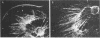
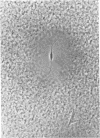
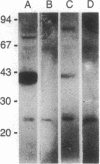
Abstract
Plasminogen activator has been implicated in tissue remodeling and cell migration during embryogenesis. In the developing nervous system, these processes are evident in the migration of neurons, axonal extension, Schwann cell migration, and the ensheathment and myelination of nerves. We have studied the production of plasminogen activator in cultures of superior cervical ganglia under conditions in which both neurons and glia are present. We have found that a principal source of the enzyme in these cultures is the glial cells and that the enzyme could not be detected at the growing tips of neurites. Plasminogen activator is also produced by Schwann cells isolated from neonatal rat sciatic nerve. The production of the enzyme by these cells is stimulated 6- to 10-fold by cholera toxin. Isolated Schwann cells and glial cells in the ganglion explant cultures produce the tissue form of plasminogen activator, a form of the enzyme not often found in nonmalignant cells. Preliminary experiments suggest that neuronal-glial interactions may regulate enzyme production by Schwann cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron-Van Evercooren A., Kleinman H. K., Seppä H. E., Rentier B., Dubois-Dalcq M. Fibronectin promotes rat Schwann cell growth and motility. J Cell Biol. 1982 Apr;93(1):211–216. doi: 10.1083/jcb.93.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockes J. P., Fields K. L., Raff M. C. A surface antigenic marker for rat Schwann cells. Nature. 1977 Mar 24;266(5600):364–366. doi: 10.1038/266364a0. [DOI] [PubMed] [Google Scholar]
- Brockes J. P., Fields K. L., Raff M. C. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979 Apr 6;165(1):105–118. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- Cornbrooks C. J., Carey D. J., McDonald J. A., Timpl R., Bunge R. P. In vivo and in vitro observations on laminin production by Schwann cells. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3850–3854. doi: 10.1073/pnas.80.12.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Vassalli J. D., Bobbitt J. L., Hull R. N., Reich E., Krane S. M. Calcitonin stimulates plasminogen activator in porcine renal tubular cells: LLC-PK1. J Cell Biol. 1981 Oct;91(1):195–200. doi: 10.1083/jcb.91.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino C., Zamboni L. Silver methenamine stain for electron microscopy. J Ultrastruct Res. 1967 Aug;19(3):273–282. doi: 10.1016/s0022-5320(67)80221-1. [DOI] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Elsdale T., Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972 Sep;54(3):626–637. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granelli-Piperno A., Reich E. A study of proteases and protease-inhibitor complexes in biological fluids. J Exp Med. 1978 Jul 1;148(1):223–234. doi: 10.1084/jem.148.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P., Benedict W., Strickland S., Reich E. Fibrin overlay methods for the detection of single transformed cells and colonies of transformed cells. Cell. 1975 Jul;5(3):323–329. doi: 10.1016/0092-8674(75)90108-7. [DOI] [PubMed] [Google Scholar]
- Kalderon N. Migration of Schwann cells and wrapping of neurites in vitro: a function of protease activity (plasmin) in the growth medium. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5992–5996. doi: 10.1073/pnas.76.11.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystosek A., Seeds N. W. Peripheral neurons and Schwann cells secrete plasminogen activator. J Cell Biol. 1984 Feb;98(2):773–776. doi: 10.1083/jcb.98.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystosek A., Seeds N. W. Plasminogen activator release at the neuronal growth cone. Science. 1981 Sep 25;213(4515):1532–1534. doi: 10.1126/science.7197054. [DOI] [PubMed] [Google Scholar]
- Krystosek A., Seeds N. W. Plasminogen activator secretion by granule neurons in cultures of developing cerebellum. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7810–7814. doi: 10.1073/pnas.78.12.7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levin E. G., Loskutoff D. J. Cultured bovine endothelial cells produce both urokinase and tissue-type plasminogen activators. J Cell Biol. 1982 Sep;94(3):631–636. doi: 10.1083/jcb.94.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loskutoff D. J. Effects of acidified fetal bovine serum on the fibrinolytic activity and growth of cells in culture. J Cell Physiol. 1978 Sep;96(3):361–369. doi: 10.1002/jcp.1040960312. [DOI] [PubMed] [Google Scholar]
- Marotti K. R., Belin D., Strickland S. The production of distinct forms of plasminogen activator by mouse embryonic cells. Dev Biol. 1982 Mar;90(1):154–159. doi: 10.1016/0012-1606(82)90220-2. [DOI] [PubMed] [Google Scholar]
- Matsuo O., Rijken D. C., Collen D. Thrombolysis by human tissue plasminogen activator and urokinase in rabbits with experimental pulmonary embolus. Nature. 1981 Jun 18;291(5816):590–591. doi: 10.1038/291590a0. [DOI] [PubMed] [Google Scholar]
- Moonen G., Grau-Wagemans M. P., Selak I. Plasminogen activator-plasmin system and neuronal migration. Nature. 1982 Aug 19;298(5876):753–755. doi: 10.1038/298753a0. [DOI] [PubMed] [Google Scholar]
- Moss J., Vaughan M. Activation of adenylate cyclase by choleragen. Annu Rev Biochem. 1979;48:581–600. doi: 10.1146/annurev.bi.48.070179.003053. [DOI] [PubMed] [Google Scholar]
- Nagamine Y., Sudol M., Reich E. Hormonal regulation of plasminogen activator mRNA production in porcine kidney cells. Cell. 1983 Apr;32(4):1181–1190. doi: 10.1016/0092-8674(83)90301-x. [DOI] [PubMed] [Google Scholar]
- Ossowski L., Biegel D., Reich E. Mammary plasminogen activator: correlation with involution, hormonal modulation and comparison between normal and neoplastic tissue. Cell. 1979 Apr;16(4):929–940. doi: 10.1016/0092-8674(79)90108-9. [DOI] [PubMed] [Google Scholar]
- Pennica D., Holmes W. E., Kohr W. J., Harkins R. N., Vehar G. A., Ward C. A., Bennett W. F., Yelverton E., Seeburg P. H., Heyneker H. L. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature. 1983 Jan 20;301(5897):214–221. doi: 10.1038/301214a0. [DOI] [PubMed] [Google Scholar]
- Quigley J. P. Phorbol ester-induced morphological changes in transformed chick fibroblasts: evidence for direct catalytic involvement of plasminogen activator. Cell. 1979 May;17(1):131–141. doi: 10.1016/0092-8674(79)90301-5. [DOI] [PubMed] [Google Scholar]
- Ratner N., Glaser L., Bunge R. P. PC12 cells as a source of neurite-derived cell surface mitogen, which stimulates Schwann cell division. J Cell Biol. 1984 Mar;98(3):1150–1155. doi: 10.1083/jcb.98.3.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer J. L., Bunge R. P., Glaser L. Studies of Schwann cell proliferation. III. Evidence for the surface localization of the neurite mitogen. J Cell Biol. 1980 Mar;84(3):767–778. doi: 10.1083/jcb.84.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. K., Strickland S. Structural components and characteristics of Reichert's membrane, an extra-embryonic basement membrane. J Biol Chem. 1981 May 10;256(9):4654–4661. [PubMed] [Google Scholar]
- Soreq H., Miskin R. Plasminogen activator in the rodent brain. Brain Res. 1981 Jul 20;216(2):361–374. doi: 10.1016/0006-8993(81)90138-4. [DOI] [PubMed] [Google Scholar]
- Soreq H., Miskin R., Zutra A., Littauer U. Z. Modulation in the levels and localization of plasminogen activator in differentiating neuroblastoma cells. Brain Res. 1983 Apr;283(2-3):257–269. doi: 10.1016/0165-3806(83)90182-7. [DOI] [PubMed] [Google Scholar]
- Strickland S., Beers W. H. Studies on the role of plasminogen activator in ovulation. In vitro response of granulosa cells to gonadotropins, cyclic nucleotides, and prostaglandins. J Biol Chem. 1976 Sep 25;251(18):5694–5702. [PubMed] [Google Scholar]
- Strickland S., Reich E., Sherman M. I. Plasminogen activator in early embryogenesis: enzyme production by trophoblast and parietal endoderm. Cell. 1976 Oct;9(2):231–240. doi: 10.1016/0092-8674(76)90114-8. [DOI] [PubMed] [Google Scholar]
- Valinsky J. E., Reich E., Le Douarin N. M. Plasminogen activator in the bursa of Fabricius: correlations with morphogenetic remodeling and cell migrations. Cell. 1981 Aug;25(2):471–476. doi: 10.1016/0092-8674(81)90065-9. [DOI] [PubMed] [Google Scholar]
- Valinsky J. E., Reich E. Plasminogen in the chick embryo. Transport and biosynthesis. J Biol Chem. 1981 Dec 10;256(23):12470–12475. [PubMed] [Google Scholar]
- Vassalli J. D., Hamilton J., Reich E. Macrophage plasminogen activator: induction by concanavalin A and phorbol myristate acetate. Cell. 1977 Jul;11(3):695–705. doi: 10.1016/0092-8674(77)90086-1. [DOI] [PubMed] [Google Scholar]
- Wilson E. L., Becker M. L., Hoal E. G., Dowdle E. B. Molecular species of plasminogen activators secreted by normal and neoplastic human cells. Cancer Res. 1980 Mar;40(3):933–938. [PubMed] [Google Scholar]
- Wood P., Okada E., Bunge R. The use of networks of dissociated rat dorsal root ganglion neurons to induce myelination by oligodencrocytes in culture. Brain Res. 1980 Aug 25;196(1):247–252. doi: 10.1016/0006-8993(80)90732-5. [DOI] [PubMed] [Google Scholar]