Abstract
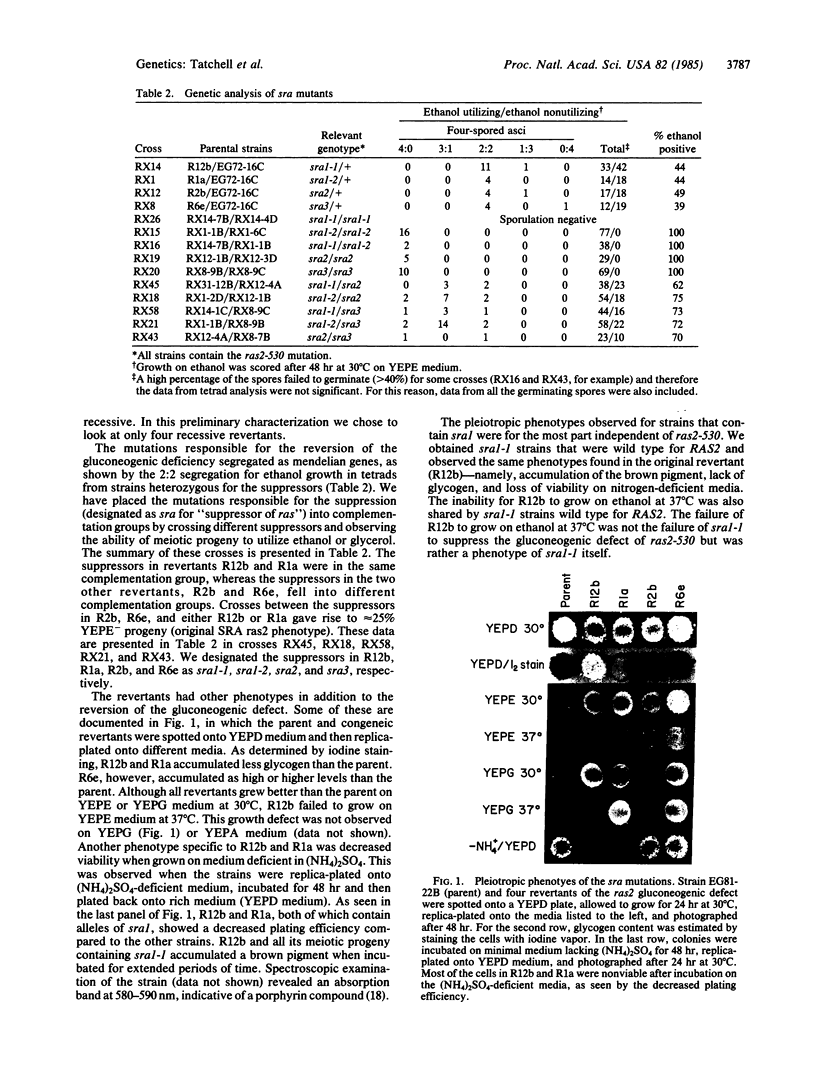
Saccharomyces cerevisiae contains two genes with remarkable homology to members of the ras oncogene family. These two genes, RAS1 and RAS2, constitute an essential gene family since spores with disruptions of both genes fail to grow. We report here that strains containing RAS2 disruptions have three distinct phenotypes. First, they fail to grow efficiently on nonfermentable carbon sources. Second, they hyperaccumulate the storage carbohydrates glycogen and trehalose. Third, diploid cells homozygous for the RAS2 disruptions sporulate on rich media. Extragenic suppressors have been isolated that suppress the gluconeogenic defect. These suppressors fall into at least three complementation groups, mutations in two of which bypass the normal requirement of RAS for cell viability, allowing cells containing neither RAS gene to grow. The phenotype of the RAS2 mutant and extragenic suppressors implicate RAS with some function in the normal response to nutrient limitation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calvert G. R., Dawes I. W. Initiation of sporulation in Saccharomyces cerevisiae. Mutations preventing initiation. J Gen Microbiol. 1984 Mar;130(3):615–624. doi: 10.1099/00221287-130-3-615. [DOI] [PubMed] [Google Scholar]
- Dawes I. W., Calvert G. R. Initiation of sporulation in Saccharomyces cerevisiae. Mutations causing derepressed sporulation and G1 arrest in the cell division cycle. J Gen Microbiol. 1984 Mar;130(3):605–613. doi: 10.1099/00221287-130-3-605. [DOI] [PubMed] [Google Scholar]
- Dawes I. W. Study of cell development using depressed mutations. Nature. 1975 Jun 26;255(5511):707–708. doi: 10.1038/255707a0. [DOI] [PubMed] [Google Scholar]
- DeFeo-Jones D., Scolnick E. M., Koller R., Dhar R. ras-Related gene sequences identified and isolated from Saccharomyces cerevisiae. Nature. 1983 Dec 15;306(5944):707–709. doi: 10.1038/306707a0. [DOI] [PubMed] [Google Scholar]
- DeFeo-Jones D., Tatchell K., Robinson L. C., Sigal I. S., Vass W. C., Lowy D. R., Scolnick E. M. Mammalian and yeast ras gene products: biological function in their heterologous systems. Science. 1985 Apr 12;228(4696):179–184. doi: 10.1126/science.3883495. [DOI] [PubMed] [Google Scholar]
- Dhar R., Nieto A., Koller R., DeFeo-Jones D., Scolnick E. M. Nucleotide sequence of two rasH related-genes isolated from the yeast Saccharomyces cerevisiae. Nucleic Acids Res. 1984 Apr 25;12(8):3611–3618. doi: 10.1093/nar/12.8.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosset M., Muir L. W., Nielsen L. D., Fischer E. H. Purification and properties of yeast glycogen phosphorylase a and b. Biochemistry. 1971 Oct 26;10(22):4105–4113. doi: 10.1021/bi00798a015. [DOI] [PubMed] [Google Scholar]
- Freese E. B., Chu M. I., Freese E. Initiation of yeast sporulation of partial carbon, nitrogen, or phosphate deprivation. J Bacteriol. 1982 Mar;149(3):840–851. doi: 10.1128/jb.149.3.840-851.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallwitz D., Donath C., Sander C. A yeast gene encoding a protein homologous to the human c-has/bas proto-oncogene product. Nature. 1983 Dec 15;306(5944):704–707. doi: 10.1038/306704a0. [DOI] [PubMed] [Google Scholar]
- Huang K. P., Cabib E. Yeast glycogen synthetase in the glucose 6-phosphate-dependent form. I. Purification and properties. J Biol Chem. 1974 Jun 25;249(12):3851–3857. [PubMed] [Google Scholar]
- Kataoka T., Powers S., Cameron S., Fasano O., Goldfarb M., Broach J., Wigler M. Functional homology of mammalian and yeast RAS genes. Cell. 1985 Jan;40(1):19–26. doi: 10.1016/0092-8674(85)90304-6. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Powers S., McGill C., Fasano O., Strathern J., Broach J., Wigler M. Genetic analysis of yeast RAS1 and RAS2 genes. Cell. 1984 Jun;37(2):437–445. doi: 10.1016/0092-8674(84)90374-x. [DOI] [PubMed] [Google Scholar]
- Lillie S. H., Pringle J. R. Reserve carbohydrate metabolism in Saccharomyces cerevisiae: responses to nutrient limitation. J Bacteriol. 1980 Sep;143(3):1384–1394. doi: 10.1128/jb.143.3.1384-1394.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Uno I., Ishikawa T. Initiation of meiosis in yeast mutants defective in adenylate cyclase and cyclic AMP-dependent protein kinase. Cell. 1983 Feb;32(2):417–423. doi: 10.1016/0092-8674(83)90461-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Uno I., Oshima Y., Ishikawa T. Isolation and characterization of yeast mutants deficient in adenylate cyclase and cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2355–2359. doi: 10.1073/pnas.79.7.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz C. H., Maia J. C., Tenan M. N., Braz-Padrão G. R., Mattoon J. R., Panek A. D. Regulation of yeast trehalase by a monocyclic, cyclic AMP-dependent phosphorylation-dephosphorylation cascade system. J Bacteriol. 1983 Feb;153(2):644–651. doi: 10.1128/jb.153.2.644-651.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padrão G. R., Malamud D. R., Panek A. D., Mattoon J. R. Regulation of energy metabolism in yeast. Inheritance of a pleiotropic mutation causing defects in metabolism of energy reserves, ethanol utilization and formation of cytochrome a.a3. Mol Gen Genet. 1982;185(2):255–261. doi: 10.1007/BF00330795. [DOI] [PubMed] [Google Scholar]
- Powers S., Kataoka T., Fasano O., Goldfarb M., Strathern J., Broach J., Wigler M. Genes in S. cerevisiae encoding proteins with domains homologous to the mammalian ras proteins. Cell. 1984 Mar;36(3):607–612. doi: 10.1016/0092-8674(84)90340-4. [DOI] [PubMed] [Google Scholar]
- Rothman-Denes L. B., Cabib E. Two forms of yeast glycogen synthetase and their role in glycogen accumulation. Proc Natl Acad Sci U S A. 1970 Jul;66(3):967–974. doi: 10.1073/pnas.66.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamanoi F., Walsh M., Kataoka T., Wigler M. A product of yeast RAS2 gene is a guanine nucleotide binding protein. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6924–6928. doi: 10.1073/pnas.81.22.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatchell K., Chaleff D. T., DeFeo-Jones D., Scolnick E. M. Requirement of either of a pair of ras-related genes of Saccharomyces cerevisiae for spore viability. Nature. 1984 Jun 7;309(5968):523–527. doi: 10.1038/309523a0. [DOI] [PubMed] [Google Scholar]
- Tatchell K., Nasmyth K. A., Hall B. D., Astell C., Smith M. In vitro mutation analysis of the mating-type locus in yeast. Cell. 1981 Nov;27(1 Pt 2):25–35. doi: 10.1016/0092-8674(81)90357-3. [DOI] [PubMed] [Google Scholar]
- Temeles G. L., DeFeo-Jones D., Tatchell K., Ellinger M. S., Scolnick E. M. Expression and characterization of ras mRNAs from Saccharomyces cerevisiae. Mol Cell Biol. 1984 Nov;4(11):2298–2305. doi: 10.1128/mcb.4.11.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temeles G. L., Gibbs J. B., D'Alonzo J. S., Sigal I. S., Scolnick E. M. Yeast and mammalian ras proteins have conserved biochemical properties. Nature. 1985 Feb 21;313(6004):700–703. doi: 10.1038/313700a0. [DOI] [PubMed] [Google Scholar]
- Toda T., Uno I., Ishikawa T., Powers S., Kataoka T., Broek D., Cameron S., Broach J., Matsumoto K., Wigler M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell. 1985 Jan;40(1):27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]
- Uno I., Matsumoto K., Adachi K., Ishikawa T. Genetic and biochemical evidence that trehalase is a substrate of cAMP-dependent protein kinase in yeast. J Biol Chem. 1983 Sep 25;258(18):10867–10872. [PubMed] [Google Scholar]
- Wingender-Drissen R., Becker J. U. Regulation of yeast phosphorylase by phosphorylase kinase and cAMP-dependent protein kinase. FEBS Lett. 1983 Oct 31;163(1):33–36. doi: 10.1016/0014-5793(83)81156-9. [DOI] [PubMed] [Google Scholar]
- Winston F., Chumley F., Fink G. R. Eviction and transplacement of mutant genes in yeast. Methods Enzymol. 1983;101:211–228. doi: 10.1016/0076-6879(83)01016-2. [DOI] [PubMed] [Google Scholar]
- van Solingen P., van der Plaat J. B. Partial purification of the protein system controlling the breakdown of trehalose in baker's yeast. Biochem Biophys Res Commun. 1975 Feb 3;62(3):553–560. doi: 10.1016/0006-291x(75)90434-9. [DOI] [PubMed] [Google Scholar]