Abstract
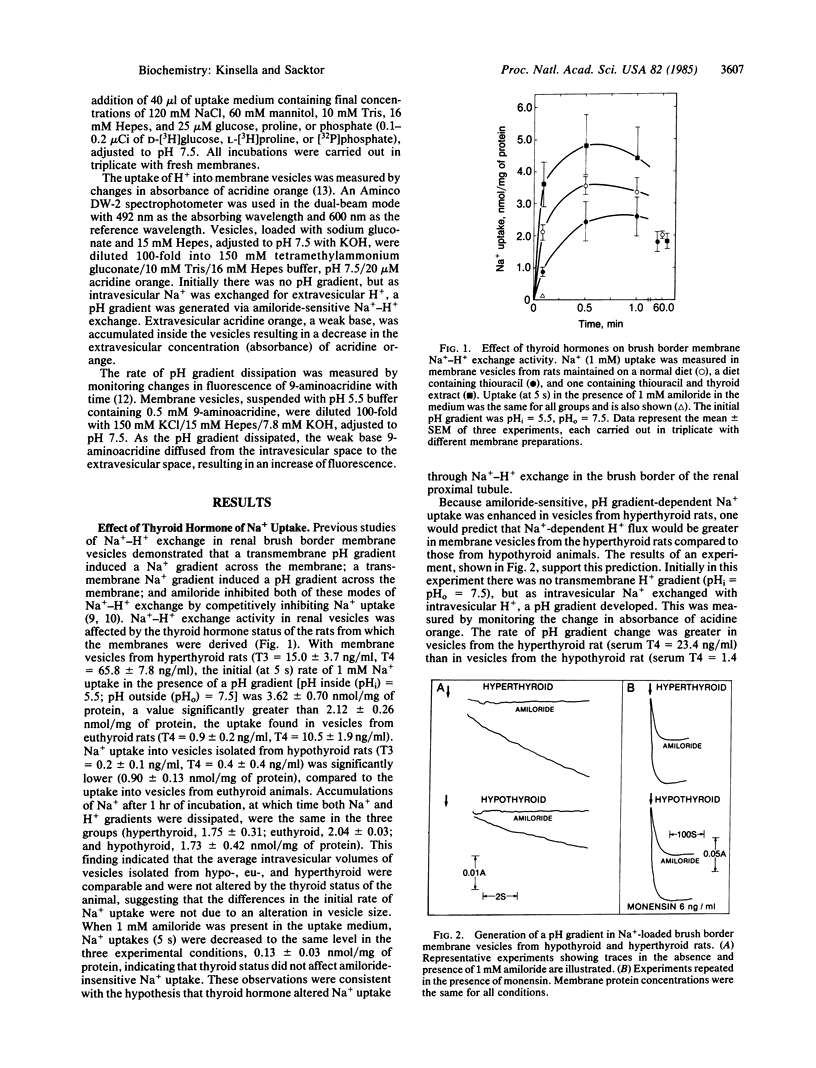
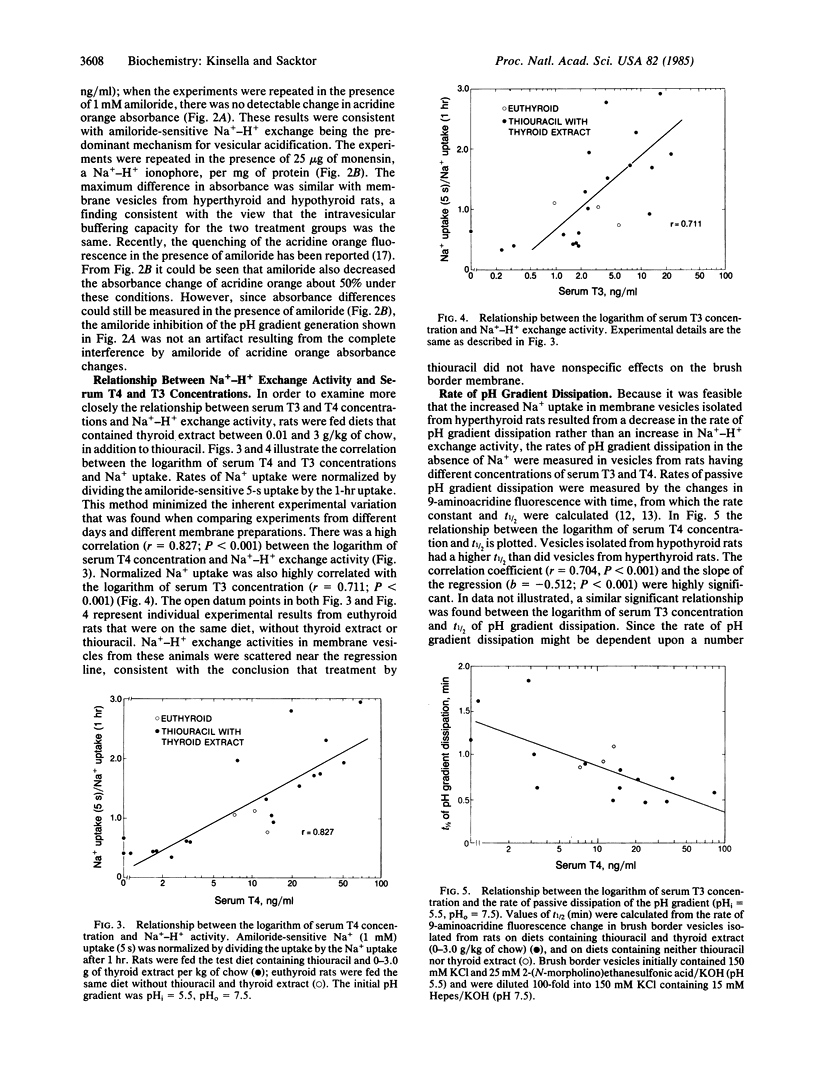
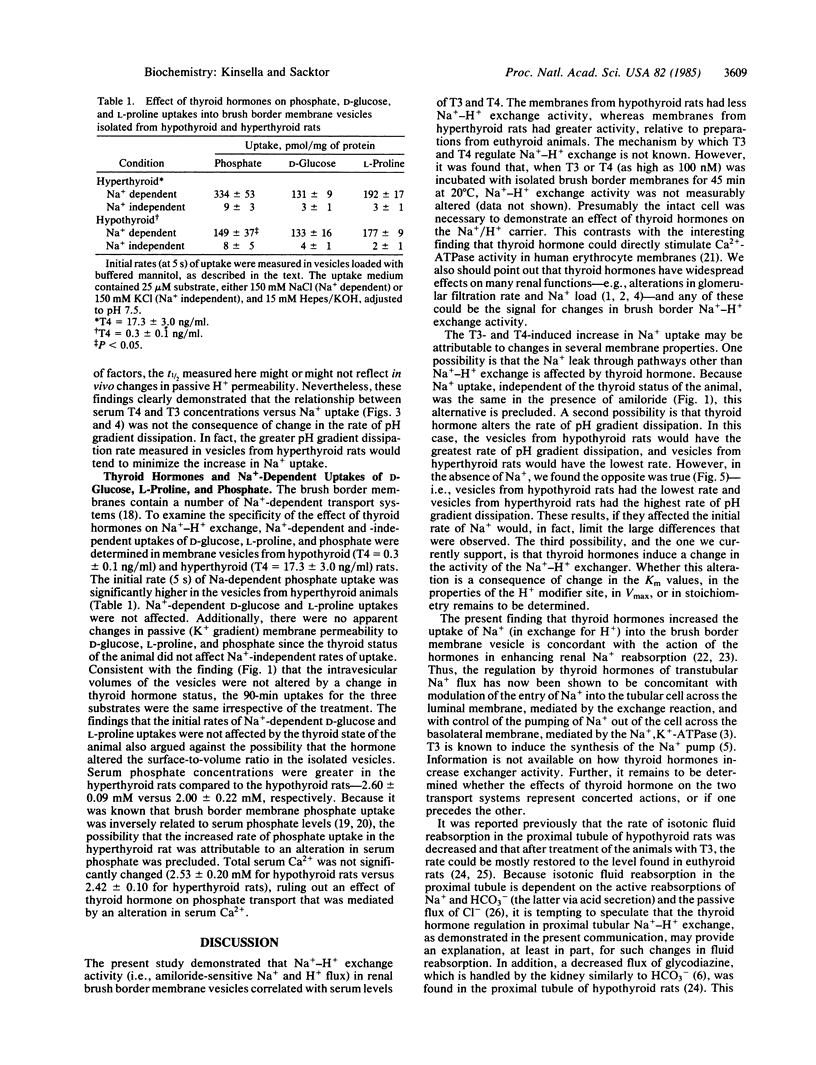
Na+-H+ exchange activity, i.e., amiloride-sensitive Na+ and H+ flux, in renal proximal tubule brush border (luminal) membrane vesicles was increased in the hyperthyroid rat and decreased in the hypothyroid rat, relative to the euthyroid animal. A positive correlation was found between Na+-H+ exchange activity and serum concentrations of thyroxine (T4) and triiodothyronine (T3). The thyroid status of the animal did not alter amiloride-insensitive Na+ uptake. The rate of passive pH gradient dissipation was higher in membrane vesicles from hyperthyroid rats compared to the rate in vesicles from hypothyroid animals, a result which would tend to limit the increase in Na+ uptake in vesicles from hyperthyroid animals. Na+-dependent phosphate uptake was increased in membrane vesicles from hyperthyroid rats; Na+-dependent D-glucose and L-proline uptakes were not changed by the thyroid status of the animal. The effect of thyroid hormones in increasing the uptake of Na+ in the brush border membrane vesicle is consistent with the action of the hormones in enhancing renal Na+ reabsorption. Further, the regulation of transtubular Na+ flux has now been shown to be concomitant with modulation of the entry of Na+ into the tubular cell across its luminal membrane, mediated by the exchange reaction, and with the previously reported control of the pumping of Na+ out of the cell across its basolateral membrane, mediated by the Na+,K+-ATPase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson P. S., Sacktor B. The Na+ gradient-dependent transport of D-glucose in renal brush border membranes. J Biol Chem. 1975 Aug 10;250(15):6032–6039. [PubMed] [Google Scholar]
- Beck J. C., Sacktor B. The sodium electrochemical potential-mediated uphill transport of D-glucose in renal brush border membrane vesicles. J Biol Chem. 1978 Aug 10;253(15):5531–5535. [PubMed] [Google Scholar]
- Berger S. J., Sacktor B. Isolation and biochemical characterization of brush borders from rabbit kidney. J Cell Biol. 1970 Dec;47(3):637–645. doi: 10.1083/jcb.47.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bommer J., Bonjour J. P., Ritz E., Fleisch H. Parathyroid-independent change in renal handling of phosphate in hyperthyroid rats. Kidney Int. 1979 Apr;15(4):325–334. doi: 10.1038/ki.1979.44. [DOI] [PubMed] [Google Scholar]
- Davis P. J., Blas S. D. In vitro stimulation of human red blood cell Ca2+-ATPase by thyroid hormone. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1073–1080. doi: 10.1016/0006-291x(81)90728-2. [DOI] [PubMed] [Google Scholar]
- De Santo N. G., Capasso G., Kinne R., Moewes B., Carella C. a., Anastasio P., Giordano C. Tubular transport processes in proximal tubules of hypothyroid rats. Lack of relationship between thyroidal dependent rise of isotonic fluid reabsorption and Na+ -K+ -ATPase activity. Pflugers Arch. 1982 Oct 1;394(4):294–301. doi: 10.1007/BF00583693. [DOI] [PubMed] [Google Scholar]
- De Santo N. G., Capasso G., Paduano C., Carella C., Giordano C. Tubular transport processes in proximal tubules of hypothyroid rats. Micropuncture studies on isotonic fluid, amino acid and buffer reabsorption. Pflugers Arch. 1980 Mar;384(2):117–122. doi: 10.1007/BF00584426. [DOI] [PubMed] [Google Scholar]
- Dennis V. W., Stead W. W., Myers J. L. Renal handling of phosphate and calcium. Annu Rev Physiol. 1979;41:257–271. doi: 10.1146/annurev.ph.41.030179.001353. [DOI] [PubMed] [Google Scholar]
- Edelman I. S. Thyroidal regulation of renal energy metabolism and (Na+ + K+)-activated adenosine triphosphatase activity. Med Clin North Am. 1975 May;59(3):605–614. doi: 10.1016/s0025-7125(16)32012-0. [DOI] [PubMed] [Google Scholar]
- Espinosa R. E., Keller M. J., Yusufi A. N., Dousa T. P. Effect of thyroxine administration on phosphate transport across renal cortical brush border membrane. Am J Physiol. 1984 Feb;246(2 Pt 2):F133–F139. doi: 10.1152/ajprenal.1984.246.2.F133. [DOI] [PubMed] [Google Scholar]
- Freiberg J. M., Kinsella J., Sacktor B. Glucocorticoids increase the Na+-H+ exchange and decrease the Na+ gradient-dependent phosphate-uptake systems in renal brush border membrane vesicles. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4932–4936. doi: 10.1073/pnas.79.16.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail-Beigi F., Edelman I. S. The mechanism of the calorigenic action of thyroid hormone. Stimulation of Na plus + K plus-activated adenosinetriphosphatase activity. J Gen Physiol. 1971 Jun;57(6):710–722. doi: 10.1085/jgp.57.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A. I., Lindheimer M. D. Actions of hormones on the kidney. Annu Rev Physiol. 1977;39:97–133. doi: 10.1146/annurev.ph.39.030177.000525. [DOI] [PubMed] [Google Scholar]
- Katz A. I., Lindheimer M. D. Renal sodium- and potassium-activated adenosine triphosphatase and sodium reabsorption in the hypothyroid rat. J Clin Invest. 1973 Apr;52(4):796–804. doi: 10.1172/JCI107243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella J. L., Aronson P. S. Amiloride inhibition of the Na+-H+ exchanger in renal microvillus membrane vesicles. Am J Physiol. 1981 Oct;241(4):F374–F379. doi: 10.1152/ajprenal.1981.241.4.F374. [DOI] [PubMed] [Google Scholar]
- Kinsella J. L., Aronson P. S. Properties of the Na+-H+ exchanger in renal microvillus membrane vesicles. Am J Physiol. 1980 Jun;238(6):F461–F469. doi: 10.1152/ajprenal.1980.238.6.F461. [DOI] [PubMed] [Google Scholar]
- Kinsella J., Cujdik T., Sacktor B. Na+-H+ exchange activity in renal brush border membrane vesicles in response to metabolic acidosis: The role of glucocorticoids. Proc Natl Acad Sci U S A. 1984 Jan;81(2):630–634. doi: 10.1073/pnas.81.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C. T., Barnes J., Cheng L., Balakir R., Sacktor B. Effects of 1,25-(OH)2D3 administered in vivo on phosphate uptake by isolated chick renal cells. Am J Physiol. 1982 May;242(5):C312–C318. doi: 10.1152/ajpcell.1982.242.5.C312. [DOI] [PubMed] [Google Scholar]
- Lo C. S., Edelman I. S. Effect of triiodothyronine on the synthesis and degradation of renal cortical (Na+ + k+)-adenosine triphosphatase. J Biol Chem. 1976 Dec 25;251(24):7834–7840. [PubMed] [Google Scholar]
- Marx S. J., Woodward C. J., Aurbach G. D. Calcitonin receptors of kidney and bone. Science. 1972 Dec 1;178(4064):999–1001. doi: 10.1126/science.178.4064.999. [DOI] [PubMed] [Google Scholar]
- Michael U. F., Barenberg R. L., Chavez R., Vaamonde C. A., Papper S. Renal handling of sodium and water in the hypothyroid rat. Clearance and micropuncture studies. J Clin Invest. 1972 Jun;51(6):1405–1412. doi: 10.1172/JCI106936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murer H., Hopfer U., Kinne R. Sodium/proton antiport in brush-border-membrane vesicles isolated from rat small intestine and kidney. Biochem J. 1976 Mar 15;154(3):597–604. [PMC free article] [PubMed] [Google Scholar]
- Sabolić I., Burckhardt G. Apparent inhibition of Na+/H+ exchange by amiloride and harmaline in acridine orange studies. Biochim Biophys Acta. 1983 Jun 10;731(2):354–360. doi: 10.1016/0005-2736(83)90028-7. [DOI] [PubMed] [Google Scholar]
- Sacktor B., Cheng L. Sodium gradient-dependent phosphate transport in renal brush border membrane vesicles. Effect of an intravesicular greater than extravesicular proton gradient. J Biol Chem. 1981 Aug 10;256(15):8080–8084. [PubMed] [Google Scholar]
- Ullrich K. J., Rumrich G., Baumann K. Renal proximal tubular buffer-(glycodiazine) transport. Inhomogeneity of local transport rate, dependence on sodium, effect of inhibitors and chronic adaptation. Pflugers Arch. 1975 Jun 26;357(3-4):149–163. doi: 10.1007/BF00585971. [DOI] [PubMed] [Google Scholar]


