Abstract
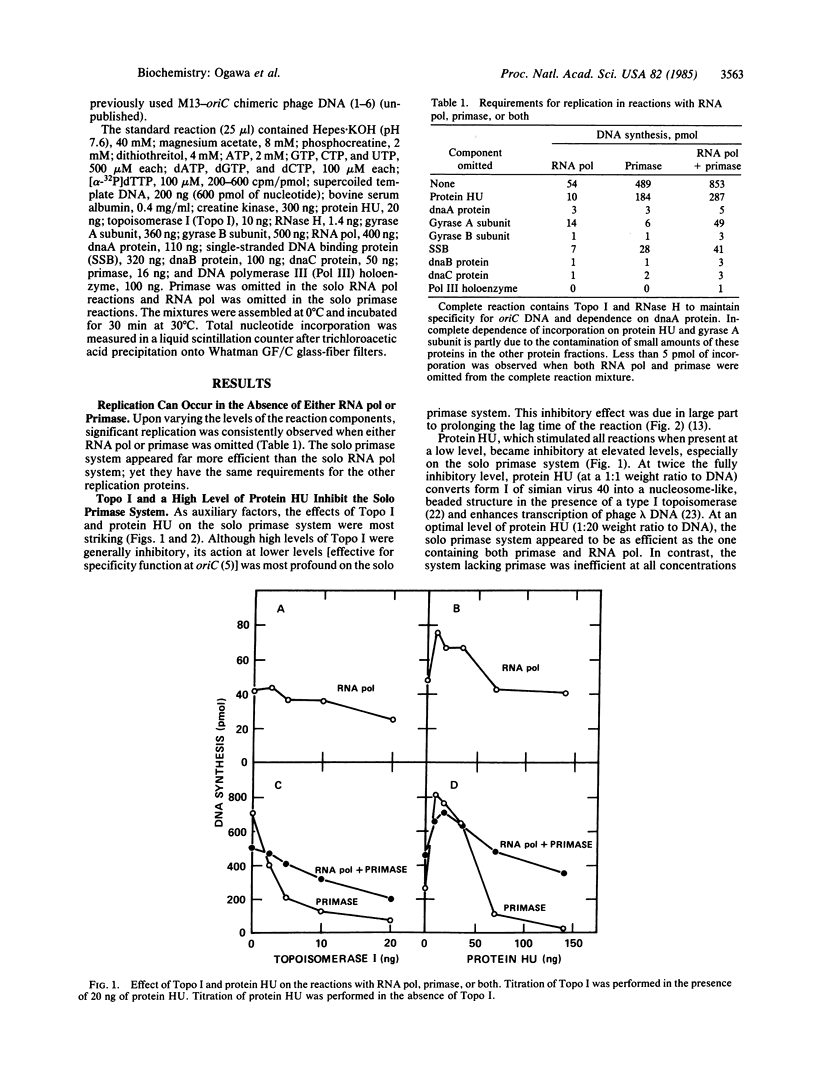
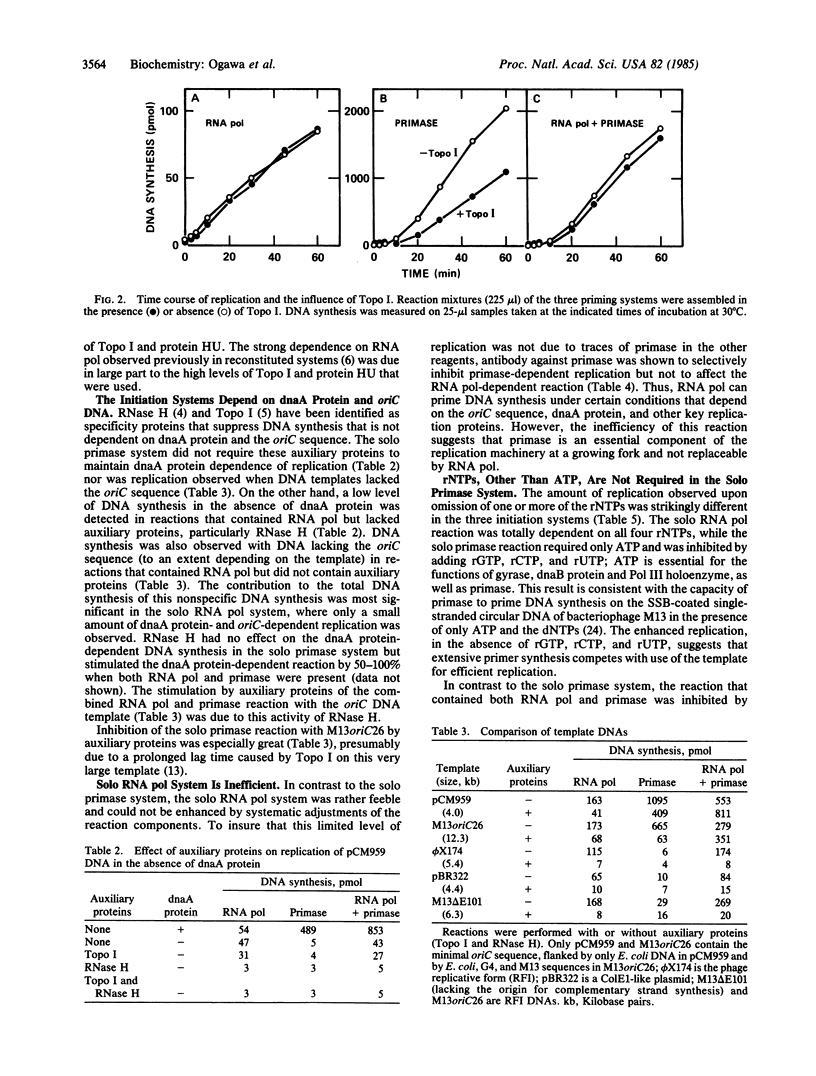
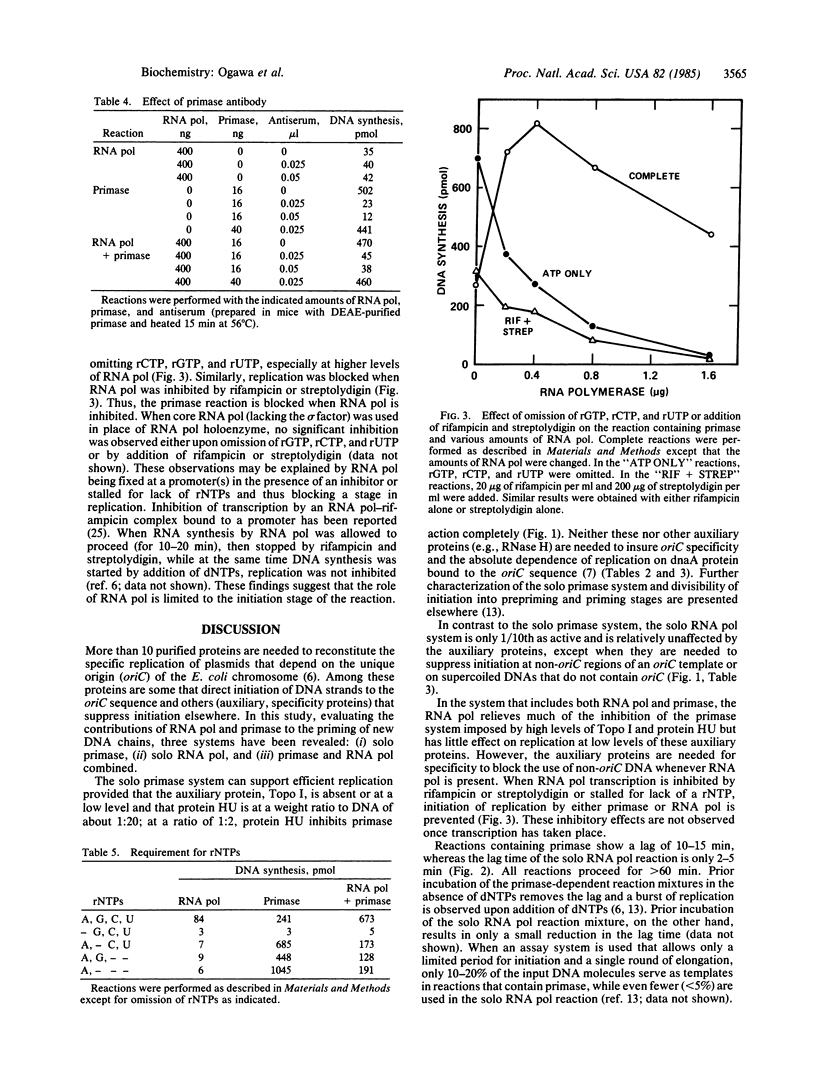
Replication of plasmids that depend on the 245-base-pair origin of the Escherichia coli chromosome (oriC) requires many purified proteins that (i) direct initiation to oriC (e.g., dnaA protein), (ii) influence initiations elsewhere (e.g., auxiliary proteins), and (iii) prime and extend DNA chains (e.g., priming and synthesis proteins). For the RNA priming and initiation of new DNA chains, the requirements for both primase and RNA polymerase (RNA pol) [Kaguni, J. M. & Kornberg, A. (1984) Cell 38, 183-190] have been further analyzed. Depending on the levels of auxiliary proteins (topoisomerase I and protein HU), three priming systems can operate: primase alone, RNA pol alone, or both combined. At low levels of auxiliary proteins, primase alone sustains an effective priming system. At higher levels, primase action is blocked, but RNA pol alone can initiate replication, albeit feebly; at these high levels of auxiliary proteins, primase and RNA pol act synergistically. When RNA pol is stalled by an inhibitor or lack of a ribonucleoside triphosphate, primase action is also inhibited. Based on these and other data [van der Ende, A., Baker, T. A., Ogawa, T. & Kornberg, A. (1985) Proc. Natl. Acad. Sci. USA 82, in press], RNA pol can counteract inhibition by auxiliary proteins and thus activate the origin for the priming by primase of the leading strand of the replication fork.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagdasarian M. M., Izakowska M., Bagdasarian M. Suppression of the DnaA phenotype by mutations in the rpoB cistron of ribonucleic acid polymerase in Salmonella typhimurium and Escherichia coli. J Bacteriol. 1977 May;130(2):577–582. doi: 10.1128/jb.130.2.577-582.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhk H. J., Messer W. The replication origin region of Escherichia coli: nucleotide sequence and functional units. Gene. 1983 Oct;24(2-3):265–279. doi: 10.1016/0378-1119(83)90087-2. [DOI] [PubMed] [Google Scholar]
- Diaz R., Ortega S. Initiation of plasmid R1 replication in vitro is independent of transcription by host RNA polymerase. Nucleic Acids Res. 1984 Jul 11;12(13):5175–5191. doi: 10.1093/nar/12.13.5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon N. E., Kornberg A. Protein HU in the enzymatic replication of the chromosomal origin of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jan;81(2):424–428. doi: 10.1073/pnas.81.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. S., Funnell B. E., Kornberg A. The dnaA protein complex with the E. coli chromosomal replication origin (oriC) and other DNA sites. Cell. 1984 Oct;38(3):889–900. doi: 10.1016/0092-8674(84)90284-8. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Kaguni J. M., Kornberg A. Enzymatic replication of the origin of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7370–7374. doi: 10.1073/pnas.78.12.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. S., Kornberg A. Purified dnaA protein in initiation of replication at the Escherichia coli chromosomal origin of replication. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5817–5821. doi: 10.1073/pnas.80.19.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaguni J. M., Kornberg A. Replication initiated at the origin (oriC) of the E. coli chromosome reconstituted with purified enzymes. Cell. 1984 Aug;38(1):183–190. doi: 10.1016/0092-8674(84)90539-7. [DOI] [PubMed] [Google Scholar]
- Kaguni J. M., Kornberg A. Topoisomerase I confers specificity in enzymatic replication of the Escherichia coli chromosomal origin. J Biol Chem. 1984 Jul 10;259(13):8578–8583. [PubMed] [Google Scholar]
- Kaguni J., LaVerne L. S., Ray D. S. Cloning and expression of the Escherichia coli replication origin in a single-stranded DNA phage. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6250–6254. doi: 10.1073/pnas.76.12.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassavetis G. A., Kaya K. M., Chamberlin M. J. Escherichia coli RNA polymerase-rifampicin complexes bound at promoter sites block RNA chain elongation by Escherichia coli RNA polymerase and T7-specific RNA polymerase. Biochemistry. 1978 Dec 26;17(26):5798–5804. doi: 10.1021/bi00619a029. [DOI] [PubMed] [Google Scholar]
- Kim M. H., Hines J. C., Ray D. S. Viable deletions of the M13 complementary strand origin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6784–6788. doi: 10.1073/pnas.78.11.6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lark K. G. Evidence for the direct involvement of RNA in the initiation of DNA replication in Escherichia coli 15T. J Mol Biol. 1972 Feb 28;64(1):47–60. doi: 10.1016/0022-2836(72)90320-8. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Pickett G. G., Kogoma T., Kornberg A. RNase H confers specificity in the dnaA-dependent initiation of replication at the unique origin of the Escherichia coli chromosome in vivo and in vitro. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1040–1044. doi: 10.1073/pnas.81.4.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen K. V., Atlung T., Kerszman G., Hansen G. E., Hansen F. G. Conditional change of DNA replication control in an RNA polymerase mutant of Escherichia coli. J Bacteriol. 1983 Apr;154(1):443–451. doi: 10.1128/jb.154.1.443-451.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvière-Yaniv J., Gros F. Characterization of a novel, low-molecular-weight DNA-binding protein from Escherichia coli. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3428–3432. doi: 10.1073/pnas.72.9.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvière-Yaniv J., Yaniv M., Germond J. E. E. coli DNA binding protein HU forms nucleosomelike structure with circular double-stranded DNA. Cell. 1979 Jun;17(2):265–274. doi: 10.1016/0092-8674(79)90152-1. [DOI] [PubMed] [Google Scholar]
- Rowen L., Kornberg A. A ribo-deoxyribonucleotide primer synthesized by primase. J Biol Chem. 1978 Feb 10;253(3):770–774. [PubMed] [Google Scholar]
- Tanaka M., Ohmori H., Hiraga S. A novel type of E. coli mutants with increased chromosomal copy number. Mol Gen Genet. 1983;192(1-2):51–60. doi: 10.1007/BF00327646. [DOI] [PubMed] [Google Scholar]
- von Meyenburg K., Hansen F. G., Riise E., Bergmans H. E., Meijer M., Messer W. Origin of replication, oriC, of the Escherichia coli K12 chromosome: genetic mapping and minichromosome replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):121–128. doi: 10.1101/sqb.1979.043.01.018. [DOI] [PubMed] [Google Scholar]


