Abstract
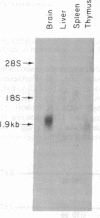
The mouse Thy-1.2 gene was isolated from a C57Bl/6 cosmid library and its nucleotide sequence was determined from an 8-kilobase-long EcoRI fragment. The predicted amino acid sequence indicates that the mouse Thy-1 molecule contains a 19 amino acid leader peptide and the 112 amino acids reported previously from protein sequence analysis, plus 31 extra amino acids at the carboxyl terminus. These 31 amino acids contain a stretch of 20 amino acids, at positions 124-143, which is highly hydrophobic. RNA transfer blot analysis of RNA from mouse tissues indicates that the sequence coding for these 31 amino acids is present on poly(A)-containing RNA of brain and thymus tissues. This hydrophobic segment very likely provides the basis for integration of Thy-1 within the plasma membrane. The entire coding sequence of Thy-1 is distributed among three exons, encoding amino acid residues -19 to 8, -7 to 106, and 107 to 143, respectively. Comparison of the mouse and rat Thy-1 genes shows that both have a similar gene organization and encode a highly conserved transmembrane segment.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acton R. T., Morris R. J., Williams A. F. Estimation of the amount and tissue distribution of rat Thy-1.1 antigen. Eur J Immunol. 1974 Sep;4(9):598–602. doi: 10.1002/eji.1830040904. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates P. F., Swift R. A. Double cos site vectors: simplified cosmid cloning. Gene. 1983 Dec;26(2-3):137–146. doi: 10.1016/0378-1119(83)90183-x. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. G., Gagnon J., Reid K. B., Williams A. F. Rat brain Thy-1 glycoprotein. The amino acid sequence, disulphide bonds and an unusual hydrophobic region. Biochem J. 1981 Apr 1;195(1):15–30. doi: 10.1042/bj1950015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cotmore S. F., Crowhurst S. A., Waterfield M. D. Purification of Thy-1-related glycoproteins from human brain and fibroblasts: comparisons between these molecules and murine glycoproteins carrying Thy-1.1 and Thy-1.2 antigens. Eur J Immunol. 1981 Aug;11(8):597–603. doi: 10.1002/eji.1830110802. [DOI] [PubMed] [Google Scholar]
- Dalchau R., Fabre J. W. Identification and unusual tissue distribution of the canine and human homologues of Thy-1 (theta). J Exp Med. 1979 Mar 1;149(3):576–591. doi: 10.1084/jem.149.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas T. C. Occurrence of a theta-like antigen in rats. J Exp Med. 1972 Nov 1;136(5):1054–1062. doi: 10.1084/jem.136.5.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld F. G., Lund T., Murray E. J., Mellor A. L., Dahl H. H., Flavell R. A. The construction of cosmid libraries which can be used to transform eukaryotic cells. Nucleic Acids Res. 1982 Nov 11;10(21):6715–6732. doi: 10.1093/nar/10.21.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt S. V., Mason D. W., Williams A. F. In rat bone marrow Thy-1 antigen is present on cells with membrane immunoglobulin and on precursors of peripheral B lymphocytes. Eur J Immunol. 1977 Nov;7(11):817–823. doi: 10.1002/eji.1830071114. [DOI] [PubMed] [Google Scholar]
- Larhammar D., Schenning L., Gustafsson K., Wiman K., Claesson L., Rask L., Peterson P. A. Complete amino acid sequence of an HLA-DR antigen-like beta chain as predicted from the nucleotide sequence: similarities with immunoglobulins and HLA-A, -B, and -C antigens. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3687–3691. doi: 10.1073/pnas.79.12.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon V. A., Unger M., Dulbecco R. Thy-1: a differentiation marker of potential mammary myoepithelial cells in vitro. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6093–6097. doi: 10.1073/pnas.75.12.6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley J. F., Lennon V. A. Transitory expression of Thy-1 antigen in skeletal muscle development. Nature. 1977 Jul 14;268(5616):163–165. doi: 10.1038/268163a0. [DOI] [PubMed] [Google Scholar]
- Letarte-Muirhead M., Barclay A. N., Williams A. F. Purification of the Thy-1 molecule, a major cell-surface glycoprotein of rat thymocytes. Biochem J. 1975 Dec;151(3):685–697. doi: 10.1042/bj1510685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour M. H., Cooper E. L. Purification and characterization of Rana pipiens brain Thy-1 glycoprotein. J Immunol. 1984 May;132(5):2515–2523. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie J. L., Fabre J. W. Studies with a monoclonal antibody on the distribution of Thy-1 in the lymphoid and extracellular connective tissues of the dog. Transplantation. 1981 Apr;31(4):275–282. doi: 10.1097/00007890-198104000-00009. [DOI] [PubMed] [Google Scholar]
- Moriuchi T., Chang H. C., Denome R., Silver J. Thy-1 cDNA sequence suggests a novel regulatory mechanism. Nature. 1983 Jan 6;301(5895):80–82. doi: 10.1038/301080a0. [DOI] [PubMed] [Google Scholar]
- Owen M. J., Knott J. C., Crumpton M. J. Labeling of lymphocyte surface antigens by the lipophilic, photoactivatable reagent hexanoyldiiodo-N-(4-azido-2-nitrophenyl)tyramine. Biochemistry. 1980 Jun 24;19(13):3092–3099. doi: 10.1021/bi00554a040. [DOI] [PubMed] [Google Scholar]
- REIF A. E., ALLEN J. M. THE AKR THYMIC ANTIGEN AND ITS DISTRIBUTION IN LEUKEMIAS AND NERVOUS TISSUES. J Exp Med. 1964 Sep 1;120:413–433. doi: 10.1084/jem.120.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostas J. A., Shevenan T. A., Sinclair C. M., Jeffrey P. L. The purification and characterization of a Thy-1-like glycoprotein from chicken brain. Biochem J. 1983 Jul 1;213(1):143–152. doi: 10.1042/bj2130143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid M., Boyse E. A., Carswell E. A., Old L. J. Serologically demonstrable alloantigens of mouse epidermal cells. J Exp Med. 1972 Apr 1;135(4):938–955. doi: 10.1084/jem.135.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T., Chang H. C., Moriuchi T., Denome R., Ploegh H., Silver J. A hydrophobic transmembrane segment at the carboxyl terminus of thy-1. Science. 1985 Feb 8;227(4687):649–651. doi: 10.1126/science.2857501. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Frelinger J. G., Fisher D., Hunkapiller T., Pereira D., Weissman S. M., Uehara H., Nathenson S., Hood L. Three cDNA clones encoding mouse transplantation antigens: homology to immunoglobulin genes. Cell. 1981 Apr;24(1):125–134. doi: 10.1016/0092-8674(81)90508-0. [DOI] [PubMed] [Google Scholar]
- Stern P. L. Theta alloantigen on mouse and rat fibroblasts. Nat New Biol. 1973 Nov 21;246(151):76–78. doi: 10.1038/newbio246076a0. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Weissman I. L., Bevan M. J. Mouse T-cell surface glycoprotein recognised by heterologous anti-thymocyte sera and its relationship to Thy-1 antigen. Nature. 1975 Aug 21;256(5519):652–654. doi: 10.1038/256652a0. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Gagnon J. Neuronal cell Thy-1 glycoprotein: homology with immunoglobulin. Science. 1982 May 14;216(4547):696–703. doi: 10.1126/science.6177036. [DOI] [PubMed] [Google Scholar]