction and T-cell memory versus peripheral deletion of T cells. Immunol Rev. 1993 Jun;133:199–223. doi: 10.1111/j.1600-065x.1993.tb01517.x. [DOI] [PubMed] [Google Scholar]
- van Essen D., Kikutani H., Gray D. CD40 ligand-transduced co-stimulation of T cells in the development of helper function. Nature. 1995 Dec 7;378(6557):620–623. doi: 10.1038/378620a0. [DOI] [PubMed] [Google Scholar]