Abstract
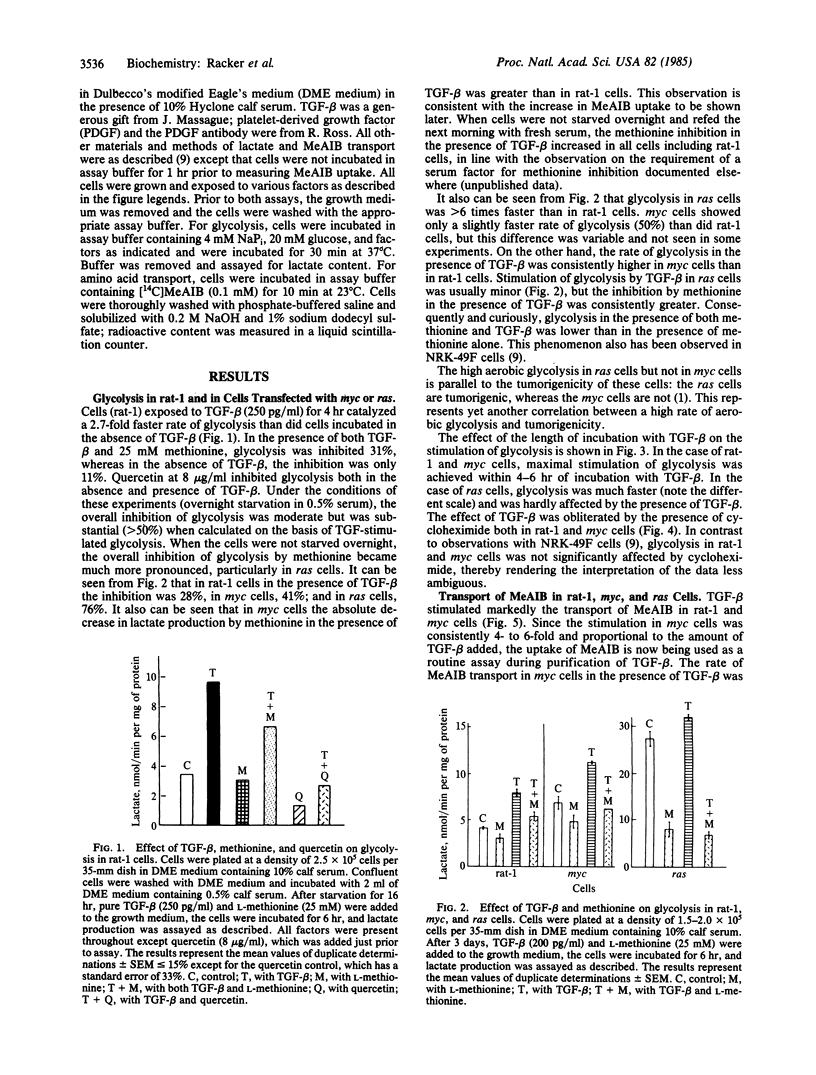
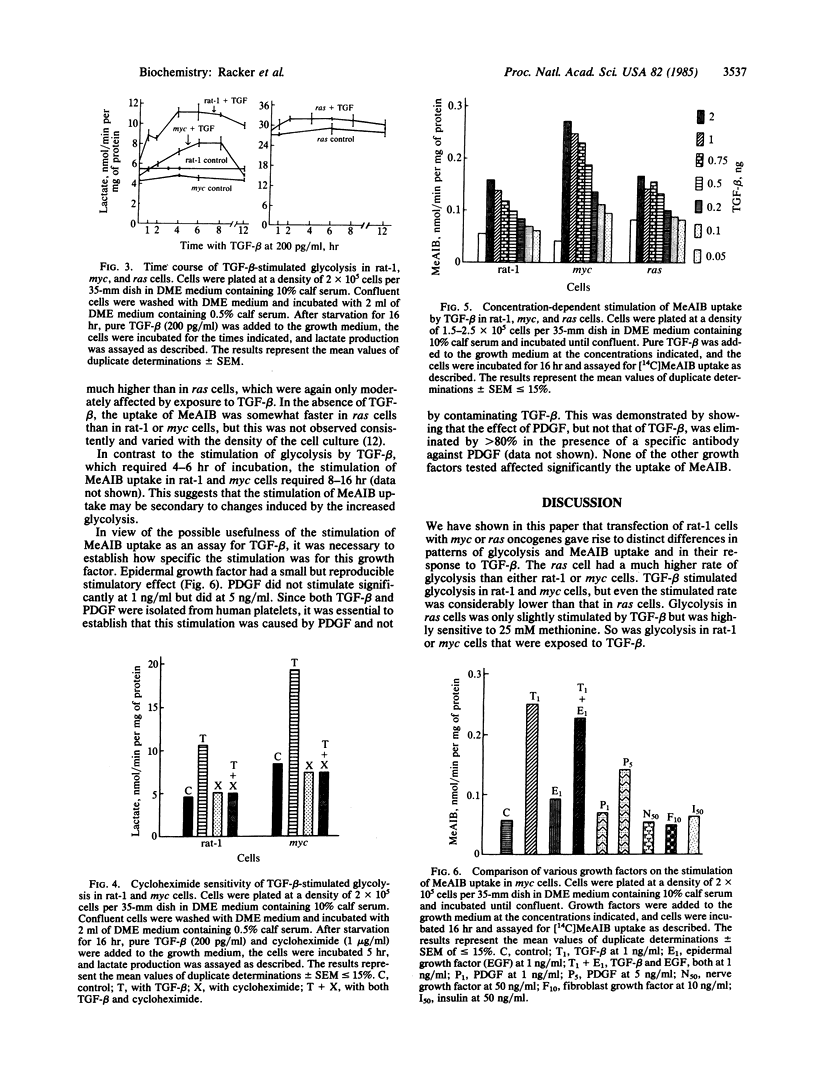
A high rate of aerobic glycolysis was catalyzed by rat-1 cells transfected with a ras oncogene (ras cells); rat-1 cells and rat-1 cells transfected with myc oncogene (myc cells) showed a low rate of glycolysis that was increased after exposure of the cells to type B transforming growth factor (TGF-beta). The uptake of radioactive methylaminoisobutyric acid or L-methionine via system A of amino acid transport also was accelerated after exposure of these cells to TGF-beta, with the myc cells being most sensitive and the ras cells least sensitive. Methionine was found to be a potent inhibitor of glycolysis in ras cells as well as in rat-1 or myc cells that were exposed to TGF-beta. We propose a relationship between the product of the ras oncogene (p21) and the protein(s) induced by exposure to TGF-beta.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boerner P., Resnick R. J., Racker E. Stimulation of glycolysis and amino acid uptake in NRK-49F cells by transforming growth factor beta and epidermal growth factor. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1350–1353. doi: 10.1073/pnas.82.5.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerner P., Saier M. H., Jr Growth regulation and amino acid transport in epithelial cells: influence of culture conditions and transformation on A, ASC, and L transport activities. J Cell Physiol. 1982 Nov;113(2):240–246. doi: 10.1002/jcp.1041130209. [DOI] [PubMed] [Google Scholar]
- Christiansen R. O., Steensland H., Loyter A., Saltzgaber J., Racker E. Energy-linked ion translocation in submitochondrial particles. II. Properties of submitochondrial particles capable of Ca++ translocation. J Biol Chem. 1969 Aug 25;244(16):4428–4436. [PubMed] [Google Scholar]
- Gazzola G. C., Dall'Asta V., Franchi-Gazzola R., Bussolati O., Longo N., Guidotti G. G. Post-translational control by carrier availability of amino acid transport in fetal human fibroblasts. Biochem Biophys Res Commun. 1984 Apr 16;120(1):172–178. doi: 10.1016/0006-291x(84)91429-3. [DOI] [PubMed] [Google Scholar]
- Inman W. H., Colowick S. P. Stimulation of glucose uptake by transforming growth factor beta: evidence for the requirement of epidermal growth factor-receptor activation. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1346–1349. doi: 10.1073/pnas.82.5.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isselbacher K. J. Increased uptake of amino acids and 2-deoxy-D-glucose by virus-transformed cells in culture. Proc Natl Acad Sci U S A. 1972 Mar;69(3):585–589. doi: 10.1073/pnas.69.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jullien M., Villaudy J., Golde A., Harel L. Inhibition by quercetin of the release of density dependent-inhibition of cell growth in RSV-transformed chicken cells. Cell Biol Int Rep. 1984 Nov;8(11):939–947. doi: 10.1016/0309-1651(84)90192-9. [DOI] [PubMed] [Google Scholar]
- Kelley D. S., Potter V. R. Regulation of amino acid transport systems by amino acid depletion and supplementation in monolayer cultures of rat hepatocytes. J Biol Chem. 1978 Dec 25;253(24):9009–9017. [PubMed] [Google Scholar]
- Kelley D. S., Potter V. R. Repression, derepression, transinhibition, and trans-stimulation of amino acid transport in rat hepatocytes and four rat hepatoma cell lines in culture. J Biol Chem. 1979 Jul 25;254(14):6691–6697. [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Racker E., Johnson J. H., Blackwell M. T. The role of ATPase in glycolysis of Ehrlich ascites tumor cells. J Biol Chem. 1983 Mar 25;258(6):3702–3705. [PubMed] [Google Scholar]
- Scholnick P., Lang D., Racker E. Regulatory mechanisms in carbohydrate metabolism. IX. Stimulation of aerobic glycolysis by energy-linked ion transport and inhibition by dextran sulfate. J Biol Chem. 1973 Jul 25;248(14):5175–5175. [PubMed] [Google Scholar]
- Stern P. H., Wallace C. D., Hoffman R. M. Altered methionine metabolism occurs in all members of a set of diverse human tumor cell lines. J Cell Physiol. 1984 Apr;119(1):29–34. doi: 10.1002/jcp.1041190106. [DOI] [PubMed] [Google Scholar]
- Tamanoi F., Walsh M., Kataoka T., Wigler M. A product of yeast RAS2 gene is a guanine nucleotide binding protein. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6924–6928. doi: 10.1073/pnas.81.22.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]


