Abstract
To investigate the role of oncogene activation in the pathogenesis of malignant tumors, we have studied the tumorigenic and metastatic properties of NIH/3T3 secondary transfectants (designated A51) containing an activated c-Ha-ras-1 gene derived from the human T24 bladder carcinoma cell line and compared them with untransfected NIH/3T3 cells. Whereas subcutaneous implantation of NIH/3T3 cells in the supraclavicular region produced palpable tumors that failed to metastasize, NIH/3T3 cells inoculated in the footpad gave rise to malignant tumors that metastasized to the lung. Under identical conditions and irrespective of the site of implantation, A51 cells formed rapidly growing primary tumors that produced pulmonary metastases. In an assay for experimental metastasis, intravenously injected NIH/3T3 cells gave rise to pulmonary nodules only at high cell inocula and in long-term survivors (90 days after injection). In contrast, A51 cells formed multiple lung tumor colonies detectable 14 days after injection. These results indicate that "normal" untransfected NIH/3T3 cultures contain subpopulations of cells that express malignant properties and that transfection of NIH/3T3 cells with activated c-Ha-ras-1 accelerates formation of metastases.
Full text
PDF

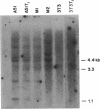
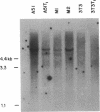
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- Blair D. G., Cooper C. S., Oskarsson M. K., Eader L. A., Vande Woude G. F. New method for detecting cellular transforming genes. Science. 1982 Dec 10;218(4577):1122–1125. doi: 10.1126/science.6293052. [DOI] [PubMed] [Google Scholar]
- Boone C. W. Malignant hemangioendotheliomas produced by subcutaneous inoculation of Balb/3T3 cells attached to glass beads. Science. 1975 Apr 4;188(4183):68–70. doi: 10.1126/science.1114343. [DOI] [PubMed] [Google Scholar]
- Fasano O., Birnbaum D., Edlund L., Fogh J., Wigler M. New human transforming genes detected by a tumorigenicity assay. Mol Cell Biol. 1984 Sep;4(9):1695–1705. doi: 10.1128/mcb.4.9.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M., Shimizu K., Perucho M., Wigler M. Isolation and preliminary characterization of a human transforming gene from T24 bladder carcinoma cells. Nature. 1982 Apr 1;296(5856):404–409. doi: 10.1038/296404a0. [DOI] [PubMed] [Google Scholar]
- Kyriazis A. P., DiPersio L., Michael G. J., Pesce A. J., Stinnett J. D. Growth patterns and metastatic behavior of human tumors growing in athymic mice. Cancer Res. 1978 Oct;38(10):3186–3190. [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Littlefield J. W. NIH 3T3 cell line. Science. 1982 Oct 15;218(4569):214–216. doi: 10.1126/science.218.4569.214-b. [DOI] [PubMed] [Google Scholar]
- Miller F. R., Medina D., Heppner G. H. Preferential growth of mammary tumors in intact mammary fatpads. Cancer Res. 1981 Oct;41(10):3863–3867. [PubMed] [Google Scholar]
- Müller R., Müller D. Co-transfection of normal NIH/3T3 DNA and retroval LTR sequences: a novel strategy for the detection of potential c-onc genes. EMBO J. 1984 May;3(5):1121–1127. doi: 10.1002/j.1460-2075.1984.tb01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold R. F., Overell R. W. Fibroblast immortality is a prerequisite for transformation by EJ c-Ha-ras oncogene. Nature. 1983 Aug 18;304(5927):648–651. doi: 10.1038/304648a0. [DOI] [PubMed] [Google Scholar]
- Perucho M., Goldfarb M., Shimizu K., Lama C., Fogh J., Wigler M. Human-tumor-derived cell lines contain common and different transforming genes. Cell. 1981 Dec;27(3 Pt 2):467–476. doi: 10.1016/0092-8674(81)90388-3. [DOI] [PubMed] [Google Scholar]
- Poste G., Doll J., Hart I. R., Fidler I. J. In vitro selection of murine B16 melanoma variants with enhanced tissue-invasive properties. Cancer Res. 1980 May;40(5):1636–1644. [PubMed] [Google Scholar]
- Poste G., Fidler I. J. The pathogenesis of cancer metastasis. Nature. 1980 Jan 10;283(5743):139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- Rubin H. Adaptive changes in spontaneously transformed Balb/3T3 cells during tumor formation and subsequent cultivation. J Natl Cancer Inst. 1984 Feb;72(2):375–381. [PubMed] [Google Scholar]
- Rubin H. Growth regulation, reverse transformation, and adaptability of 3T3 cells in decreased Mg2+ concentration. Proc Natl Acad Sci U S A. 1981 Jan;78(1):328–332. doi: 10.1073/pnas.78.1.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos E., Tronick S. R., Aaronson S. A., Pulciani S., Barbacid M. T24 human bladder carcinoma oncogene is an activated form of the normal human homologue of BALB- and Harvey-MSV transforming genes. Nature. 1982 Jul 22;298(5872):343–347. doi: 10.1038/298343a0. [DOI] [PubMed] [Google Scholar]
- Shih C., Padhy L. C., Murray M., Weinberg R. A. Transforming genes of carcinomas and neuroblastomas introduced into mouse fibroblasts. Nature. 1981 Mar 19;290(5803):261–264. doi: 10.1038/290261a0. [DOI] [PubMed] [Google Scholar]
- Shih C., Shilo B. Z., Goldfarb M. P., Dannenberg A., Weinberg R. A. Passage of phenotypes of chemically transformed cells via transfection of DNA and chromatin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5714–5718. doi: 10.1073/pnas.76.11.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotkin D., Gianni A. M., Rozenblatt S., Weinberg R. A. Infectious viral DNA of murine leukemia virus. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4910–4913. doi: 10.1073/pnas.72.12.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sweet R. W., Chao M. V., Axel R. The structure of the thymidine kinase gene promoter: nuclease hypersensitivity correlates with expression. Cell. 1982 Dec;31(2 Pt 1):347–353. doi: 10.1016/0092-8674(82)90128-3. [DOI] [PubMed] [Google Scholar]
- Vaage J. Relationship between tumor growth characteristics and preferential sites of growth. J Natl Cancer Inst. 1984 May;72(5):1199–1203. [PubMed] [Google Scholar]
- Watson R., Oskarsson M., Vande Woude G. F. Human DNA sequence homologous to the transforming gene (mos) of Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4078–4082. doi: 10.1073/pnas.79.13.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]