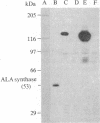
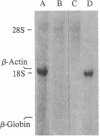
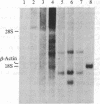
Abstract
We report the isolation of cDNA clones encoding delta-aminolevulinate synthase (ALA synthase; EC 2.3.1.37), the first enzyme in the heme biosynthetic pathway in animal cells. The gene was isolated from a chicken erythroid cDNA library prepared in the bacteriophage lambda fusion/expression vector gt11, using rabbit antibody raised against the relatively abundant chicken liver enzyme. The chicken liver and red cell ALA synthase isozymes share substantial crossreactivity to the antibody, thereby allowing isolation of the erythroid-specific gene by using the heterologous antibody in immune screening of the red cell cDNA library. Preliminary analysis documenting the tissue specificity of transcription indicates that the enzyme is encoded by a highly homologous set of messages, which appear to differ in size in various avian tissues. From analysis using strand-specific RNA probes, it appears that the different ALA synthase mRNAs detected may be transcribed from a family of genes that are closely related in nucleotide sequence and are each regulated in a developmentally specific manner.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama Y., Kato S. Two cell lines from lymphomas of Marek's disease. Biken J. 1974 Sep;17(3):105–116. [PubMed] [Google Scholar]
- Beug H., Palmieri S., Freudenstein C., Zentgraf H., Graf T. Hormone-dependent terminal differentiation in vitro of chicken erythroleukemia cells transformed by ts mutants of avian erythroblastosis virus. Cell. 1982 Apr;28(4):907–919. doi: 10.1016/0092-8674(82)90070-8. [DOI] [PubMed] [Google Scholar]
- Bishop D. F., Kitchen H., Wood W. A. Evidence for erythroid and nonerythroid forms of delta-aminolevulinate synthetase. Arch Biochem Biophys. 1981 Feb;206(2):380–391. doi: 10.1016/0003-9861(81)90105-3. [DOI] [PubMed] [Google Scholar]
- Bittner M., Kupferer P., Morris C. F. Electrophoretic transfer of proteins and nucleic acids from slab gels to diazobenzyloxymethyl cellulose or nitrocellulose sheets. Anal Biochem. 1980 Mar 1;102(2):459–471. doi: 10.1016/0003-2697(80)90182-7. [DOI] [PubMed] [Google Scholar]
- Bottomley S. S., Smithee G. A. Characterization and measurement of delta-aminolaevulinate synthetase in bone marrow cell mitochondria. Biochim Biophys Acta. 1968 Apr 24;159(1):27–37. doi: 10.1016/0005-2744(68)90241-6. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Dodgson J. B., Strommer J., Engel J. D. Isolation of the chicken beta-globin gene and a linked embryonic beta-like globin gene from a chicken DNA recombinant library. Cell. 1979 Aug;17(4):879–887. doi: 10.1016/0092-8674(79)90328-3. [DOI] [PubMed] [Google Scholar]
- Dolan M., Dodgson J. B., Engel J. D. Analysis of the adult chicken beta-globin gene. Nucleotide sequence of the locus, microheterogeneity at the 5'-end of beta-globin mRNA, and aberrant nuclear RNA species. J Biol Chem. 1983 Mar 25;258(6):3983–3990. [PubMed] [Google Scholar]
- Dreyfuss G., Adam S. A., Choi Y. D. Physical change in cytoplasmic messenger ribonucleoproteins in cells treated with inhibitors of mRNA transcription. Mol Cell Biol. 1984 Mar;4(3):415–423. doi: 10.1128/mcb.4.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock K., Tsang V. C. India ink staining of proteins on nitrocellulose paper. Anal Biochem. 1983 Aug;133(1):157–162. doi: 10.1016/0003-2697(83)90237-3. [DOI] [PubMed] [Google Scholar]
- Hayashi N., Kurashima Y., Kikuchi G. Mechanism of allylisopropylacetamide-induced increase of -aminolevulinate synthetase in liver mitochondria. V. Mechanism of regulation by hemin of the level of -aminolevulinate synthetase in rat liver mitochondria. Arch Biochem Biophys. 1972 Jan;148(1):10–21. doi: 10.1016/0003-9861(72)90109-9. [DOI] [PubMed] [Google Scholar]
- Hayashi N., Terasawa M., Yamauchi K., Kikuchi G. Effects of hemin on the synthesis and intracellular translocation of delta-aminolevulinate synthase in the liver of rats treated with 3,5-dicarbethoxy-1,4-dihydrocollidine. J Biochem. 1980 Nov;88(5):1537–1543. doi: 10.1093/oxfordjournals.jbchem.a133124. [DOI] [PubMed] [Google Scholar]
- Howlett G. J., Birch H., Dickson P. W., Schreiber G. Determination of the molecular weight of detergent-solubilized enzymes by sedimentation equilibrium in an air-driven ultracentrifuge. Biochem Biophys Res Commun. 1982 Apr 14;105(3):895–901. doi: 10.1016/0006-291x(82)91054-3. [DOI] [PubMed] [Google Scholar]
- Hoyt M. A., Knight D. M., Das A., Miller H. I., Echols H. Control of phage lambda development by stability and synthesis of cII protein: role of the viral cIII and host hflA, himA and himD genes. Cell. 1982 Dec;31(3 Pt 2):565–573. doi: 10.1016/0092-8674(82)90312-9. [DOI] [PubMed] [Google Scholar]
- KIKUCHI G., KUMAR A., TALMAGE P., SHEMIN D. The enzymatic synthesis of delta-aminolevulinic acid. J Biol Chem. 1958 Nov;233(5):1214–1219. [PubMed] [Google Scholar]
- Kikuchi G., Hayashi N. Regulation by heme of synthesis and intracellular translocation of delta-aminolevulinate synthase in the liver. Mol Cell Biochem. 1981 Jun 9;37(1):27–41. doi: 10.1007/BF02355885. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- McClements W., Skalka A. M. Analysis of chicken ribosomal RNA genes and construction of lambda hybrids containing gene fragments. Science. 1977 Apr 8;196(4286):195–197. doi: 10.1126/science.557836. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S., Granick S. Induction of -aminolevulinic acid synthetase in chick embryo liver cells in cluture. Proc Natl Acad Sci U S A. 1970 Oct;67(2):517–522. doi: 10.1073/pnas.67.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S. Sequential induction of heme pathway enzymes during erythroid differentiation of mouse Friend leukemia virus-infected cells. J Exp Med. 1976 Feb 1;143(2):305–315. doi: 10.1084/jem.143.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava G., Brooker J. D., May B. K., Elliott W. H. Haem control in experimental porphyria. The effect of haemin on the induction of delta-aminolaevulinate synthase in isolated chick-embryo liver cells. Biochem J. 1980 Jun 15;188(3):781–788. doi: 10.1042/bj1880781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storti R. V., Szwast A. E. Molecular cloning and characterization of Drosophila genes and their expression during embryonic development and in primary muscle cell cultures. Dev Biol. 1982 Apr;90(2):272–283. doi: 10.1016/0012-1606(82)90376-1. [DOI] [PubMed] [Google Scholar]
- Tonita Y., Oashi A., Kikuchi G. Induction of delta-aminolevulinate synthetase in organ culture of chick embryo liver by allylisopropylacetamide and 3,5-dicarbethoxy-1,4-dihydrocollidine. J Biochem. 1974 May;75(5):1007–1007. doi: 10.1093/oxfordjournals.jbchem.a130472. [DOI] [PubMed] [Google Scholar]
- Tyrrell D. L., Marks G. S. Drug-induced porphyrin biosynthesis. V. Effect of protohemin on the transcriptional and post-transcriptional phases of -aminolevulinic acid synthetase induction. Biochem Pharmacol. 1972 Aug 1;21(15):2077–2093. doi: 10.1016/0006-2952(72)90161-x. [DOI] [PubMed] [Google Scholar]
- Wada O., Sassa S., Takaku F., Yano Y., Uratta G., Nakao K. Different responses of the hepatic and erythropoietic delta-aminolevulinic acid synthetase of mice. Biochim Biophys Acta. 1967 Nov 28;148(2):585–587. doi: 10.1016/0304-4165(67)90165-1. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Hayashi N., Kikuchi G. delta-Aminolevulinate synthase isozymes in the liver and erythroid cells of chicken. Biochem Biophys Res Commun. 1983 Jun 15;113(2):377–383. doi: 10.1016/0006-291x(83)91737-0. [DOI] [PubMed] [Google Scholar]
- Whiting M. J. Synthesis of delta-aminolaevulinate synthase by isolated liver polyribosomes. Biochem J. 1976 Aug 15;158(2):391–400. doi: 10.1042/bj1580391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods J. S., Dixon R. L. Studies of the perinatal differences in the activity of hepatic -aminolevulinic acid synthetase. Biochem Pharmacol. 1972 Jun 15;21(12):1735–1744. doi: 10.1016/0006-2952(72)90080-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Hayashi N., Kikuchi G. Regulation of synthesis and intracellular translocation of delta-aminolevulinate synthase by heme and its relation to the heme saturation of tryptophan pyrrolase in rat liver. Arch Biochem Biophys. 1981 Jul;209(2):451–459. doi: 10.1016/0003-9861(81)90302-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Hayashi N., Kikuchi G. Translational inhibition by heme of the synthesis of hepatic delta-aminolevulinate synthase in a cell-free system. Biochem Biophys Res Commun. 1983 Aug 30;115(1):225–231. doi: 10.1016/0006-291x(83)90993-2. [DOI] [PubMed] [Google Scholar]
- Yamauchi K., Hayashi N., Kikuchi G. Translocation of delta-aminolevulinate synthase from the cytosol to the mitochondria and its regulation by hemin in the rat liver. J Biol Chem. 1980 Feb 25;255(4):1746–1751. [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]