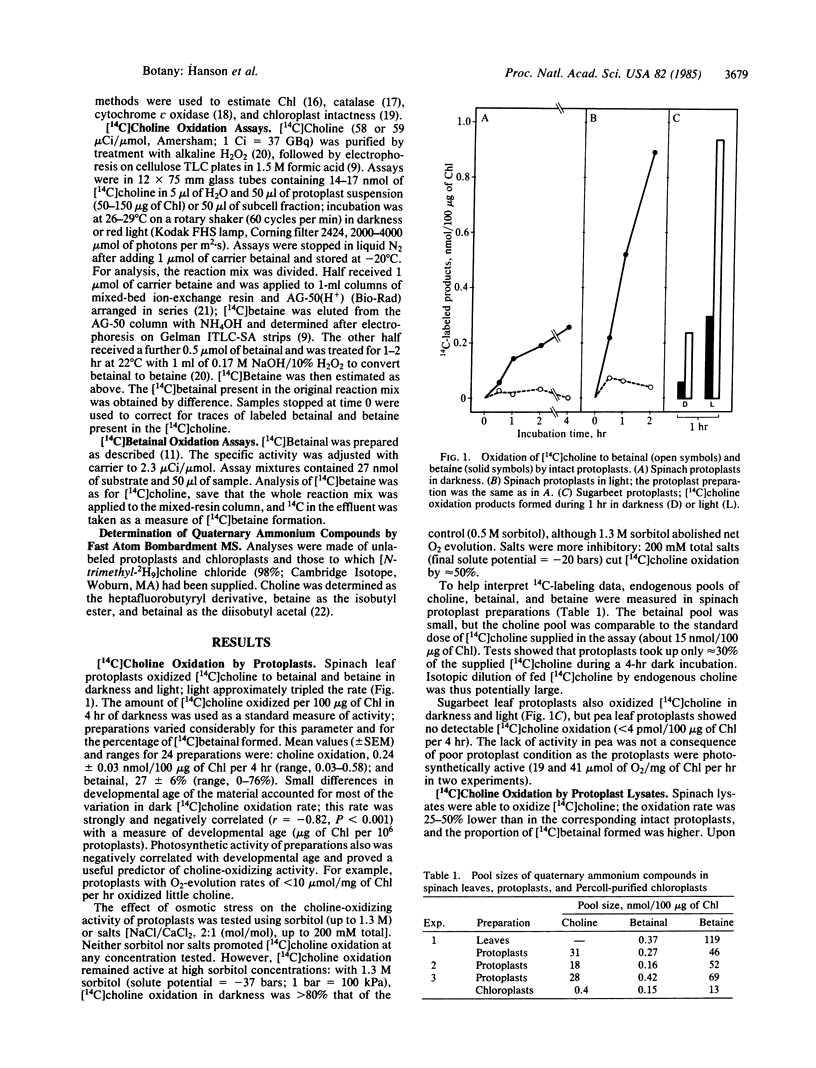
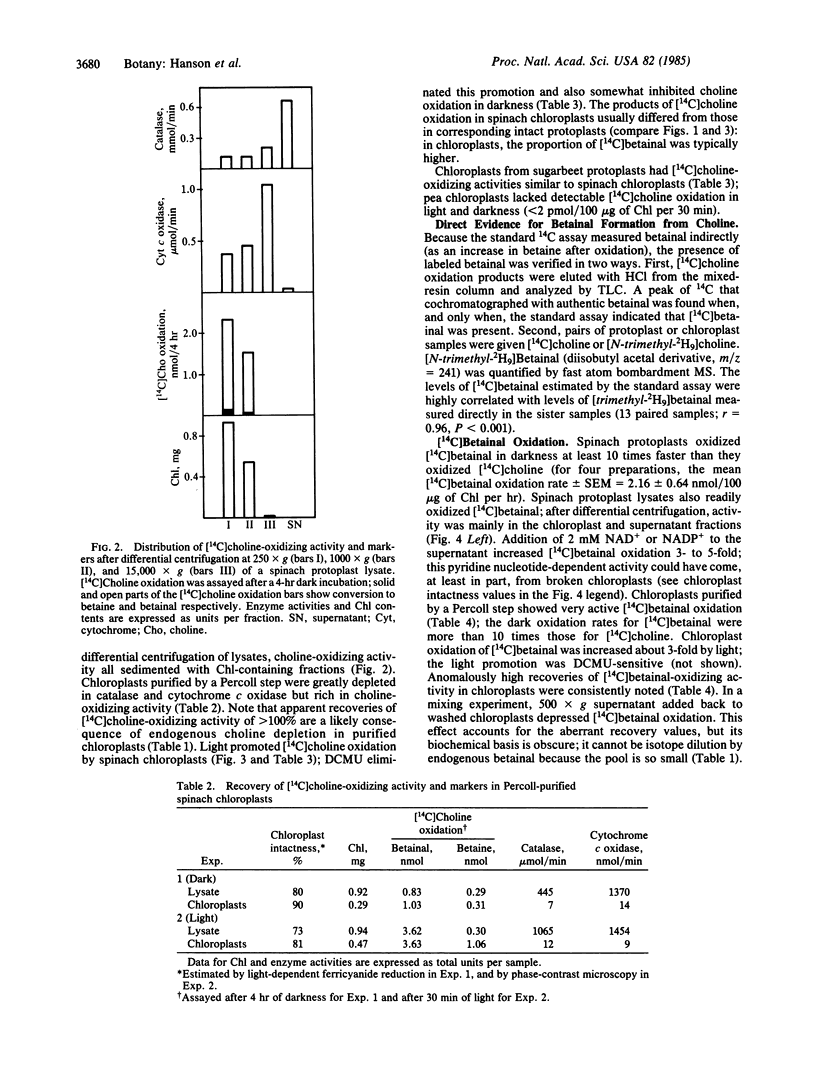
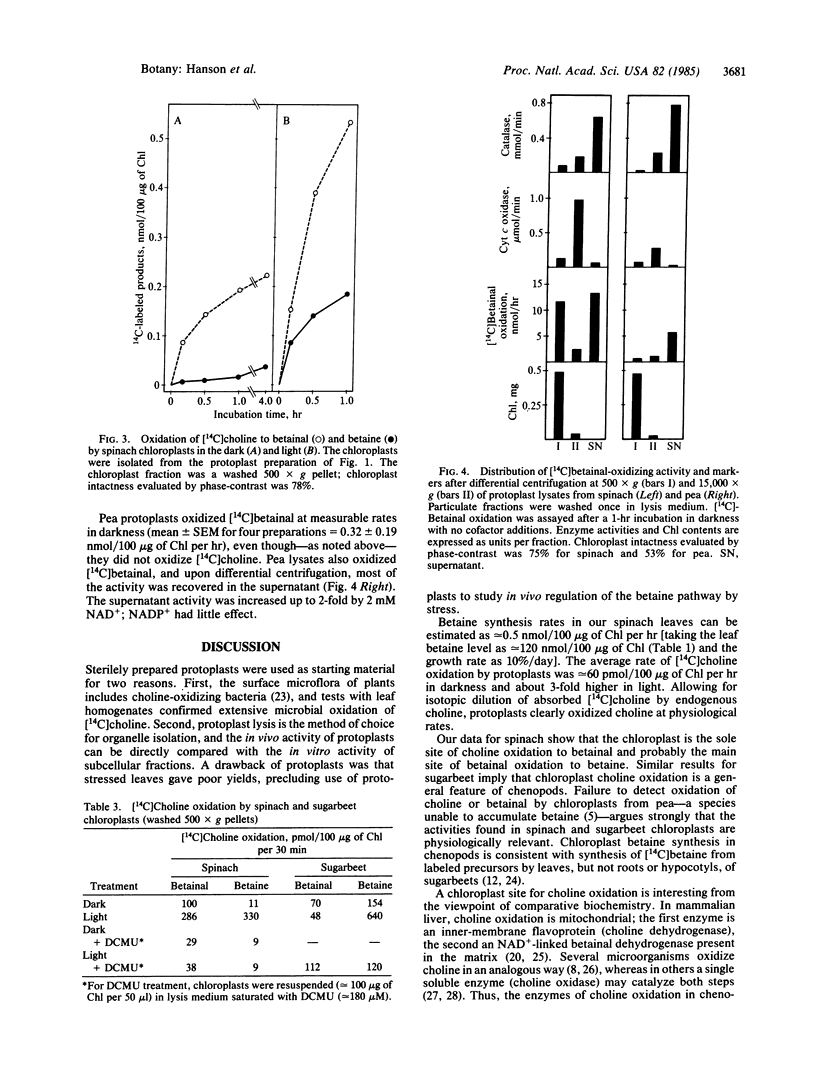
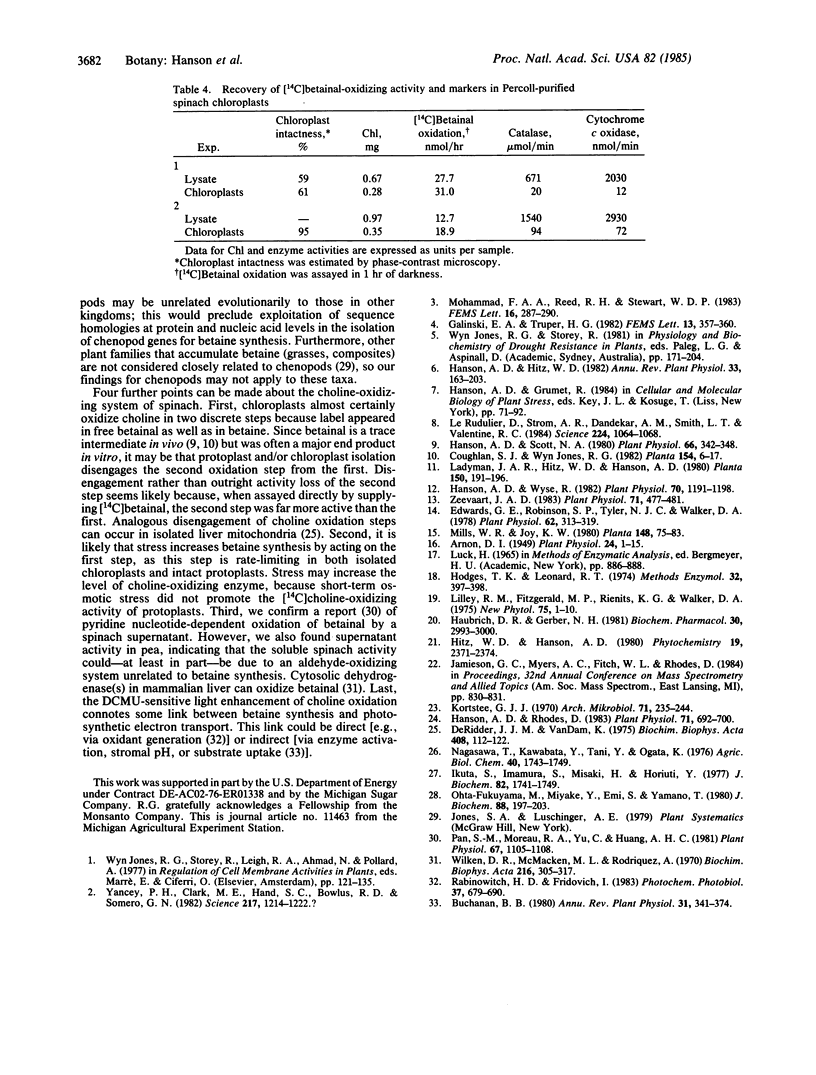
Abstract
Plants from several families (Chenopodiaceae, Gramineae, Compositae) accumulate betaine (glycine betaine) in response to salt or water stress via the pathway: choline → betainal (betaine aldehyde) → betaine. Betaine accumulation is probably a metabolic adaptation to stress. Intact protoplasts from leaves of spinach (Spinacia oleracea) oxidized [14C]choline to betainal and betaine, as did protoplast lysates. Upon differential centrifugation, the [14C]choline-oxidizing activity of lysates sedimented with chloroplasts. Chloroplasts purified from protoplast lysates by a Percoll cushion procedure retained strong [14C]choline-oxidizing activity (1-3 nmol/mg of chlorophyll per hr), although the proportion of the intermediate, [14C]betainal, in the reaction products was usually higher than for protoplasts. Isolated chloroplasts also readily oxidized [14C]betainal to betaine (20-100 nmol/mg of chlorophyll per hr). Light increased the oxidation of both [14C]choline and [14C]betainal by isolated chloroplasts ≈3-fold; this light-stimulation was abolished by 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU). Similar results were obtained with another chenopod (Beta vulgaris) but not with pea (Pisum sativum), a species that accumulates no betaine. The chloroplast site for betaine synthesis in chenopods contrasts with the mitochondrial site in mammals.
Keywords: stress resistance, Spinacia oleracea, choline oxidation, betaine aldehyde oxidation, protoplasts
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G. E., Robinson S. P., Tyler N. J., Walker D. A. Photosynthesis by isolated protoplasts, protoplast extracts, and chloroplasts of wheat: influence of orthophosphate, pyrophosphate, and adenylates. Plant Physiol. 1978 Aug;62(2):313–319. doi: 10.1104/pp.62.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson A. D., Rhodes D. C Tracer Evidence for Synthesis of Choline and Betaine via Phosphoryl Base Intermediates in Salinized Sugarbeet Leaves. Plant Physiol. 1983 Mar;71(3):692–700. doi: 10.1104/pp.71.3.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson A. D., Scott N. A. Betaine Synthesis from Radioactive Precursors in Attached, Water-stressed Barley Leaves. Plant Physiol. 1980 Aug;66(2):342–348. doi: 10.1104/pp.66.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson A. D., Wyse R. Biosynthesis, translocation, and accumulation of betaine in sugar beet and its progenitors in relation to salinity. Plant Physiol. 1982 Oct;70(4):1191–1198. doi: 10.1104/pp.70.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubrich D. R., Gerber N. H. Choline dehydrogenase. Assay, properties and inhibitors. Biochem Pharmacol. 1981 Nov 1;30(21):2993–3000. doi: 10.1016/0006-2952(81)90265-3. [DOI] [PubMed] [Google Scholar]
- Ikuta S., Imamura S., Misaki H., Horiuti Y. Purification and characterization of choline oxidase from Arthrobacter globiformis. J Biochem. 1977 Dec;82(6):1741–1749. doi: 10.1093/oxfordjournals.jbchem.a131872. [DOI] [PubMed] [Google Scholar]
- Kortstee G. J. The aerobic decomposition of choline by microorganisms. I. The ability of aerobic organisms, particularly coryneform bacteria, to utilize choline as the sole carbon and nitrogen source. Arch Mikrobiol. 1970;71(3):235–244. [PubMed] [Google Scholar]
- Le Rudulier D., Strom A. R., Dandekar A. M., Smith L. T., Valentine R. C. Molecular biology of osmoregulation. Science. 1984 Jun 8;224(4653):1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- Ohta-Fukuyama M., Miyake Y., Emi S., Yamano T. Identification and properties of the prosthetic group of choline oxidase from Alcaligenes sp. J Biochem. 1980 Jul;88(1):197–203. [PubMed] [Google Scholar]
- Pan S. M., Moreau R. A., Yu C., Huang A. H. Betaine accumulation and betaine-aldehyde dehydrogenase in spinach leaves. Plant Physiol. 1981 Jun;67(6):1105–1108. doi: 10.1104/pp.67.6.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilken D. R., McMacken M. L., Rodriquez A. Choline and betaine aldehyde oxidation by rat liver mitochondria. Biochim Biophys Acta. 1970 Sep 1;216(2):305–317. doi: 10.1016/0005-2728(70)90222-7. [DOI] [PubMed] [Google Scholar]
- Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Living with water stress: evolution of osmolyte systems. Science. 1982 Sep 24;217(4566):1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- Zeevaart J. A. Metabolism of Abscisic Acid and Its Regulation in Xanthium Leaves during and after Water Stress. Plant Physiol. 1983 Mar;71(3):477–481. doi: 10.1104/pp.71.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ridder J. J., van Dam K. Control of choline oxidation by rat-liver mitochondria. Biochim Biophys Acta. 1975 Nov 11;408(2):112–122. doi: 10.1016/0005-2728(75)90003-1. [DOI] [PubMed] [Google Scholar]


