Abstract
Flavaglines are a class of natural products with potent insecticidal and anticancer activities. β-Lactones are a privileged structural motif found in both therapeutic agents and chemical probes. Herein, we report the synthesis, unexpected light-driven di-epimerization, and activity-based protein profiling of a novel rocaglate-derived β-lactone. In addition to in vitro inhibition of the serine hydrolases ABHD10 and ACOT1/2, the most potent β-lactone enantiomer was also found to inhibit these enzymes, as well as the serine peptidases CTSA and SCPEP1, in PC3 cells.
Introduction
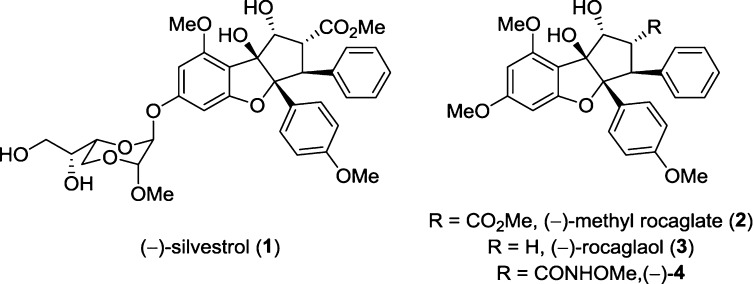
Flavaglines, specifically rocaglate derivatives, are cyclopenta[b]benzofuran natural products isolated from the genus Aglaia that have been shown to be potent anticancer agents.1 Figure 1 shows three natural products: silvestrol (1),2 methyl rocaglate (2),3a,3b and rocaglaol (3)3b as well as the fully synthetic protein translation inhibitor hydroxamate 4.4 Rocaglates have also been shown to have other pharmacological activities including anti-inflammatory, neuroprotective, and cardioprotective effects.1j As methyl rocaglate (2) has a secondary hydroxyl syn-facial and β-substituted to its ester moiety, we considered that a β-lactone structure 5 may be installed on the rocaglate core (Figure 2) and the resulting “remodeled”5 natural product may possess distinct pharmacological activity from other rocaglates and β-lactones.6
Figure 1.
Rocaglates that exhibit potent biological activity.
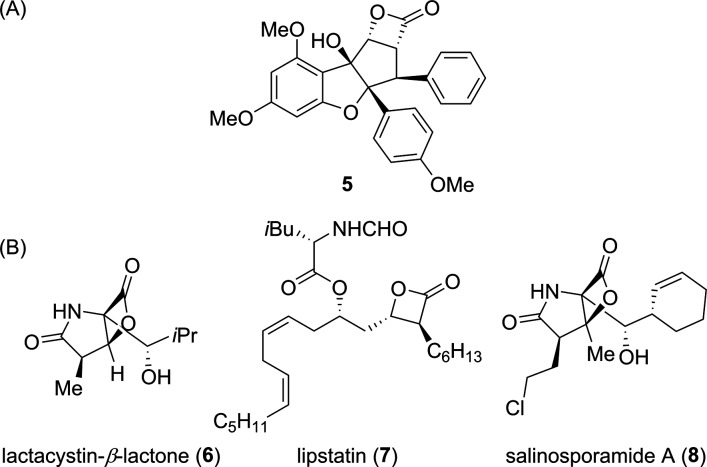
Figure 2.
(A) β-Lactone 5 possessing a rocaglate scaffold. (B) β-Lactone-containing natural products.
β-Lactones are an important class of enzyme inhibitors that have found broad utility as both chemical probes7 and drugs.8 There are numerous bioactive β-lactones found in nature, several of which are widely used in biomedical research, including omuralide (lactacystin-β-lactone, 6)6b and lipstatin9 (or lipostatin, 7, Figure 2). Furthermore, these chemotypes have appeared in the clinical agents salinosporamide A (marizomib or NPI-0052, 8), a phase I clinical candidate for cancer,10 and tetrahydrolipstatin (THL or orlistat), which is currently an approved treatment for obesity.11 The mechanism of biological action of β-lactones is typically through covalent acylation of a functional nucleophilic residue in the active sites of enzymes to produce stable and inactive acyl-enzyme adducts.12 Thus, while β-lactones can target a wide array of protein families,13 enzymes possessing catalytically essential nucleophiles are particularly susceptible to inactivation. Because these chemotypes show a wide range of activities across serine hydrolase class,7,14 we wondered if β-lactones derived from rocaglates would display unique pharmacology and present new opportunities for probe development for serine hydrolases.
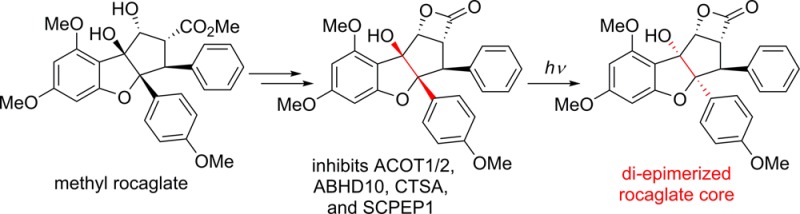
Herein, we report the synthesis of β-lactone 5 and its novel light-driven di-epimerization to afford an unprecedented cyclopenta[b]benzofuran scaffold. To identify targets of β-lactone 5, we profiled this compound against human and mouse proteomes by competitive activity-based protein profiling (ABPP) using fluorophosphonate (FP) probes that show broad-spectrum reactivity with serine hydrolases.15 From this analysis, we identified several serine hydrolase targets of rocaglate β-lactones, including α/β hydrolase domain-containing protein 10 (ABHD10), retinoid-inducible serine carboxypeptidase (SCPEP1), and acyl-coenzyme A thioesterase 1/2 (ACOT1/2), showing that the structural features of rocaglate β-lactones direct its activity to a unique subset of serine hydrolases. Finally, we show that (−)-5, the most active enantiomer, inhibits these enzymes in cells and thus may serve as a valuable probe for exploring their functions in cells. More generally, this work underscores the versatility of β-lactones as a chemotype for serine hydrolase inhibition.
Results and Discussion
Synthesis
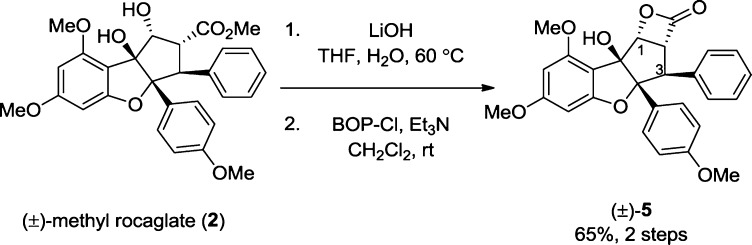
We first evaluated the synthesis of the target rocaglate β-lactone structure. Using bis(2-oxo-3-oxazolidinyl)phosphinic chloride (BOP-Cl)/triethylamine,16 we observed the transformation of rocagloic acid17 derived from (±)-methyl rocaglate (2)18 to β-lactone (±)-5 (Scheme 1). Interestingly, the chemical shifts for H1 and H2 (δ = 5.41 and 4.55 ppm, respectively) were found to be further downfield from their respective shifts observed for methyl rocaglate (2) (5.01 and 3.88 ppm, respectively) consistent with deshielding by the β-lactone. The coupling constants for H1–3 were lower than the respective coupling constants observed for 2 consistent with ring strain of the β-lactone. IR analysis (C=O stretch, 1830 cm–1)6a and high-resolution mass spectrometry supported the proposed structure of β-lactone 5 which was later unambiguously confirmed by X-ray analysis (Figure 3).19
Scheme 1. Synthesis of β-Lactone (±)-5 from (±)-Methyl Rocaglate (2).
Figure 3.
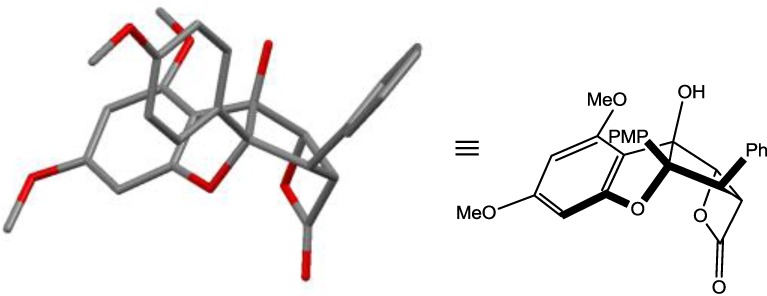
X-ray structure of β-lactone (±)-5. Hydrogens omitted for clarity. PMP = para-methoxyphenyl.
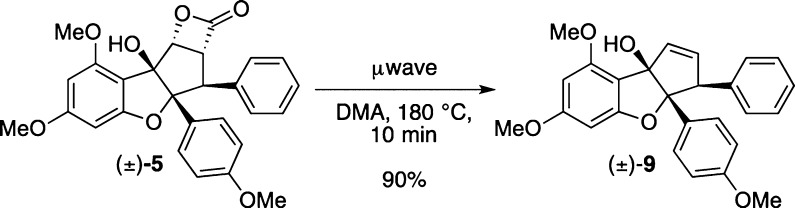
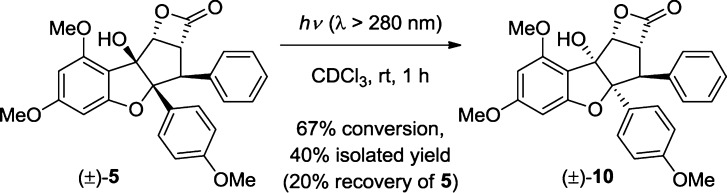
With β-lactone 5 in hand, we next studied its reactivity. We first evaluated thermolysis of β-lactone (±)-5 to facilitate extrusion of carbon dioxide and observed formation of alkene (±)-9 using microwave conditions (Scheme 2).20,21 Additionally, we explored photochemical methods to extrude carbon dioxide.6c,22 When exposed to UV light (>280 nm), β-lactone (±)-5 underwent an unexpected rearrangement to form the corresponding di-epi-β-lactone (±)-10 (Scheme 3). The relative configuration of isomer 10 was unambiguously confirmed through X-ray crystal structure analysis (cf. Figure 4).19 The reaction did not go to complete conversion, and irradiation for more than 4 h led to decomposition. We considered whether 10 was thermodynamically more stable. Indeed, computational analysis of 5 and 10 showed that β-lactone 10 was 7.0 kcal/mol lower in energy.23 This is most likely due to the release of strain from caged structure 5 to the staircase structure of stereoisomer 10. Additionally, the aromatic substituents of 10 are no longer syn.
Scheme 2. Extrusion of CO2 Using Microwave Conditions.
Scheme 3. Photoisomerization of Rocaglate β-lactone (±)-5 to Di-epi Isomer (±)-10.
Figure 4.
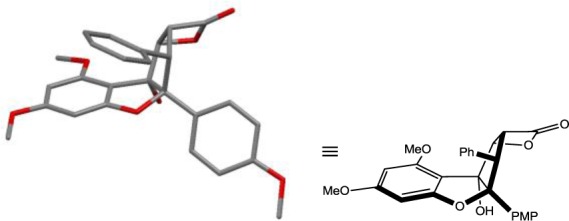
X-ray structure of β-lactone (±)-10. Hydrogens omitted for clarity. PMP = para-methoxyphenyl.
Further experiments were performed to probe the mechanism of the di-epimerization process. We considered whether β-lactone 5 could isomerize to 10 in the presence of Brønsted acid. However, 5 did not undergo the observed rearrangement when subjected to catalytic amounts of camphorsulfonic acid or para-toluenesulfonic acid (p-TsOH) without irradiation with UV light.24 Furthermore, we did not observe a reaction when aqueous HCl was added. Previously, Ullman and Wan studied the photosolvolysis of benzylic alcohols in methanol.25 We considered that 5 may be undergoing a similar transformation as outlined in Figure 5A. A benzylic cation intermediate may be formed from excitation of 5 which can then form an ortho-quinone methide cation.26 The electron-rich para-methoxyphenyl moiety of 5 may stabilize the ortho-quinone methide cation for di-epimerization. Attack by water then gives the di-epimerized product 10. To test this mechanistic hypothesis, we conducted photochemical solvolysis experiments. However, treatment of 5 with methanol (in the presence of heat from the lamp) ring-opened the β-lactone to form methyl rocaglate (2) which further reacted to form multiple products. Use of the mildly acidic solvent 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) (Table 1, entry 1) did not afford isomerization product 10. If the reaction is indeed proceeding through photodehydroxylation, acidic/hydrogen-bonding solvents such as HFIP should facilitate the reaction outlined in Figure 5A.25d Irradiation of rocaglaol (3)27 (cf. Figure 1) with and without solvent additives also led to complex mixtures that we were unable to separate and characterize.
Figure 5.
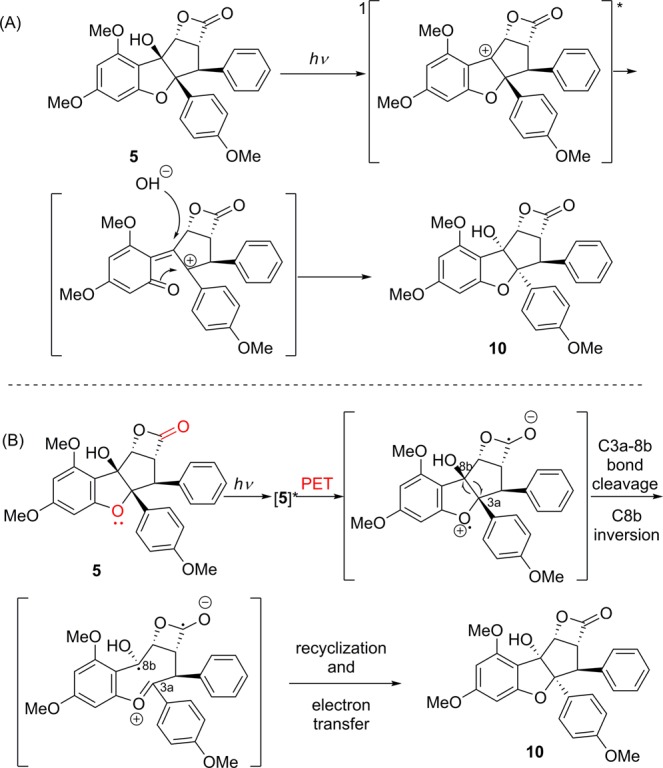
Possible mechanisms of the photochemical diepimerization via (A) singlet cation or (B) PET.
Table 1. Evidence for a PET Mechanism Involved in Transformation of (±)-5 to (±)-10.
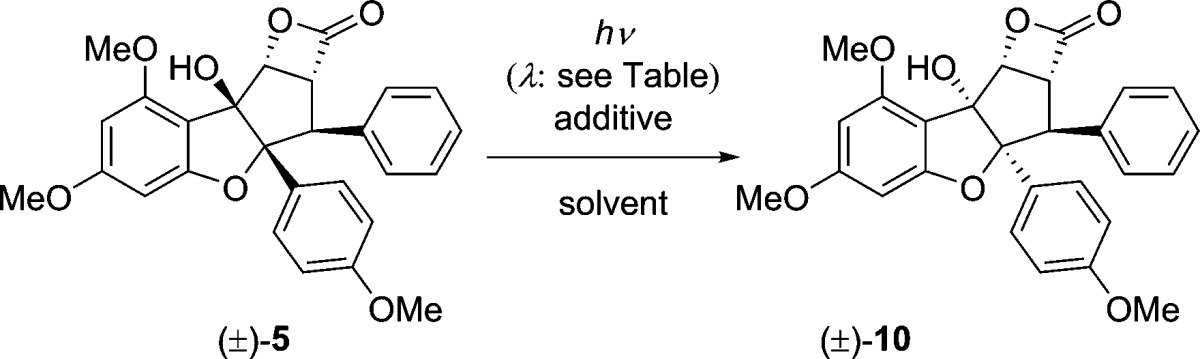
| entry | λ (nm) | additivea | solvent | yield (%)b |
|---|---|---|---|---|
| 1 | >280 | – | HFIP | –c |
| 2 | >280 | – | CDCl3 | 40 |
| 3 | >280 | – | PhCH3 | – |
| 4 | >280 | Ph2CO | PhCH3 | 69 |
| 5 | 315–400 | – | CDCl3 | – |
| 6 | 315–400 | Ph2CO | CDCl3 | 81 |
1 equiv of additive used.
Isolated yield after column chromatography.
Quantitative recovery of starting material.
We also considered an alternative mechanism in which photoexcited 5 could undergo photochemical electron transfer (PET)28 from the benzofuran ring oxygen to the β-lactone carbonyl (Figure 5B). Examination of the X-ray structure of 5 (Figure 3) indicates that the cyclopenta[b]benzofuran oxygen of 5 is 2.9 Å from the β-lactone carbonyl carbon. The resulting radical ion pair can then undergo C3a–8b cleavage, C8b inversion, and subsequent recyclization/electron transfer. By screening different solvents, we found that the reaction does not proceed when irradiated in toluene (Table 1, entry 3). However, addition of benzophenone (1 equiv) resulted in a 69% yield of (±)-10 (Table 1, entry 4). Other sensitizing additives such as 1,4-dicyanonaphthalene and Michler’s ketone did not afford rearranged 10 (λ > 280 nm, toluene used as solvent).28c Selective irradiation of benzophenone also sensitized the transformation when using lower energy light (Table 1, entries 5 and 6). When the electron-accepting sensitizer benzophenone was used, we propose that electron transfer from 5 to triplet-excited benzophenone occurs, thereby generating a radical cation rocaglate species that can undergo di-epimerization. Although we cannot rule out the benzylic cation mechanism (Figure 5A), the reaction is most likely proceeding via a PET mechanism (Figure 5B) since we achieved highest yields of 10 employing benzophenone as sensitizer.
Activity-Based Protein Profiling of β-Lactone 5
With β-lactone (±)-5 in hand, we next assessed its activity across the serine hydrolase class using competitive ABPP, a technique that allows class-wide profiling of serine hydrolase activity in native proteomes. We treated proteomes derived from human cell lines (PC3 and LNCaP) and mouse tissues (brain, liver and testes) with (±)-5 (1.0 μM or 25 μM) or DMSO for 30 min followed by the serine hydrolase-directed activity-based probe fluorophosphonate-rhodamine (FP-Rh).29 FP-Rh-labeled proteomes were then resolved by SDS-PAGE and imaged using a fluorescent gel scanner. This analysis identified multiple serine hydrolase targets of (±)-5, some of which were prominently inhibited at 1.0 μM (Figure 6A). We chose to further analyze the soluble proteome of PC3 cells (a human prostate cancer cell line) in more detail, as (±)-5 inhibited two enzymes in these cells that migrated at ∼30 and ∼45 kDa (red asterisks, Figure 6A). Analyzing (±)-5 over a wider concentration range (Figure 6B) revealed inhibition of the 30 kDa band (IC50 ∼100 nM) with a ∼10 fold selectivity window over other observable serine hydrolases. Notably, these targets were not inhibited by (±)-methyl rocaglate (2) and were less sensitive to inhibition by diastereomeric β-lactone (±)-10 and THL (Supplementary Figure 1).19
Figure 6.
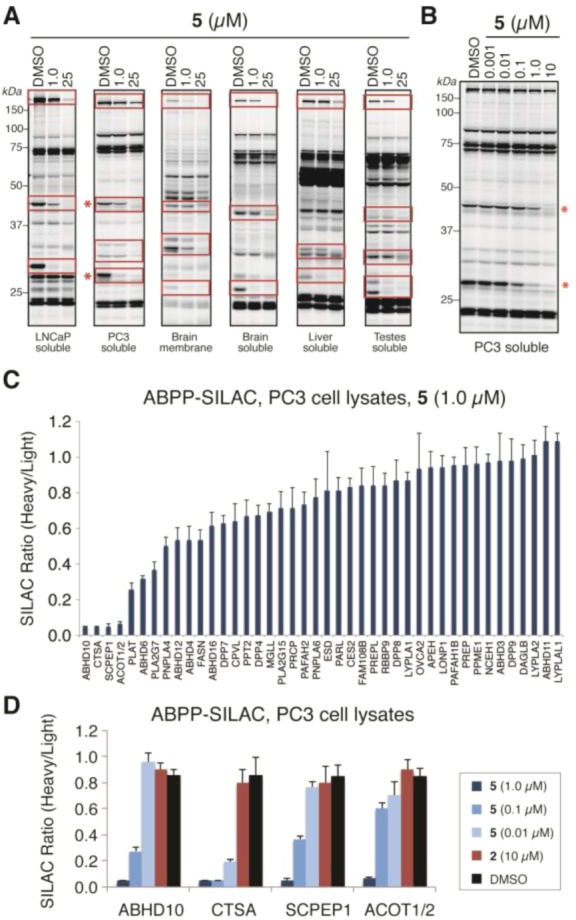
In vitro competitive ABPP of β-lactone (±)-5. (A) Gel-based ABPP of (±)-5 in various human cancer cell and mouse tissue proteomes showing inhibition of multiple serine hydrolases (highlighted in red boxes). Proteomes were treated with DMSO or (±)-5 (1.0 or 25 μM) for 30 min followed by FP-Rh (30 min). (B) Concentration-dependent inhibition of serine hydrolase activities in PC3 cell proteomes treated with DMSO or (±)-5 (0.001–10 μM), showing significant inhibition of serine hydrolase activities migrating at 30 and 45 kDa (red asterisks). (C) ABPP-SILAC analysis of (±)-5 at 1.0 μM (heavy amino acid-labeled proteome) versus DMSO (light amino acid-labeled proteome) in PC3 cell proteomes, revealing inhibition of ABHD10, CTSA, SCPEP1, and ACOT1/2. (D) ABPP-SILAC analysis of (±)-5 (1.0, 0.1, and 0.01 μM), 2 (10 μM), or DMSO (heavy) versus DMSO (light) in PC3 cell proteomes showing dose-dependent inhibition of the four primary targets of (±)-5 observed at 1.0 μM. For C and D, data are presented as the mean ± standard deviations of heavy/light ratios for multiple unique peptides from each serine hydrolase.
We next applied the quantitative mass spectrometry (MS)-based platform ABPP-SILAC30 to identify the targets of (±)-5. PC3 cells grown in media containing isotopically heavy or light amino acids were harvested and lysed. Whole cell lysates derived from the “light” cells were treated with DMSO, whereas the “heavy” cells were treated with (±)-5 (0.01, 0.1, and 1.0 μM), (±)-2, or DMSO. After 30 min, each proteome was treated with a biotinylated FP probe (FP-biotin)29 and then combined allowing selective enrichment, identification, and quantification of serine hydrolase activities by LC/LC-MS/MS analysis. This analysis identified ∼40 serine hydrolases in PC3 proteomes, four of which were inhibited >75% at 1.0 μM of (±)-5: ABHD10, CTSA, SCPEP1, and ACOT1/2 (Figure 6C). Notably, these targets were dose dependently inhibited by (±)-5 but not by methyl ester (±)-2 (Figure 6D and Supplementary Figure 2), indicating that the β-lactone moiety is essential for serine hydrolase inhibition. Based on the predicted molecular weights for each enzyme, relative spectral counts (as an estimate of the relative abundance of serine hydrolases), and concentration-dependent inhibition profiles from both gel- and MS-ABPP experiments, we postulate that the ∼30 and 45 kDa gel bands (Figure 6B) are likely ABHD10 and ACOT1/2, respectively, while CTSA and SCPEP1 did not appear to be adequately resolved for detection by gel-based ABPP (see below). It should be noted that ACOT1 and ACOT2, while distinct enzymes, share very high sequence identity (>90%), precluding their differentiation in our MS-based experiments.
Interestingly, CTSA or cathepsin A, one of the primary targets of (±)-5, is also known to be inhibited by other β-lactones including omuralide (lactacystin-β-lactone, 6) and salinosporamide A (8).14b,31 Inhibitors for ABHD10 have only recently been discovered in competitive ABPP studies of aza-β-lactams.32 That ABHD10 is also sensitive to β-lactones, as noted herein and by List et al.,33 suggests that strained cyclic esters and amides are particularly well-suited for ABHD10 inhibition. SCPEP1 is a poorly characterized secreted carboxypeptidase that appears to be involved in vascular remodeling.34 While lacking selective small-molecule inhibitors, SCPEP1 has recently been shown to be targeted by the β-lactones omuralide and vibralactone.33 We are not aware of any reported inhibitors for ACOT1 or 2. These enzymes appear to be important for lipid homeostasis through the hydrolysis of acyl-CoA species; however, the biological consequences of disrupting ACOT1/2 activity is unknown, warranting the pursuit of pharmacological inhibitors. Notably, ACOT1 and ACOT2 were previously screened against a large panel of carbamates, none of which were found to exhibit inhibitory activity.35
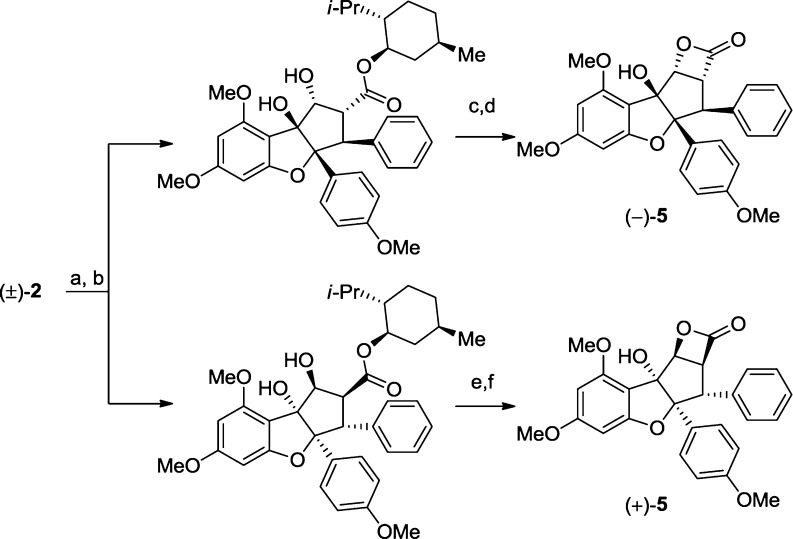
Having identified several serine hydrolase targets of (±)-5, we next wondered if its pure enantiomers would exhibit distinct potency and specificity profiles. Both enantiomers of 5 were accessed via hydrolysis of (±)-methyl rocaglate (2), menthyl ester formation/separation, and subsequent hydrolysis/BOP-Cl coupling (Scheme 4).4 Testing both enantiomers by competitive ABPP revealed that (−)-5 more potently inhibited ABHD10 and ACOT1/2 than (+)-5 (Figure 7A).
Scheme 4. Synthesis of Both Enantiomers of 5.
Conditions: (a) LiOH, THF, H2O, 60 °C; (b) L-menthol, DCC, DMAP, CH2Cl2, rt (60% combined yield, 2 steps); (c) NaOH, DMSO, H2O, 60 °C, 53% yield; (d) BOP-Cl, Et3N, CH2Cl2, rt, 65% yield; (e) NaOH, DMSO, H2O, 60 °C, 55% yield; (f) BOP-Cl, Et3N, CH2Cl2, rt, 65% yield. DCC = N,N-dicyclohexylcarbodiimide; DMAP = N,N-dimethylaminopyridine; BOP-Cl = bis(2-oxo-3-oxazolidinyl)phosphinic chloride; DMSO = dimethylsulfoxide.
Figure 7.
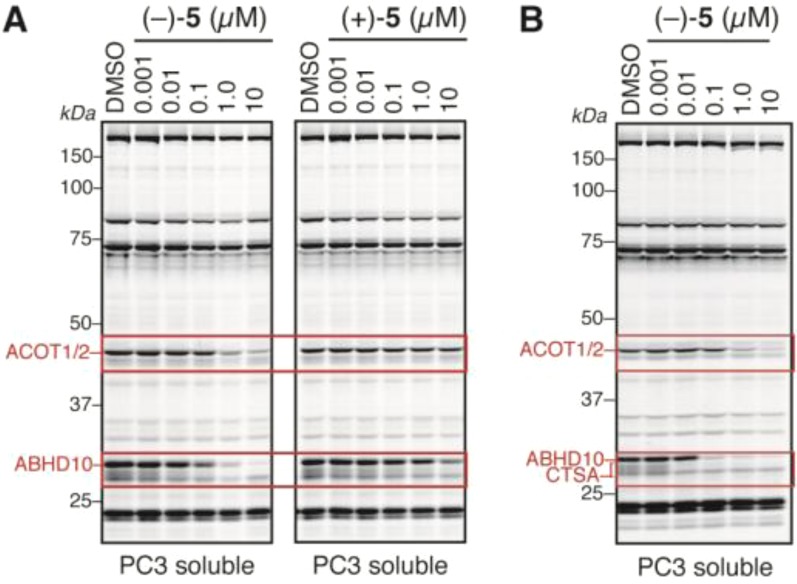
In vitro and in situ competitive ABPP of isolated enantiomers of 5. (A) In vitro gel-based competitive ABPP of pure enantiomers of 5 in PC3 cell proteomes showing that (−)-5 is more potent than (+)-5 for inhibiting ABHD10 and ACOT1/2. (B) In situ gel-based competitive ABPP of PC3 cells treated with DMSO or (−)-5 (0.001–10 μM) for 2 h, showing that (−)-5 retains its activity in cells.
The activity displayed by 5 for poorly characterized enzymes that lack selective inhibitors (or even leads) suggests that this agent, as well as derivatives thereof, could serve as valuable chemical probes. To achieve this goal, however, 5 would need to exhibit inhibitory activity in situ. Accordingly, we next treated PC3 cells with DMSO or increasing concentrations of (−)-5, and after 2 h, the cells were harvested, lysed, and treated with FP-Rh for gel-based ABPP. From this analysis, we observed greater than 90% blockage of ABHD10 activity at 100 nM with greater than 10-fold selectivity over ACOT1/2, which was also inhibited at higher in situ concentrations of 5 (Figure 7B). Notably, a faint band migrating just below ABHD10 was fully competed at only 10 nM, which is likely the active, 32 kDa fragment of CTSA36 given its expected migration by gel37 and inhibitory profile which was consistent with ABPP-SILAC data (>80% inhibition at 10 nM of 5; Figure 6D). These data demonstrate that (−)-5 is highly active as a serine hydrolase inhibitor in cells.
Conclusion
In summary, we have synthesized a novel β-lactone derived from the cyclopenta[b]benzofuran natural product methyl rocaglate. Attempts to decarboxylate the β-lactone led to the discovery of a photochemical di-epimerization producing novel stereoisomers of the rocaglate core which most likely proceeds through PET. Furthermore, this β-lactone rocaglate undergoes clean photochemical di-epimerization, while rocaglate natural products (e.g. methyl rocaglate, rocaglaol) do not. ABPP of the rocaglate β-lactone in complex proteomes and cells revealed an inhibitory profile unique among known β-lactone inhibitors targeting several enzymes including CTSA, SCPEP1, ABHD10, and ACOT1/2. While we speculate that 5 inhibits these enzymes through acylation of the active site serine nucleophile, as has been reported for other β-lactones,12 additional studies will be needed to elucidate the precise mechanism of inhibition. Tagged analogues of 5 allowing direct detection of covalent targets may facilitate these efforts as well as aid identification of potential nonserine hydrolase targets, which have not been addressed herein. Nevertheless, these findings suggest that 5 and similar compounds may serve as valuable probes for exploring the function of specific serine hydrolases in cells.
Acknowledgments
We thank the National Institutes of Health (R01 GM073855, J.A.P., Jr.; DA033760, B.F.C.; and DA032541, M.J.N.) and the Skaggs Institute for Chemical Biology for research support. This work was supported in part by a grant from Abide Therapeutics. We thank Dr. Jeffrey Bacon (Boston University) for X-ray crystal structure analyses and Professor John Snyder (Boston University), Professors Kevin Moeller (Washington University at St. Louis) and David Nicewicz (University of North Carolina at Chapel Hill), Dr. Kiel Lazarski, and Mr. Steven Stone (Boston University) for extremely helpful and stimulating discussions.
Supporting Information Available
Experimental procedures and characterization data for all new compounds described herein, including CIF files for compounds 5 and 10. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- For select reviews of the biology and syntheses of flavaglines:; a Ebada S. S.; Lajkiewicz N.; Porco J. A. Jr.; Li-Weber M.; Proksch P. Prog. Chem. Org. Nat. Prod. 2011, 94, 1–58. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ribeiro N.; Thuaud F.; Nebigil C.; Désaubry L. Bioorg. Med. Chem. 2012, 20, 1857–1864. [DOI] [PubMed] [Google Scholar]; For recent articles on the synthesis, isolation and biology of flavaglines and derivatives:; c Lajkiewicz N. J.; Roche S. P.; Gerard B.; Porco J. A. Jr. J. Am. Chem. Soc. 2012, 134, 13108–13113. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Liu T.; Nair S. J.; Lescarbeau A.; Belani J.; Peluso S.; Conley J.; Tillotson B.; O’Hearn P.; Smith S.; Slocum K.; West K.; Helble J.; Douglas M.; Bahadoor A.; Ali J.; McGovern K.; Fritz C.; Palombella V. J.; Wylie A.; Castro A. C.; Tremblay M. R. J. Med. Chem. 2012, 55, 8859–8878. [DOI] [PubMed] [Google Scholar]; e Ribeiro N.; Thuaud F.; Bernard Y.; Gaiddon C.; Cresteil T.; Hild A.; Hirsch E. C.; Michel P. P.; Nebigil C. G.; Désaubry L. J. Med. Chem. 2012, 55, 10064–10073. [DOI] [PubMed] [Google Scholar]; f Chambers J. M.; Huang D. C. S.; Lindqvist L. M.; Savage G. P.; White J. M.; Rizzacasa M. A. J. Nat. Prod. 2012, 75, 1500–1504. [DOI] [PubMed] [Google Scholar]; g Pan L.; Acuña U. M.; Li J.; Jena N.; Ninh T. N.; Pannell C. M.; Chai H.; Fuchs J. R.; Carcache de Blanco E. J.; Soejarto D. D.; Kinghorn A. D. J. Nat. Prod. 2013, 76, 394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Chambers J. M.; Lindqvist L. M.; Webb A.; Huang D. C. S.; Savage G. P.; Rizzacasa M. A. Org. Lett. 2013, 15, 1406–1409. [DOI] [PubMed] [Google Scholar]; i Santagata S.; Mendillo M. L.; Tang Y.-C.; Subramanian A.; Perley C. C.; Roche S. P.; Wong B.; Narayan R.; Kwon H.; Koeva M.; Amon A.; Golub T. R.; Porco J. A. Jr.; Whitesell L.; Lindquist S. Science 2013, 341, 242–243. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Basmadjian C.; Thuaud F.; Ribeiro N.; Désaubry L. Future Med. Chem. 2013, 5, 2185–2197. [DOI] [PubMed] [Google Scholar]
- Hwang B. Y.; Su B.-N.; Chai H.; Mi Q.; Kardono L. B. S.; Afriastini J. J.; Riswan S.; Santarsiero B. D.; Mesecar A. D.; Wild R.; Fairchild C. R.; Vite G. D.; Rose W. C.; Farnsworth N. R.; Cordell G. A.; Pezzuto J. M.; Swanson S. M.; Kinghorn A. D. J. Org. Chem. 2004, 69, 3350–3358. [DOI] [PubMed] [Google Scholar]
- a Ko F.-N.; Wu T.-S.; Liou M.-J.; Huang T.-F.; Teng C.-M. Eur. J. Pharmacol. 1992, 218, 129–135. [DOI] [PubMed] [Google Scholar]; b Ishibashi F.; Satasook C.; Isman M. B.; Towers G. H. N. Phytochemistry 1993, 32, 307–310. [Google Scholar]
- Rodrigo C. M.; Cencic R.; Roche S. P.; Pelletier J.; Porco J. A. Jr. J. Med. Chem. 2012, 55, 558–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Szpilman A. M.; Carreira E. M. Angew. Chem., Int. Ed. 2010, 49, 9592–9628. [DOI] [PubMed] [Google Scholar]; b Balthaser B. R.; Maloney M. C.; Beeler A. B.; Porco J. A. Jr.; Snyder J. K. Nat. Chem. 2011, 3, 969–973. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Kawamura T.; Matsubara K.; Otaka H.; Tashiro E.; Shindo K.; Yanagita R. C.; Irie K.; Imoto M. Bioorg. Med. Chem. 2011, 19, 4377–4385. [DOI] [PubMed] [Google Scholar]; d Li J.; Cisar J. S.; Zhou C.-Y.; Vera B.; Williams H.; Rodríguez A. D.; Cravatt B. F.; Romo D. Nat. Chem. 2013, 5, 510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Grenning A. J.; Snyder J. K.; Porco J. A. Jr.. Org. Lett. 2014, 16, article ASAP. [DOI] [PMC free article] [PubMed]
- For select references and reviews on β-lactones:; a Pommier A.; Pons J.-M. Synthesis 1993, 441–459. [Google Scholar]; b Fenteany G.; Standaert R. F.; Reichard G. A.; Corey E. J.; Schreiber S. L. Proc. Natl. Acad. Sci. U.S.A. 1994, 91, 3358–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Wang Y.; Tennyson R. L.; Romo D. Heterocycles 2004, 64, 605–658. [Google Scholar]; d Nguyen H.; Ma G.; Gladysheva T.; Fremgen T.; Romo D. J. Org. Chem. 2010, 76, 2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Böttcher T.; Sieber S. A. Med. Chem. Commun. 2012, 3, 408–417. [Google Scholar]
- Bottcher T.; Sieber S. A. Angew. Chem., Int. Ed. 2008, 47, 4600–4603. [DOI] [PubMed] [Google Scholar]
- Sjöström L.; Rissanen A.; Andersen T.; Boldrin M.; Golay A.; Koppeschaar H. P. F.; Krempf M. Lancet 1998, 352, 167–172. [DOI] [PubMed] [Google Scholar]
- Isolation, biological evaluation, and structure elucidation of lipstatin:; a Weibel E. K.; Hadvary P.; Hochuli E.; Kupfer E.; Lengsfeld H. J. Antibiot. 1987, 40, 1081–1085. [DOI] [PubMed] [Google Scholar]; b Hochuli E.; Kupfer E.; Maurer R.; Meister W.; Mercadal Y.; Schmidt K. J. Antibiot. 1987, 40, 1086–1091. [DOI] [PubMed] [Google Scholar]
- Isolation and anticancer drug development of salinosporamide A:; a Feling R. H.; Buchanan G. O.; Mincer T. J.; Kauffman C. A.; Jensen P. R.; Fenical W. Angew. Chem., Int. Ed. 2003, 42, 355–357. [DOI] [PubMed] [Google Scholar]; b Fenical W.; Jensen P. R.; Palladino M. A.; Lam K. S.; Lloyd G. K.; Potts B. C. Bioorg. Med. Chem. 2009, 17, 2175–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Potts B. C.; Albitar M. X.; Anderson K. C.; Baritaki S.; Berkers C.; Bonavida B.; Chandra J.; Chauhan D.; Cusack J. C.; Fenical W.; Ghobrial I. M.; Groll M.; Jensen P. R.; Lam K. S.; Lloyd G. K.; McBride W.; McConkey D. J.; Miller C. P.; Neuteboom S. T.; Oki Y.; Ovaa H.; Pajonk F.; Richardson P. G.; Roccaro A. M.; Sloss C. M.; Spear M. A.; Valashi E.; Younes A.; Palladino M. A. Curr. Cancer Drug 2011, 11, 254–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For reviews on tetrahydrolipstatin:; a McNeely W.; Benfield P. Drugs 1998, 56, 241–249. [DOI] [PubMed] [Google Scholar]; b Padwal R. S.; Majumdar S. R. Lancet 2007, 369, 71–77. [DOI] [PubMed] [Google Scholar]
- a Guerciolini R. Int. J. Obes. Relat. Metab. Disord. 1997, 21Suppl 3S12–S23. [PubMed] [Google Scholar]; b Groll M.; Huber R.; Potts B. C. M. J. Am. Chem. Soc. 2006, 128, 5136–5141. [DOI] [PubMed] [Google Scholar]; c Zeiler E.; List A.; Alte F.; Gersch M.; Wachtel R.; Poreba M.; Drag M.; Groll M.; Sieber S. A. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 11302–11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Böttcher T.; Sieber S. Angew. Chem., Int. Ed. 2007, 47, 4600–3. [DOI] [PubMed] [Google Scholar]; b Yang P.-Y.; Liu K.; Ngai M. H.; Lear M. J.; Wenk M. R.; Yao S. Q. J. Am. Chem. Soc. 2010, 132, 656–666. [DOI] [PubMed] [Google Scholar]
- a Hoover H. S.; Blankman J. L.; Niessen S.; Cravatt B. F. Bioorg. Med. Chem. Lett. 2008, 18, 5838–5841. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Chauhan D.; Catley L.; Li G.; Podar K.; Hideshima T.; Velankar M.; Mitsiades C.; Mitsiades N.; Yasui H.; Letai A.; Ovaa H.; Berkers C.; Nicholson B.; Chao T.-H.; Neuteboom S. T. C.; Richardson P.; Palladino M. A.; Anderson K. C. Cancer Cell 2005, 8, 407–419. [DOI] [PubMed] [Google Scholar]
- Leung D.; Hardouin C.; Boger D. L.; Cravatt B. F. Nat. Biotechnol. 2003, 21, 687–691. [DOI] [PubMed] [Google Scholar]
- Corey E. J.; Reichard G. A.; Kania R. Tetrahedron Lett. 1993, 34, 6977–6980. [Google Scholar]
- Wang S.-K.; Cheng Y.-J.; Duh C.-Y. J. Nat. Prod. 2001, 64, 92–94. [DOI] [PubMed] [Google Scholar]
- Procedures to synthesize methyl rocaglate (2) were done using optimized procedures from:Roche S. P.; Cencic R.; Pelletier J.; Porco J. A. Jr. Angew. Chem., Int. Ed. 2010, 49, 6533–6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See SI for complete experimental details.
- Mulzer J.; Zippel M. J. Chem. Soc., Chem. Commun. 1981, 891–892. [Google Scholar]
- For the synthesis of a similar compound, 1,2-anhydro methyl rocaglate:Magnus P.; Stent M. A. H. Org. Lett. 2005, 7, 3853–3855. [DOI] [PubMed] [Google Scholar]
- Schnapp K. A.; Motz P. L.; Stoeckel S. M.; Wilson R. M.; Bauer J. A. K.; Bohne C. Tetrahedron Lett. 1996, 37, 2317–2320. [Google Scholar]
- Computational analysis was performed using Spartan ’10 (Wavefunction, Inc., Irvine, CA). Single point energies were computed for the X-ray structures of 5 and 10 using the B3LYP/6-31G* level of theory.
- Bringmann G.; Mühlbacher J.; Messer K.; Dreyer M.; Ebel R.; Nugroho B. W.; Wray V.; Proksch P. J. Nat. Prod. 2003, 66, 80–85. [DOI] [PubMed] [Google Scholar]
- a Lin C.-I.; Singh P.; Ullman E. F. J. Am. Chem. Soc. 1976, 98, 6711–6712. [Google Scholar]; b Lin C.-I.; Singh P.; Ullman E. F. J. Am. Chem. Soc. 1976, 98, 7848–7850. [Google Scholar]; c Photodehydroxylation of benzylic alcohols: Irie M. J. Am. Chem. Soc. 1983, 105, 2078–2079. [Google Scholar]; d Wan P.; Chak B. J. Chem. Soc. Perkin Trans. II 1986, 1751–1756. [Google Scholar]
- a Van De Water R. W.; Pettus T. R. R. Tetrahedron 2002, 58, 5367–5405. [Google Scholar]; b Veljković J.; Uzelac L.; Molčanov K.; Mlinarić-Majerski K.; Kralj M.; Wan P.; Basarić N. J. Org. Chem. 2012, 77, 4596–4610. [DOI] [PubMed] [Google Scholar]
- Gerard B.; Sangji S.; O’Leary D. J.; Porco J. A. Jr. J. Am. Chem. Soc. 2006, 128, 7754–7755. [DOI] [PubMed] [Google Scholar]
- For PET of 1,3,5-trimethoxybenzene to generate a radical cation:; a Cai X.; Sakamoto M.; Fujitsuka M.; Majima T. J. Phys. Chem. A 2007, 111, 1788–1791. [DOI] [PubMed] [Google Scholar]; b Cai X.; Fujitsuka M.; Majima T. J. Phys. Chem. A 2007, 111, 4743–4747. [DOI] [PubMed] [Google Scholar]; For a review on PET:; c Hoffmann N. J. Photochem. Photobiol. C 2008, 9, 43–60. [Google Scholar]; d Hoffmann N. Chem. Rev. 2008, 108, 1052–1103. [DOI] [PubMed] [Google Scholar]
- Patricelli M. P.; Giang D. K.; Stamp L. M.; Burbaum J. J. Proteomics 2001, 1, 1067–1071. [DOI] [PubMed] [Google Scholar]
- a Adibekian A.; Martin B. R.; Wang C.; Hsu K.-L.; Bachovchin D. A.; Niessen S.; Hoover H.; Cravatt B. F. Nat. Chem. Biol. 2011, 7, 469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Bachovchin D. A.; Mohr J. T.; Speers A. E.; Wang C.; Berlin J. M.; Spicer T. P.; Fernandez-Vega V.; Chase P.; Hodder P. S.; Schürer S. C.; Nomura D. K.; Rosen H.; Fu G. C.; Cravatt B. F. Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 6811–6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowska H.; Wojcik C.; Omura S.; Worowski K. Biochem. Biophys. Res. Commun. 1997, 234, 729–732. [DOI] [PubMed] [Google Scholar]
- a Berlin J. M.; Fu G. C. Angew. Chem., Int. Ed. 2008, 47, 7048–7050. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zuhl A. M.; Mohr J. T.; Bachovchin D. A.; Niessen S.; Hsu K.-L.; Berlin J. M.; Dochnahl M.; López–Alberca M. P.; Fu G. C.; Cravatt B. F. J. Am. Chem. Soc. 2012, 134, 5068–5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- List A.; Zeiler E.; Gallastegui N.; Rusch M.; Hedberg C.; Sieber S. A.; Groll M. Angew. Chem., Int. Ed. 2013, 53, 571–574. [DOI] [PubMed] [Google Scholar]
- Lee T.-H.; Chen J.; Miano J. M. Circ. Res. 2009, 105, 271–278. [DOI] [PubMed] [Google Scholar]
- Bachovchin D. A.; Ji T.; Li W.; Simon G. M.; Blankman J. L.; Adibekian A.; Hoover H.; Niessen S.; Cravatt B. F. Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 20941–20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galjart N. J.; Gillemans N.; Harris A.; van der Horst G. T.; Verheijen F. W.; Galjaard H.; d’Azzo A. Cell 1988, 54, 755–764. [DOI] [PubMed] [Google Scholar]
- Jessani N.; Liu Y.; Humphrey M.; Cravatt B. F. Proc. Natl. Acad. Sci. U.S.A. 2002, 99, 10335–10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.