Abstract
Evasion of apoptosis is a hallmark of cancer, and reversing this process by inhibition of survival signaling pathways is a potential therapeutic strategy. Phosphoinositide 3-kinase (PI3K) signaling can promote cell survival and is upregulated in solid tumor types, including colorectal cancer (CRC), although these effects are context dependent. The role of PI3K in tumorigenesis combined with their amenability to specific inhibition makes them attractive drug targets. However, we observed that inhibition of PI3K in HCT116, DLD-1, and SW620 CRC cells did not induce apoptotic cell death. Moreover, these cells were relatively resistant to the Bcl-2 homology domain 3 (BH3) mimetic ABT-737, which directly targets the Bcl-2 family of apoptosis regulators. To test the hypothesis that PI3K inhibition lowers the apoptotic threshold without causing apoptosis per se, PI3K inhibitors were combined with ABT-737. PI3K inhibition enhanced ABT-737-induced apoptosis by 2.3- to 4.5-fold and reduced expression levels of MCL-1, the resistance biomarker for ABT-737. PI3K inhibition enhanced ABT-737-induced apoptosis a further 1.4- to 2.4-fold in CRC cells with small interfering RNA-depleted MCL-1, indicative of additional sensitizing mechanisms. The observation that ABT-737-induced apoptosis was unaffected by inhibition of PI3K downstream effectors AKT and mTOR, implicated a novel PI3K-dependant pathway. To elucidate this, an RNA interference (RNAi) screen of potential downstream effectors of PI3K signaling was conducted, which demonstrated that knockdown of the TEC kinase BMX sensitized to ABT-737. This suggests that BMX is an antiapoptotic downstream effector of PI3K, independent of AKT.
Introduction
Single-agent treatments rarely prove sufficient for cancer cure. This is in part due to a variety of innate or acquired, drug-specific, or pleiotropic drug resistance mechanism(s), one of which is suppression of drug-induced cell death. Consequently, there is considerable motivation to overcome drug resistance mechanisms by identifying rational combinations of molecular targeted drugs. To aid this, the US Food and Drug Administration (FDA) is considering early-phase drug combination trials without the necessity for prior single-agent approval [1]. Historically, choice of drug combinations is predicated on nonoverlapping drug toxicities; however, the concept of targeting multiple different hallmarks of cancer is an emerging approach [2]. Two such hallmarks are sustained inappropriate proliferative signaling and suppression of apoptotic cell death [2]. Molecular features that contribute to these hallmarks in several human tumors, including colorectal cancer (CRC), are aberrations in the phosphoinositide 3-kinase (PI3K) signaling pathway and up-regulation of antiapoptotic Bcl-2 family proteins. This study examines the combined effect of inhibiting PI3K signaling and interrupting the protein-protein interactions between proapoptotic and antiapoptotic members of the Bcl-2 family in CRC cells.
PI3K phosphorylates the 3-hydroxy group of phosphatidylinositol lipid rings to generate a secondary messenger that is implicated in many intracellular signaling pathways. The most studied are the class I PI3Ks, which phosphorylate phosphatidylinositol-4,5-bisphosphate generating phosphatidylinositol-3,4,5-triphosphate [PtdIns(3,4,5)3] [3]. PtdIns(3,4,5)3 is a docking site for a number of proteins that contain PtdIns(3,4,5)3-binding motifs such as pleckstrin homology (PH) domains, frequently leading to activation of the docked protein. The best characterized effectors of the pathway are phosphoinositide-dependent kinase 1 (PDK1) and AKT (AKA protein kinase B). PDK1 and AKT bind to PtdIns(3,4,5)3, allowing PDK1 to phosphorylate and activate AKT [4]. Activation of AKT has multiple cell fate outcomes including increased cell survival, sustained cell proliferation, and enhanced cell migration, all of which have potential to promote oncogenesis [5]. Aberrant PI3K signaling is implicated in many cancer types. For example, loss of the PI3K antagonistic phosphatase PTEN and activating mutations in PIK3CA, the gene encoding the catalytic PI3K subunit p110α, are among the most common genetic aberrations in cancer [6]. Consequently, the PI3K signaling pathway is a major focus of drug discovery programs, with multiple small-molecule inhibitors targeting PI3K, AKT, and other PI3K pathway components undergoing clinical trials [6]. Multiple lines of preclinical evidence suggest that PI3K signaling acts to suppress apoptosis through mechanisms including the modulation of Bcl-2 family proteins that control the release of potent apoptogens from mitochondria [5]. However, despite this body of evidence, apoptosis is not induced in many cancer cell lines after specific inhibition of PI3K pathway signaling [7,8]. Furthermore, emerging evidence shows that, whereas in some cell types, combining PI3K inhibition with conventional chemotherapeutic agents induces apoptosis [9,10], this is not the case with CRC cells [7], a disease where aberrant PI3K activation is common.
Drug development efforts to disrupt interactions between proapoptotic and antiapoptotic proteins of the Bcl-2 family yielded the Bcl-2 homology domain 3 (BH3) mimetic class of drugs [11]. The “poster-child” BH3 mimetic ABT-737 and its related clinical candidate navitoclax readily induce apoptosis in small cell lung cancer in vitro and in vivo as a single agent [12] and kill lymphoma cell lines and primary lymphoma cells ex vivo [12,13], and navitoclax has demonstrated promising results in a phase I clinical trial in patients with chronic lymphocytic leukemia [14]. However, in several cancer cell types, including CRC, ABT-737 treatment alone does not induce apoptosis at clinically relevant concentrations [15]. In a broad range of cancer cell types, ABT-737 acts synergistically with a variety of conventional and novel chemotherapeutic agents [16], including agents that target the PI3K pathway [17,18]. This suggests that a lowering of the apoptotic threshold by ABT-737 facilitates the coupling of druginduced damage and/or the interruption of survival signaling events to the commitment to apoptotic cell death. Therefore, the hypothesis tested in this study was that PI3K pathway ablation using small-molecule inhibitors could “prime” CRC cells for apoptosis but that cell death would only be realized if the actions of antiapoptotic Bcl-2 family proteins were negated by a BH3 mimetic.
Materials and Methods
Cell Culture and Drugs
HCT116, DLD-1 [American Type Culture Collection (ATCC), Manassas, VA], and isogenic pairs of HCT116 and DLD-1 expressing only wild-type or mutant PIK3CA (a kind gift from B. Vogelstein) were cultured in McCoy's 5A media (Life Technologies, Inc, Paisley, United Kingdom) supplemented with 10% FBS (BioWest, Nuaillé, France). SW620 (ATCC) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% FBS and glutamine (Life Technologies, Inc). All cells weremaintained in a humidified atmosphere at 37°C and 5% CO2. Cell lines were authenticated using the AmpFlSTR system (Applied Biosystems, Paisley, United Kingdom) during the study. ABT-737 (a kind gift from AbbVie, Chicago, IL), PI-103, rapamycin, Akti1/2, KU-0063794 (Merck, Nottingham, United Kingdom), GDC-0941, MK-2206, and PCI-32765 (Selleck Chemicals, Houston, TX) were all dissolved to 10 mM in DMSO (Sigma, Dorset, United Kingdom) and stored as single use aliquots at -20°C/-80°C (Figure W1).
Concentration Response
Cells were seeded into 96-well plates. After 24 hours, cells were treated with the indicated concentration of drug(s) and cultured for a further 72 hours in the presence of drug(s). Plates were stained with sulforhodamine B (SRB) and processed as previously described [7] to give an indication of cellular biomass. To determine logGI50, log drug concentration was plotted against raw absorbance, and nonlinear curve fit analysis was performed (GraphPad Prism; GraphPad Software, La Jolla, CA). Statistical analysis was carried out on three independent logGI50 readings and transformed to growth inhibition 50 (GI50) for presentation. For display purposes only, drug concentration (log scale) has been plotted against normalized absorbance.
Western Blot Analysis
Cell lysis and Western blot analysis were carried out as previously described [7]. The following primary antibodies were used: rabbit anti-pS473AKT (No. 4058), rabbit anti-AKT (No. 9297), rabbit anti-pT246 40-kDa proline-rich AKT substrate (PRAS40) (No. 2997), rabbit anti-PRAS40 (No. 2691), pS240/244S6 (No. 4838), rabbit anti-S6 (No. 2217), rabbit anti-cleaved caspase 3 (No. 9661), rabbit *P (No. 9542), rabbit anti-Bax (No. 2774; all from Cell Signaling Technology, Danvers, MA), mouse anti-Bcl-2 (M0887; Dako, Glostrup, Denmark), rabbit anti-BCL-XL (No. 610211; Becton Dickinson, Oxford, United Kingdom), mouse anti-human MCL-1 (No. 559027; Becton Dickenson), rabbit anti-MCL-1 (sc819; Santa Cruz Biotechnology, Inc, Dallas, TX), rabbit anti-Bad (AF819; R&D Systems, Minneapolis, MN), rabbit anti-Bim (No. 202000; Merck), mouse anti-Bak (AM03; Merck), mouse anti-α-tubulin (CP06; Merck), and mouse anti-GAPDH (G9545; Sigma).
Measurement of Apoptosis
Annexin V/7-aminoactinomycin D (7AAD) flow cytometry was performed as previously described [7]. For assessment of Bak conformational change, cells were cultured in a 96-well plate and treated with the indicated drug(s) for 24 hours. Cells were fixed with 1% formaldehyde and sent to Imagen Biotech (Cheshire, United Kingdom) where immunofluorescent staining for conformationally changed Bak and high-content analysis were carried out using proprietary protocols using Bak conformation-specific antibodies. Realtime assessment of cells with activated Caspase 3/7 was carried out using the CellPlayer apoptosis Caspase 3/7 reagent (Essen BioScience, Ann Arbor, MI) following manufacturer's recommendations. Cells were placed in an IncuCyte (Essen BioScience) and imaged every 2 hours. The number of fluorescent cells per field of view was determined using IncuCyte software (Essen BioScience) following manufacturer's recommendations.
RNA Interference (RNAi)
siRNA SMARTpools or individual oligos (Thermo Scientific, Leicestershire, United Kingdom) were transfected into SW620 or HCT116 cells using DharmaFECT 2 (Thermo Scientific) according to manufacturer's instructions. For the small interfering RNA (siRNA) library screen, cells were reverse transfected with 5 pmol of siRNA in 6 wells of a 96-well plate per SMARTpool and left for 48 hours, 3 wells were treated with 4 µM ABT-737, and 3 wells were treated with DMSO equivalent. For other siRNA experiments, siRNA were transfected in six-well plates, reseeded into appropriate culture vessels 24 hours later, and drug treated after another 24 hours. Calculation of robust z score is described in Supplemental Materials and Methods section.
Real-Time Quantitative Polymerase Chain Reaction
Quantitative polymerase chain reaction (qPCR) was carried out as previously described [19]. Assays for bone marrow tyrosine kinase gene in chromosome X protein (BMX), SOS1, and SGK1 were designed with the Roche (West Sussex, United Kingdom) Universal ProbeLibrary Assay Design Center.
Statistical Analysis
Unpaired, two-tailed t tests were performed in Excel (Microsoft, Redmond, WA) to determine significance. P < .05 was considered significant.
Results
PI-103 Sensitized CRC Cell Lines to ABT-737
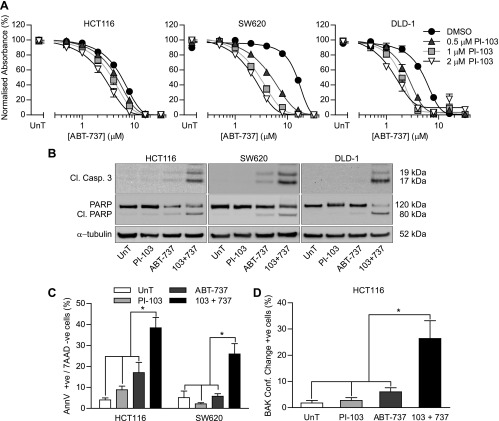
The purpose of this study was to investigate the effect of combining ABT-737 with PI3K pathway inhibitors in CRC cell lines. The lines used were HCT116 and DLD-1, which carry oncogenic activating mutations in PIK3CA, and SW620 cells that are wild type for PIK3CA [20]. All three cell lines also harbor mutant KRAS (www.sanger.ac.uk/genetics/CGP/cosmic/), which can activate the PI3K signaling pathway [4]. All cell lines were responsive to the PI3K/mammalian target of rapamycin (mTOR) inhibitor PI-103 in the SRB assay, which measures cellular biomass, with GI50 values of 288 nM in HCT116 [95% confidence interval (CI) = 255–325 nM], 184 nM in DLD-1 (95% CI = 132–256 nM), and 636 nM in SW620 cells (95% CI = 508–797 nM) (Figure W2A). The phosphorylation of PI3K and/or mTOR effectors AKT, PRAS40, and S6 was inhibited by PI-103 in all cell lines (Figure W2B), confirming that PI-103 inhibited PI3K and mTOR. To determine whether PI-103 treatment affected CRC cell sensitivity to ABT-737, HCT116, SW620, and DLD-1 cells were treated concomitantly with PI-103 and/or ABT-737 (Figure 1A and Table 1). All cell lines exhibited a concentration-dependent response to ABT-737 alone, and the ABT-737 GI50 was reduced significantly in a concentration-dependent manner by PI-103. This is consistent with PI3K inhibition sensitizing CRC cells to ABT-737. The observation that PI-103 increased the sensitivity toward ABT-737 irrespective of PIK3CA mutation status suggests that PIK3CA mutation is not essential for this effect.
Figure 1.
PI-103 sensitized CRC Cell Lines to ABT-737-induced apoptosis. (A) Cells were exposed to DMSO or the indicated concentration of PI-103 and the indicated concentration of ABT-737 for 3 days. Cells were fixed and stained with SRB, and the absorbance relative to untreated (UnT) cells was determined relative to DMSO or PI-103 only-treated cells as appropriate for individual concentration response curves. (B-D) Cells were treated with the indicated combinations of 4 µMABT-737 (A and B) or 8 µMABT-737 (C) and 2 µMPI-103 for 24 hours. (A) The level of cleaved caspase 3, full-length PARP, cleaved PARP, and β-tubulin was assessed by Western blot analysis. Results are representative of three independent experiments. (B) Cells were stained with allophycocyanin (APC)-conjugated annexin V and 7AAD, and the percentage of annexin V-positive/7AAD-negative cells was determined by flow cytometry. (C) Cells were fixed and stained for conformationally changed BAK, and the percentage of positive cells was determined by immunofluorescence. All graphs represent the means of three independent experiments carried out in triplicate (A) or duplicate (C and D) ± SEM. *P < .05 according to two-tailed unpaired t test.
Table 1.
Effect of PI-103 Treatment on ABT-737 GI50.
| Cell Line | Treatment | ABT-737 GI50 (µM ± 95% CI) | Significance* |
| HCT116 | DMSO | 5.80 (5.16–6.52) | |
| 0.5 µM PI-103 | 4.70 (4.30–5.15) | 0.0495 | |
| 1 µM PI-103 | 4.39 (3.78–5.09) | 0.0439 | |
| 2 µM PI-103 | 3.11 (2.26–4.29) | 0.0231 | |
| SW620 | DMSO | 15.5 (14.2–16.8) | |
| 0.5 µM PI-103 | 5.07 (3.75–6.84) | 0.0022 | |
| 1 µM PI-103 | 2.88 (2.14–3.88) | 0.0004 | |
| 2 µM PI-103 | 2.22 (1.86–2.66) | <0.0001 | |
| DLD-1 | DMSO | 5.35 (5.15–5.55) | |
| 0.5 µM PI-103 | 2.44 (2.19–2.71) | 0.0002 | |
| 1 µM PI-103 | 1.82 (1.60–2.07) | 0.0001 | |
| 2 µM PI-103 | 1.36 (0.99–1.87) | 0.0011 |
Two-tailed unpaired t test versus DMSO-treated GI50 for same cell line.
PI-103 Enhanced ABT-737-Induced Apoptosis
The effect of PI-103 on the levels of ABT-737-induced apoptosis was determined by several methods. First, the effect of ABT-737 and/or PI-103 on caspase 3 and PARP cleavage was determined by Western blot analysis (Figure 1B). ABT-737 alone induced some caspase 3 and PARP cleavage, whereas PI-103-only treatment had no effect. Combining the two agents increased caspase 3 and PARP cleavage, suggesting that the combination caused more apoptosis than either agent alone. The effect of ABT-737 and PI-103 on the externalization of phosphatidylserine, another classic biomarker of apoptosis, was determined by flow cytometry in HCT116 and SW620 cells (Figure 1C). In both cases, neither PI-103 nor ABT-737 alone caused a significant level of apoptosis compared to untreated cells, whereas combining ABT-737 and PI-103 gave significantly more apoptosis than any other condition.
PI3K signaling can promote numerous antiapoptotic mechanisms that act upstream or downstream of cytochrome c release from mitochondria, for example, Bad sequestration and caspase 9 inhibition, respectively [21,22]. To determine whether the PI-103-induced ABT-737 sensitization was upstream of cytochrome c release, the percentage of HCT116 cells exhibiting the early activating N-terminal conformational change in the multidomain proapoptotic protein Bak [23] was assessed by immunofluorescence. The combination of PI-103 and ABT-737 resulted in a significantly higher proportion of cells with activated Bak than either agent alone (Figure 1D). This confirmed that PI-103 sensitized CRC cells to ABT-737-induced apoptosis and that this effect was mediated upstream of cytochrome c release.
ABT-737 Sensitization Was Not Solely due to MCL-1 Down-Regulation
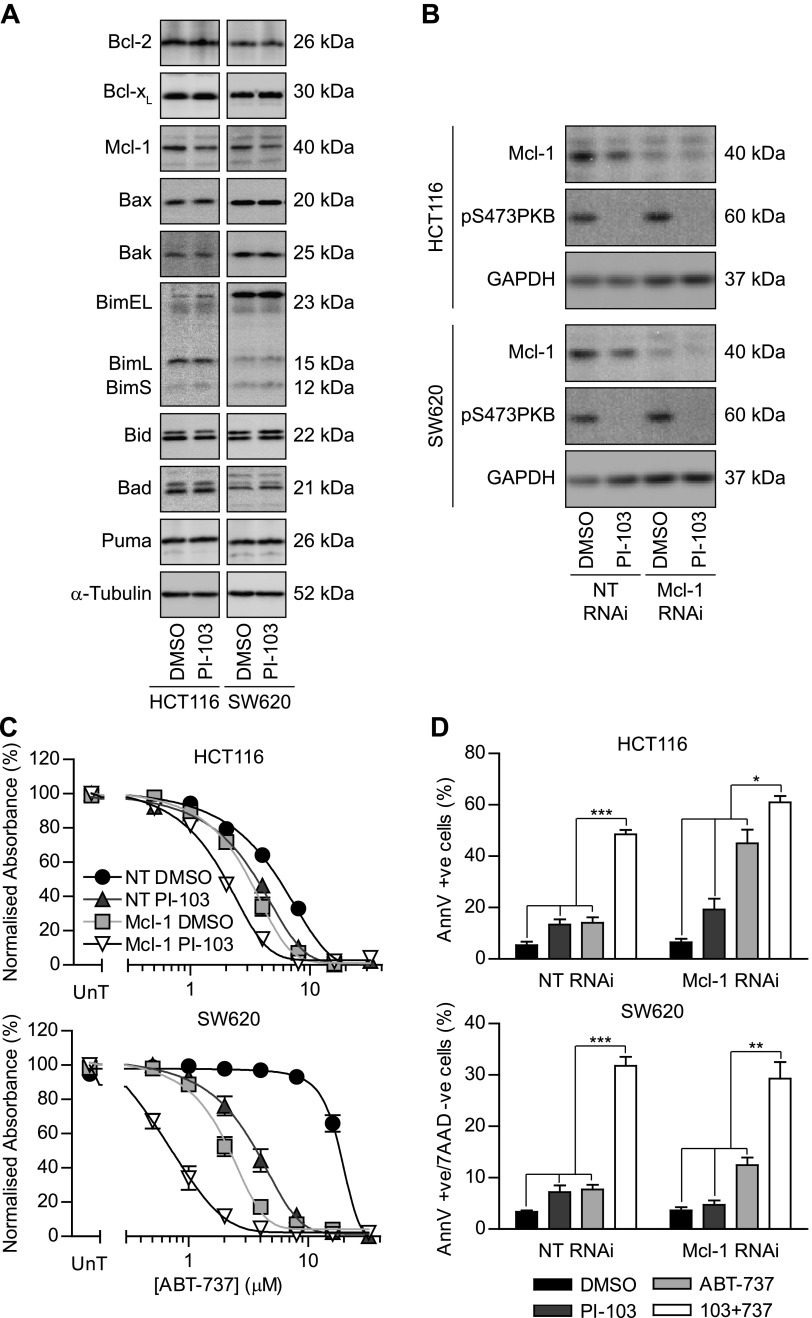
There is precedence for PI3K inhibition affecting several Bcl-2 family members including Bad, Bim, Bcl-2, MCL-1, and Bax [5]. When the effect of PI-103 on expression levels of 10 Bcl-2 family members was assessed by Western blot analysis inHCT116 and SW620 cells, the only observed change was a reduction in MCL-1 level (Figure 2A). MCL-1 is an established resistance biomarker for ABT-737, due to the poor affinity of ABT-737 for this antiapoptotic Bcl-2 family member [24,25]. Moreover, MCL-1 stability is known to be decreased by PI3K inhibition due to activation of GSK3β [26]. To test the hypothesis that PI-103-induced sensitivity to ABT-737 was solely due to a reduction of MCL-1 levels, MCL-1 was depleted by siRNA (Figure 2B), and the effect of PI-103 on ABT-737 sensitivity was reassessed. If the effect of PI3K inhibition on ABT-737 sensitivity is mediated only through MCL-1 down-regulation, treatment with PI-103 should not further sensitize MCL-1-depleted cells to ABT-737. MCL-1 knockdown significantly sensitized both HCT116 and SW620 to ABT-737, reducing the GI50 to a similar extent to that in cells transfected with nontargeting siRNA and treated with PI-103 (Figure 2C and Table W1). However, PI-103 significantly increased sensitivity to ABT-737 in MCL-1 knockdown cells. To determine whether this additional increase in ABT-737 sensitivity was due to apoptosis, cells were transfected with nontargeting siRNA or MCL-1-specific siRNA, treated with combinations of ABT-737 and/or PI-103, and analyzed by annexin V/7AAD flow cytometry (Figure 2D). This confirmed that, in MCL-1-depleted HCT116 and SW620 cells, PI-103 treatment increased ABT-737-induced apoptosis. One experimental caveat is that, whereasMCL-1 was clearly depleted by siRNA, a detectable level remained, and PI-103 could be reducing the MCL-1 levels further (beyond the resolution of the assay). Thus, the increased apoptosis observed could still be MCL-1 dependent. To address this, similar studies were performed in MCL-1-/- mouse embryonic fibroblasts (MEFs). MCL-1 knockout was confirmed (Figure W3A), and combining ABT-737 and PI-103 gave a significantly greater decrease in colony formation than either agent alone (Figure W3B). Furthermore, the combination gave enhanced cell death in parental and MCL-1-/- MEFs (Figure W3C). Although the mechanism of PI-103-induced sensitization to ABT-737 may be different between CRC cell lines and MEFs, this further demonstrates that MCL-1 is not essential for PI-103-induced sensitization. Taken together, these data suggest that, although reduced levels of MCL-1 in PI-103-treated cells caused sensitization to ABT-737, there are additional MCL-1-independent events that influence the response to ABT-737.
Figure 2.
Reduced MCL-1 level is not solely responsible for increased ABT-737 sensitivity. (A) HCT116 and SW620 cells were treated with DMSO equivalent or 2 µM PI-103 for 24 hours, and the level of Bcl-2, BCL-XL, MCL-1, Bax, Bak, Bim, Bid, Puma, Bad, and Noxa was determined by Western blot analysis. (B–D) HCT116 and SW620 cells were transfected with nontargeting siRNA (NT RNAi) or siRNA targeting MCL-1 (MCL-1 RNAi) and plated for experiments 24 hours later. Cells were treated with 2 µM PI-103 for 24 hours, and the effect on levels of MCL-1, pS473 AKT, and GAPDH was determined by Western blot analysis (B). RNAi cells were treated with 2 µM PI-103 or DMSO equivalent and the indicated concentration of ABT-737 for 3 days and processed as in Figure 1A (C). RNAi cells were treated with 2 µMPI-103 and/or 4 µMABT-737 (NT RNAi), 2 µMABT-737 (HCT116 MCL-1 RNAi), or 1 µMABT-737 (SW620 MCL-1 RNAi) for 24 hours. Cells were stained with APC-conjugated annexin V and 7AAD and analyzed by flow cytometry (D). All blots are representative of three independent experiments, and all graphs represent the means of three independent experiments carried out in triplicate (C) or duplicate (D) ± SEM. *P < .05, **P < .01, and ***P < .001 according to two-tailed unpaired t test.
ABT-737 Sensitization Was due to Inhibition of PI3K but Not AKT or mTOR
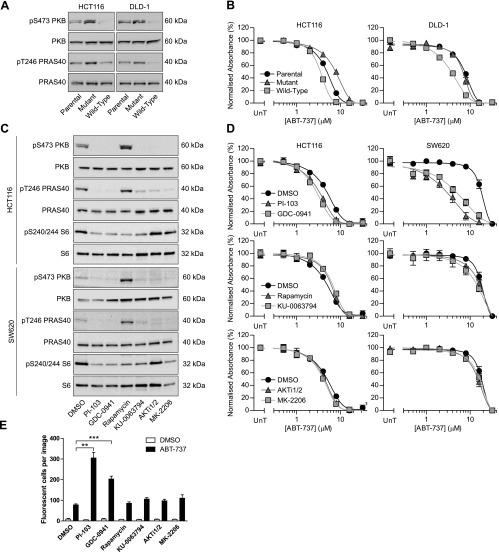
PI-103 is a dual PI3K/mTOR inhibitor; therefore, it was investigated whether the effect of PI-103 on ABT-737 sensitivity was due to PI3K inhibition, mTOR inhibition, and/or off-target effects. To assess this, PI3K signaling was genetically altered. This was achieved using HCT116 and DLD-1 cells, both of which normally express one mutant and one wild-type PIK3CA allele (labeled Parental). In the isogenic cell lines, one allele had been silenced by insertion of an adeno-associated virus (AAV)-targeting system into exon 1 of either the wild-type or mutant PIK3CA [27]. If the AAV inserted into the wild-type PIK3CA allele, only the mutant protein was expressed, resulting in higher PI3K activity compared to parental cells (labeled Mutant). Conversely, if the AAV inserted into the mutant PIK3CA allele, only the wild-type protein was expressed, resulting in lower PI3K activity (labeled Wild-Type). This was confirmed by assessing the level of AKT and PRAS40 phosphorylation in the isogenic cells (Figure 3A). HCT116 and DLD-1 cells that only expressed wildtype PIK3CA (low PI3K activity) were significantly more sensitive to ABT-737 than corresponding parental cells (Figure 3B and Table W2). HCT116 cells that only expressed mutant PIK3CA (high PI3K activity) were significantly more resistant to ABT-737 than HCT116 parental cells, and DLD-1 cells with mutant PIK3CA were as resistant as parental cells. These data demonstrate that reduced PI3K activity correlates with increased ABT-737 sensitivity, suggesting that the observed effect of PI-103 was not due to off-target effects. However, as both targets of PI-103 are components of the PI3K signaling pathway, it remained unclear which target of PI-103 was responsible for the enhanced ABT-737 sensitivity. To investigate this further, the effect of a panel of PI3K pathway inhibitors on ABT-737 sensitivity was assessed in HCT116 and SW620 cells, specifically GDC-0941, a class I PI3K-specific inhibitor [28], rapamycin, an mTOR complex 1 (mTORC1)-specific inhibitor [29], KU-0063794, an ATP-competitive mTOR inhibitor that inhibits mTORC1 and mTORC2 but not PI3K [30], and AKTi1/2 and MK-2206, two allosteric AKT inhibitors [31,32]. All agents were used at concentrations that demonstrably inhibited their primary targets: GDC-0941 and KU-0063794 inhibited phosphorylation of AKT, PRAS40, and S6; rapamycin inhibited S6 phosphorylation but increased AKT and PRAS40 phosphorylation; and AKTi1/2 and MK-2206 inhibited AKT and PRAS40 but not S6 phosphorylation (Figure 3C). In HCT116 and SW620 cells, GDC-0941 significantly sensitized to ABT-737, whereas rapamycin and KU-0063794 were without effect (Figure 3D and Table 2), suggesting that PI3K inhibition, rather than mTORC1/2 inhibition, was responsible for ABT-737 sensitization. Contrary to expectations, KU-0063794 did not sensitize to ABT-737 despite the fact that AKT is a target of mTORC2 (Figure 3C) and AKT is considered the main downstream effector of PI3K [6]. To explore this further, the effect of AKT inhibition on ABT-737 sensitivity was investigated. In both cell lines, neither AKTi1/2 nor MK-2206 had a significant effect on the sensitivity to ABT-737 (Figure 3D and Table 2), strongly suggesting that the downstream target of PI3K responsible for ABT-737 sensitization is AKT independent and implicating a novel, antiapoptotic PI3K-dependent, AKT-independent signaling pathway. The ABT-737 concentration-response data were verified by combining ABT-737 with each of the PI3K pathway inhibitors and monitoring caspase activation in SW620 cells (Figure 3E). Only PI-103 and GDC-0941 when combined with ABT-737 caused a significant increase in the number of cells with activated caspase compared to ABT-737 alone, consistent with the ABT-737 concentrationresponse data. Furthermore, the PI-103-induced down-regulation of MCL-1 was not observed with Akti1/2 or rapamycin treatment (Figure W4), and neither Akti1/2 nor rapamycin was able to further sensitize MCL-1-depleted SW620 cells to ABT-737 (Figure W5 and Table W3). These data are consistent with at least two antiapoptotic AKT/mTOR-independent pathways acting downstream of PI3K, only one of which affects MCL-1 levels.
Figure 3.
ABT-737 sensitization was PI3K dependent but AKT and mTOR independent. (A and B) Parental HCT116 and DLD-1 cells or cells expressing only the mutant or wild-type PIK3CA allele were assessed for the level of pS473 AKT, total AKT, pT246 PRAS40, or total PRAS40 by Western blot analysis (A) or exposed to the indicated concentrations of ABT-737 and processed as in Figure 1A (B). (C) HCT116 and SW620 cells were exposed to 2 µM PI-103, 2 µM GDC-0941, 10 nM rapamycin, 2 µM KU-0063794, 1 µM AKTi1/2, or 1 µM MK-2206 for 4 hours, and the effect on level of pS473 AKT, total AKT, pT246 PRAS40, total PRAS40, pS240/244 S6, and total S6 was determined by Western blot analysis. (D) HCT116 and SW620 cells were treated with the same concentrations of PI3K pathway inhibitors as in C and the indicated concentrations of ABT-737 for 3 days and processed as in Figure 1A. (E) SW620 cells were treated with the same concentrations of PI3K pathway inhibitors as in C with or without 4 µM ABT-737 plus CellPlayer Caspase 3/7 reagent, which fluoresces after cleavage by caspases. Number of fluorescent cells was determined 8 hours after start of treatment. All blots are representative of three independent experiments, and all graphs represent the means of three independent experiments carried out in triplicate (B and D) or duplicate (E) ± SEM. **P < .001 and ***P < .001 according to two-tailed unpaired t test.
Table 2.
Effect of PI3K Pathway Inhibitors on ABT-737 GI50.
| Cell Line | Treatment | ABT-737 GI50 (µM ± 95% CI) | Significance* |
| HCT116 | DMSO | 4.95 (4.15–5.90) | |
| 2 µM PI-103 | 3.33 (2.88–3.84) | 0.0268 | |
| 2 µM GDC0941 | 2.73 (2.54–2.93) | 0.0036 | |
| 1 µM AKTi1/2 | 4.32 (4.17–4.46) | 0.2092 | |
| 1 µM MK2206 | 4.06 (3.72–4.43) | 0.1189 | |
| 10 nM rapamycin | 6.19 (5.77–6.64) | 0.0821 | |
| 2 µM KU0063794 | 6.57 (6.10–7.07) | 0.0441 | |
| SW620 | DMSO | 16.9 (15.4–18.5) | |
| 2 µM PI-103 | 3.15 (2.45–4.05) | 0.0002 | |
| 2 µM GDC0941 | 6.09 (3.10–11.9) | 0.0424 | |
| 1 µM AKTi1/2 | 14.5 (11.4–18.5) | 0.3140 | |
| 1 µM MK2206 | 20.0 (10.6–37.6) | 0.6317 | |
| 10 nM rapamycin | 15.3 (12.8–18.4) | 0.4049 | |
| 2 µM KU0063794 | 16.4 (12.9–20.8) | 0.8369 |
Two-tailed unpaired t test versus DMSO-treated GI50 for same cell line.
BMX Down-Regulation Sensitized to ABT-737
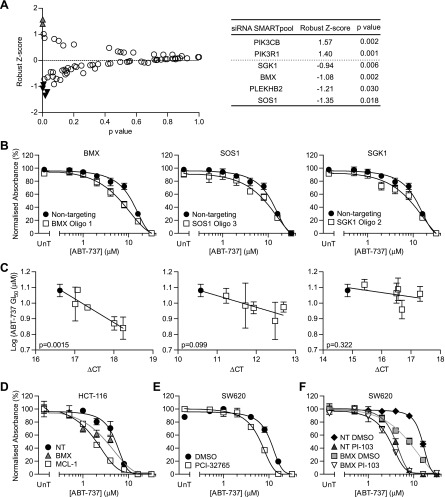
The secondary messenger generated by class I PI3K, PtdIns(3,4,5) P3, has the potential to regulate many proteins in addition to AKT. Indeed, there are reported to be >50 proteins that can bind to PtdIns (3,4,5)P3 [3]. To determine whether any of these proteins may be involved in the PI3K inhibition-induced sensitization to ABT-737, a SMARTpool siRNA library targeting mRNA for each of 52 proteins containing a PH domain that interacts with PtdIns(3,4,5)P3 and other core PI3K pathway proteins was designed (Table W4). SW620 cells, which exhibited the greatest degree of sensitization to ABT-737 when PI3K signaling was inhibited, were transfected with each siRNA, and the effect of ABT-737 treatment determined relative to non-targeting siRNA-transfected cells (Figure 4A, left panel). Four siRNAs were shown to induce a significant (P < .05; robust z score < -0.9) increase in ABT-737 sensitivity (Figure 4A, right panel, and Table W4). Of these four siRNAs, BMX, SOS1, and SGK1 were chosen for further analysis. A complete ABT-737 concentration response was carried out on cells transfected with each of the individual siRNA oligos that made up the SMARTpool with some oligos demonstrating a degree of sensitization for all three targets (Figures 4B and W6). However, only oligos 1 and 3 targeting BMX produced a significant sensitization to ABT-737 (P = .041 and .022, respectively; Table W5). Furthermore, when the association between the level of mRNA knockdown for each oligo set and the sensitization toward ABT-737 was assessed, only knockdown of BMX significantly correlated with ABT-737 efficacy (P = .0015; Figures 4C and W6B). The BMX siRNA oligos that gave the greatest degree of ABT-737 sensitization, SMARTpool, oligos 1 and 3, also caused the greatest reduction of BMX protein expression (Figure W7). BMX knockdown in HCT116 cells also sensitized to ABT-737 (Figure 4D and Table W6). Moreover, pharmacological inhibition of BMX with the TEC family kinase inhibitor PCI-32765 (ibrutinib [33]) in SW620, HCT116, and DLD-1 cells sensitized to ABT-737 (Figures 4E and W8, and Table W7). To determine whether BMX acted downstream of PI3K with regard to ABT-737 sensitization, the effect of BMX knockdown and PI-103 treatment on ABT-737 sensitivity was assessed. Knockdown of BMX did not further sensitize PI-103-treated SW620 cells to ABT-737 (Figure 4F and Table W8), suggesting that BMX-induced sensitization to ABT-737 is downstream of PI3K. BMX inhibition, either by RNAi or treatment with PCI-32765, did not affect expression of MCL-1 (Figure W9), suggesting that sensitization to ABT-737 is through an MCL-1-independent mechanism. Together, these data suggest that inhibition of BMX activity, either through knockdown or pharmacological inhibition, can sensitize CRC cell lines to ABT-737. This indicates that this PH domain-containing protein may represent a key AKT-independent effector downstream of PI3K that is responsible for PI3K inhibition-induced ABT-737 sensitization.
Figure 4.
BMX knockdown and inhibition sensitized to ABT-737. (A) SW620 cells were transfected with an siRNA SMARTpool targeting 1 of 65 mRNA that encode proteins potentially involved in PI3K signaling or a nontargeting control. Forty-eight hours later, cells were treated with 4 µM ABT-737 or DMSO equivalent for 72 hours, and cellular biomass was determined by SRB staining. Robust z score of ABT-737-treated cells compared to DMSO control cells was calculated, and the P value for each SMARTpool compared to nontargeting control was determined by two-tailed unpaired t test. Values for SMARTpool that induce ABT-737 resistance ( ) and sensitivity (▼) are shown in right-hand table. (B and C) SW620 cells were transfected with nontargeting siRNA (●), SMARTpool siRNA, or individual oligos targeting BMX, SOS1, or SGK1 (□), and an ABT-737 concentration response was carried out, or the appropriate mRNA level was determined by qPCR. B shows the concentration response curve for the most effective siRNA oligo, and C shows the correlation between knockdown efficiency and ABT-737 efficacy. (D) HCT116 cells were transfected with nontargeting siRNA, BMX siRNA, or MCL-1 siRNA and the indicated concentration of ABT-737 for 3 days and processed as in Figure 1A. (E) SW620 cells were treated with 4 µM PCI-32765 or DMSO equivalent and the indicated concentration of ABT-737 for 3 days and processed as in Figure 1A. (F) SW620 cells were transfected with nontargeting siRNA or BMX oligo 1, treated with 2 µM PI-103 or DMSO equivalent and the indicated concentration of ABT-737 for 3 days, and processed as in Figure 1A. All graphs represent the means of three independent experiments carried out in triplicate ± SEM.
) and sensitivity (▼) are shown in right-hand table. (B and C) SW620 cells were transfected with nontargeting siRNA (●), SMARTpool siRNA, or individual oligos targeting BMX, SOS1, or SGK1 (□), and an ABT-737 concentration response was carried out, or the appropriate mRNA level was determined by qPCR. B shows the concentration response curve for the most effective siRNA oligo, and C shows the correlation between knockdown efficiency and ABT-737 efficacy. (D) HCT116 cells were transfected with nontargeting siRNA, BMX siRNA, or MCL-1 siRNA and the indicated concentration of ABT-737 for 3 days and processed as in Figure 1A. (E) SW620 cells were treated with 4 µM PCI-32765 or DMSO equivalent and the indicated concentration of ABT-737 for 3 days and processed as in Figure 1A. (F) SW620 cells were transfected with nontargeting siRNA or BMX oligo 1, treated with 2 µM PI-103 or DMSO equivalent and the indicated concentration of ABT-737 for 3 days, and processed as in Figure 1A. All graphs represent the means of three independent experiments carried out in triplicate ± SEM.
Discussion
In this study, we presented evidence that the inhibition of PI3K signaling increased the sensitivity of CRC cells to ABT-737-induced apoptosis. This effect was shown to be independent of AKT inhibition and in part due to factors additional to the observed MCL-1 down-regulation. Furthermore, we present data demonstrating that inhibition of the TEC kinase BMX also sensitizes to ABT-737. This raises the possibility that BMX is a key downstream target of PI3K signaling mediating ABT-737 sensitivity and suggests that the PI3K/BMX axis may have antiapoptotic activity in CRC cells.
Other studies in non-small cell lung cancer [18] and lymphoma cell lines [17] have demonstrated that canonical PI3K pathway inhibition downstream of PI3K increased apoptosis in response to ABT-737 (or navitoclax). BCL-XL prevented PI3K inhibition-induced apoptosis in non-small cell lung cancer cells, and this was overcome by ABT-737; however, PI3K inhibition was phenocopied in AKT-depleted cells, suggesting that the antiapoptotic effect of PI3K signaling was AKT dependent [18]. Rapamycin increased navitoclax-induced apoptosis in lymphoma cells in vitro and in vivo, although the mechanism of action was not investigated [17]. Taken together, these three studies suggest that there are different mechanisms downstream of PI3K signaling to suppress ABT-737-induced apoptosis and that these mechanisms are context dependent. In support of this, a study investigating the importance of AKT in cellular proliferation/survival across a panel of human tumor cell lines revealed that a subset of cell lines (including HCT116 and DLD-1) was dependent on PI3K but not on AKT for proliferation and survival [34]. The lack of an absolute requirement for AKT in CRC cells has also been reported. When either PDK1 or AKT1 and AKT2 were knocked out in both HCT116 and DLD-1 cells [35], the cells were able to survive in standard culture conditions. However, inhibition of PI3K signaling by pharmacological intervention or expression of a dominant negative PI3K subunit in the same cell lines ([7] and Figure W2) caused a profound proliferation delay. Overall, there are clearly cell line-dependent differences in signaling downstream of PI3K, and this emphasizes the need to broaden our understanding of this important signaling pathway beyond AKT and its known downstream targets.
The TEC family of kinases is the second largest family of non-receptor protein tyrosine kinases, comprising five members, namely, TEC, BTK, ITK, TXK, and BMX (also known as ETK). Whereas TEC kinases are primarily expressed in hematopoietic cells, BMX and TEC have a broader expression profile. Specifically, BMX is expressed in endothelial linages as well as epithelial cancers such as breast and prostate [36]. All TEC kinases except TXK have a PH domain that interacts with PtdIns(3,4,5)3 and can be activated by PI3K signaling [37–39]. Furthermore, BMX has recently been implicated in mutant PIK3CA transformation [40]. BMX has been suggested to have an antiapoptotic function in prostate cancer cell lines [41], where expression of dominant negative BMX enhances chemotherapy- and radiotherapy-induced apoptosis. The downstream targets of BMX are not fully elucidated; although research has demonstrated that BMX can activate STAT3 [42] and also bind to and activate PAK1 [43], whether these targets are responsible for the antiapoptotic effect of BMX is unclear. BMX has been reported to bind to BCL-XL in bladder cancer cell lines [44], although the functional consequences of this interaction and how it is regulated have not been investigated. In the study reported here, we were unable to detect any change in phosphorylation of STAT proteins after treatment with PI3K or TEC kinase inhibitors (data not shown), and further investigation is now required to understand the antiapoptotic role of BMX in CRC better.
In addition to BMX, the RNAi library screen identified three other potential sensitizers to ABT-737, namely, SOS1, SGK1, and PLEKHB2. Further study with deconvolved SMARTpool siRNA oligos (Figures 4C and W5) suggested that the efficacy of SOS1 and SGK1 RNAi was probably due to off-target effects of some of the oligos, rather than knockdown of the intended target. PLEKHB2 was not investigated further as PLEKHB2's PH domain has recently been suggested to bind preferentially to phosphatidylserine rather than PtdIns(3,4,5)3 [45], and therefore, it is unlikely that PLEKHB2 is a downstream target of PI3K signaling. It is also interesting to note that knockdown of two PI3K subunits, PIK3CB and PIK3R1, was implicated in causing resistance to ABT-737 (Figure 4A). One possibility is that down-regulation of specific PI3K subunits (e.g., PIK3CB) leads to a compensatory up-regulation of other subunits (e.g., PIK3CA), and it is the upregulated subunit that drives ABT-737 resistance, a hypothesis that will be tested in future studies.
From a clinical perspective, the data presented here suggest that combining navitoclax with PI3K inhibitors or TEC kinase inhibitors may prove beneficial to patients with metastatic CRC, a population of patients with a 6% chance of 5-year survival (Colorectal Cancer Survival by Stage—NCIN Data Briefing 2009, http://tinyurl.com/pf5hl45), exemplifying the clinical need for improved therapy. However, more research is needed to identify phenotypic and/or genotypic traits that predict for combinatorial efficacy. The data presented here demonstrate that PI3K inhibition increased apoptosis induced by ABT-737 more in SW620 cells (PIK3CA wild type) than in DLD-1 or HCT116 cells (PIK3CA mutant). However, we lack sufficient statistical power to conclude that these differences are due to PIK3CA status due to the plethora of other genetic differences between the cell lines. *P predictive biomarkers for this drug combination expansion to a larger panel of CRC cell lines with known genetic aberrations is required.
Supplemental Materials and Methods
Robust Z Score Calculation
Sensitivity to ABT-737 for each siRNA SMARTPool was assessed calculating the surviving fraction following treatment with 4 µM ABT-737 for 72 hours for each siRNA target. Surviving fraction = replicate well with ABT-737/(average of three replicate wells with DMSO). The robust z score was used to analyze siRNA screen data. Robust z score is the number of median absolute deviations (MADs); a value is from the median value of the data set. The robust z score is used because it reduces the effect of outliers on the results and prevents missing potential significant changes in sensitivity to ABT-737. First, the new surviving fractions are calculated. New surviving fraction = median surviving fraction for all 65 siRNAs - individual surviving fraction. Negative new surviving fractions are converted to positive values, and theMAD is then calculated. MAD = SD (new surviving fractions) x; 1.4826. The robust z score for each replicate can be calculated. Robust z score = [(surviving fraction - median surviving fraction)/MAD] x; 1.4826.
Supplementary Material
Acknowledgments
The authors thank John Brognard for advice on data interpretation and manuscript preparation and Becky Bola and Matthew Lancashire for editorial advice.
Abbreviations
- CRC
colorectal cancer
- PI3K
phosphoinositide 3-kinase
- PtdIns(3,4,5)P3
phosphatidylinositol-3,4,5-triphosphate
- PH
pleckstrin homology
- siRNA
small interfering RNA
- SRB
sulforhodamine B
Footnotes
This work was supported by Cancer Research UK (CR-UK) core funding to the CR-UK Manchester Institute (grant No. C147/A12328). O.D. was supported through funding from the Cancer Research UK Experimental Cancer Medicine Centre grant to the Manchester Cancer Research Centre.
This article refers to supplementary materials, which are designated by Tables W1 to W8 and Figures W1 to W9 and are available online at www.neoplasia.com.
References
- 1.FDA, author. Regulatory watch: FDA guidance on co-developing investigational drugs. Nat Rev Drug Discov. 2011;10:86. doi: 10.1038/nrd3363. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Stephens L, Hawkins P. Signalling via class IA PI3Ks. Adv Enzyme Regul. 2010;51:27–36. doi: 10.1016/j.advenzreg.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase-AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 5.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 7.Martin-Fernandez C, Bales J, Hodgkinson C, Welman A, Welham MJ, Dive C, Morrow CJ. Blocking phosphoinositide 3-kinase activity in colorectal cancer cells reduces proliferation but does not increase apoptosis alone or in combination with cytotoxic drugs. Mol Cancer Res. 2009;7:955–965. doi: 10.1158/1541-7786.MCR-08-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raynaud FI, Eccles S, Clarke PA, Hayes A, Nutley B, Alix S, Henley A, Di-Stefano F, Ahmad Z, Guillard S, et al. Pharmacologic characterization of a potent inhibitor of class I phosphatidylinositide 3-kinases. Cancer Res. 2007;67:5840–5850. doi: 10.1158/0008-5472.CAN-06-4615. [DOI] [PubMed] [Google Scholar]
- 9.Guillard S, Clarke PA, Te Poele R, Mohri Z, Bjerke L, Valenti M, Raynaud F, Eccles SA, Workman P. Molecular pharmacology of phosphatidylinositol 3-kinase inhibition in human glioma. Cell Cycle. 2009;8:443–453. doi: 10.4161/cc.8.3.7643. [DOI] [PubMed] [Google Scholar]
- 10.Opel D, Westhoff MA, Bender A, Braun V, Debatin KM, Fulda S. Phosphatidylinositol 3-kinase inhibition broadly sensitizes glioblastoma cells to death receptor- and drug-induced apoptosis. Cancer Res. 2008;68:6271–6280. doi: 10.1158/0008-5472.CAN-07-6769. [DOI] [PubMed] [Google Scholar]
- 11.Chonghaile TN, Letai A. Mimicking the BH3 domain to kill cancer cells. Oncogene. 2008;27(suppl 1):S149–S157. doi: 10.1038/onc.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 13.Del Gaizo Moore V, Schlis KD, Sallan SE, Armstrong SA, Letai A. BCL-2 dependence and ABT-737 sensitivity in acute lymphoblastic leukemia. Blood. 2008;111:2300–2309. doi: 10.1182/blood-2007-06-098012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson WH, O'Connor OA, Czuczman MS, LaCasce AS, Gerecitano JF, Leonard JP, Tulpule A, Dunleavy K, Xiong H, Chiu YL, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11:1149–1159. doi: 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison LR, Micha D, Brandenburg M, Simpson KL, Morrow CJ, Denneny O, Hodgkinson C, Yunus Z, Dempsey C, Roberts D, et al. Hypoxic human cancer cells are sensitized to BH-3 mimetic-induced apoptosis via downregulation of the Bcl-2 protein Mcl-1. J Clin Invest. 2011;121:1075–1087. doi: 10.1172/JCI43505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cragg MS, Harris C, Strasser A, Scott CL. Unleashing the power of inhibitors of oncogenic kinases through BH3 mimetics. Nat Rev Cancer. 2009;9:321–326. doi: 10.1038/nrc2615. [DOI] [PubMed] [Google Scholar]
- 17.Ackler S, Xiao Y, Mitten MJ, Foster K, Oleksijew A, Refici M, Schlessinger S, Wang B, Chemburkar SR, Bauch J, et al. ABT-263 and rapamycin act cooperatively to kill lymphoma cells in vitro and in vivo. Mol Cancer Ther. 2008;7:3265–3274. doi: 10.1158/1535-7163.MCT-08-0268. [DOI] [PubMed] [Google Scholar]
- 18.Qian J, Zou Y, Rahman JS, Lu B, Massion PP. Synergy between phosphatidylinositol 3-kinase/Akt pathway and Bcl-xL in the control of apoptosis in adenocarcinoma cells of the lung. Mol Cancer Ther. 2009;8:101–109. doi: 10.1158/1535-7163.MCT-08-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klymenko T, Brandenburg M, Morrow C, Dive C, Makin G. The novel Bcl-2 inhibitor ABT-737 is more effective in hypoxia and is able to reverse hypoxia-induced drug resistance in neuroblastoma cells. Mol Cancer Ther. 2011;10:2373–2383. doi: 10.1158/1535-7163.MCT-11-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrow CJ, Gray A, Dive C. Comparison of phosphatidylinositol-3-kinase signalling within a panel of human colorectal cancer cell lines with mutant or wild-type PIK3CA. FEBS Lett. 2005;579:5123–5128. doi: 10.1016/j.febslet.2005.07.096. [DOI] [PubMed] [Google Scholar]
- 21.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 22.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 23.Griffiths GJ, Dubrez L, Morgan CP, Jones NA, Whitehouse J, Corfe BM, Dive C, Hickman JA. Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J Cell Biol. 1999;144:903–914. doi: 10.1083/jcb.144.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, Deng X, Zhai D, Shi YX, Sneed T, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 25.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, Willis SN, Scott CL, Day CL, Cory S, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faber AC, Li D, Song Y, Liang MC, Yeap BY, Bronson RT, Lifshits E, Chen Z, Maira SM, Garcia-Echeverria C, et al. Differential induction of apoptosis in HER2 and EGFR addicted cancers following PI3K inhibition. Proc Natl Acad Sci USA. 2009;106:19503–19508. doi: 10.1073/pnas.0905056106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samuels Y, Diaz LA, Jr, Schmidt-Kittler O, Cummins JM, Delong L, Cheong I, Rago C, Huso DL, Lengauer C, Kinzler KW, et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005;7:561–573. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 28.Folkes AJ, Ahmadi K, Alderton WK, Alix S, Baker SJ, Box G, Chuckowree IS, Clarke PA, Depledge P, Eccles SA, et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem. 2008;51:5522–5532. doi: 10.1021/jm800295d. [DOI] [PubMed] [Google Scholar]
- 29.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Martinez JM, Moran J, Clarke RG, Gray A, Cosulich SC, Chresta CM, Alessi DR. Ku-0063794 is a specific inhibitor of the mammalian target of rapamycin (mTOR) Biochem J. 2009;421:29–42. doi: 10.1042/BJ20090489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnett SF, Defeo-Jones D, Fu S, Hancock PJ, Haskell KM, Jones RE, Kahana JA, Kral AM, Leander K, Lee LL, et al. Identification and characterization of pleckstrin-homology-domain-dependent and isoenzyme-specific Akt inhibitors. Biochem J. 2005;385:399–408. doi: 10.1042/BJ20041140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, Ueno Y, Hatch H, Majumder PK, Pan BS, et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther. 2010;9:1956–1967. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- 33.Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, Li S, Pan Z, Thamm DH, Miller RA, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci USA. 2010;107:13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vasudevan KM, Barbie DA, Davies MA, Rabinovsky R, McNear CJ, Kim JJ, Hennessy BT, Tseng H, Pochanard P, Kim SY, et al. AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell. 2009;16:21–32. doi: 10.1016/j.ccr.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ericson K, Gan C, Cheong I, Rago C, Samuels Y, Velculescu VE, Kinzler KW, Huso DL, Vogelstein B, Papadopoulos N. Genetic inactivation of AKT1, AKT2, and PDPK1 in human colorectal cancer cells clarifies their roles in tumor growth regulation. Proc Natl Acad Sci USA. 2010;107:2598–2603. doi: 10.1073/pnas.0914018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cenni B, Gutmann S, Gottar-Guillier M. BMX and its role in inflammation, cardiovascular disease, and cancer. Int Rev Immunol. 2012;31:166–173. doi: 10.3109/08830185.2012.663838. [DOI] [PubMed] [Google Scholar]
- 37.August A, Sadra A, Dupont B, Hanafusa H. Src-induced activation of inducible T cell kinase (ITK) requires phosphatidylinositol 3-kinase activity and the Pleckstrin homology domain of inducible T cell kinase. Proc Natl Acad Sci USA. 1997;94:11227–11232. doi: 10.1073/pnas.94.21.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Z, Wahl MI, Eguinoa A, Stephens LR, Hawkins PT, Witte ON. Phosphatidylinositol 3-kinase-γ activates Bruton's tyrosine kinase in concert with Src family kinases. Proc Natl Acad Sci USA. 1997;94:13820–13825. doi: 10.1073/pnas.94.25.13820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiu Y, Robinson D, Pretlow TG, Kung HJ. Etk/Bmx, a tyrosine kinase with a pleckstrin-homology domain, is an effector of phosphatidylinositol 3′-kinase and is involved in interleukin 6-induced neuroendocrine differentiation of prostate cancer cells. Proc Natl Acad Sci USA. 1998;95:3644–3649. doi: 10.1073/pnas.95.7.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hart JR, Liao L, Yates JR, III, Vogt PK. Essential role of Stat3 in PI3K-induced oncogenic transformation. Proc Natl Acad Sci USA. 2011;108:13247–13252. doi: 10.1073/pnas.1110486108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xue LY, Qiu Y, He J, Kung HJ, Oleinick NL. Etk/Bmx, a PH-domain containing tyrosine kinase, protects prostate cancer cells from apoptosis induced by photodynamic therapy or thapsigargin. Oncogene. 1999;18:3391–3398. doi: 10.1038/sj.onc.1202687. [DOI] [PubMed] [Google Scholar]
- 42.Tsai YT, Su YH, Fang SS, Huang TN, Qiu Y, Jou YS, Shih HM, Kung HJ, Chen RH. Etk, a Btk family tyrosine kinase, mediates cellular transformation by linking Src to STAT3 activation. Mol Cell Biol. 2000;20:2043–2054. doi: 10.1128/mcb.20.6.2043-2054.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bagheri-Yarmand R, Mandal M, Taludker AH, Wang RA, Vadlamudi RK, Kung HJ, Kumar R. Etk/Bmx tyrosine kinase activates Pak1 and regulates tumorigenicity of breast cancer cells. J Biol Chem. 2001;276:29403–29409. doi: 10.1074/jbc.M103129200. [DOI] [PubMed] [Google Scholar]
- 44.Guo S, Sun F, Guo Z, Li W, Alfano A, Chen H, Magyar CE, Huang J, Chai TC, Qiu S, et al. Tyrosine kinase ETK/BMX is up-regulated in bladder cancer and predicts poor prognosis in patients with cystectomy. PLoS One. 2011;6:e17778. doi: 10.1371/journal.pone.0017778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uchida Y, Hasegawa J, Chinnapen D, Inoue T, Okazaki S, Kato R, Wakatsuki S, Misaki R, Koike M, Uchiyama Y, et al. Intracellular phosphatidylserine is essential for retrograde membrane traffic through endosomes. Proc Natl Acad Sci USA. 2011;108:15846–15851. doi: 10.1073/pnas.1109101108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.