Abstract
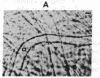
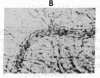
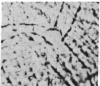
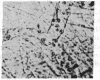
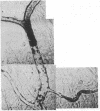
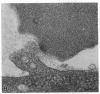
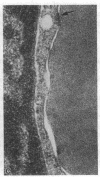
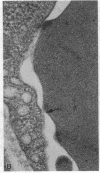
We have studied the pathophysiology of the vascular obstruction induced by Plasmodium falciparum-parasitized erythrocytes with the use of an ex vivo microcirculatory preparation perfused with red cells infected with knobless and knobby clones of the FCR-3 strain. We find that parasitized erythrocyte membrane knobs are indispensable for the generation of the circulatory obstruction. Uninfected erythrocytes incubated in culture and erythrocytes infected with early or late forms of the knobless clones or the early forms of the knobby clone all failed to obstruct the microcirculation, although exhibiting various effects on bulk viscosity and peripheral resistance during flow. In contrast, late forms of the knobby clone produced significantly higher peripheral resistance during flow and significant obstruction as detected by changes in time of pressure flow recovery as well as by direct videorecorded microscopic observation. Optical and electron microscopy showed that the adherence of parasitized cells to the endothelium was limited to the venules and involved the knobs in junctions. In addition, we were able to follow the sequence of events during obstruction: initial red-cell adherence to the venular endothelium (sometimes only transitory) followed by progressive recruitment at the venule surface, finally leading to total obstruction that involved parasitized and nonparasitized erythrocytes. Sometimes, retrograde aggregation would extend the obstruction to the capillaries or even precapillary arterioles. These results show that knobs are necessary and sufficient to produce vascular obstruction and that other factors (spleen, immunological, etc.) can only have a modulating role. These results also exclude the possibility that the exclusive adherence to venules is the consequence of "plasma factors" found in the malaric patients.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa M., Rabbege J. R., Wellde B. T. Junctional apparatus in erythrocytes infected with malarial parasites. Z Zellforsch Mikrosk Anat. 1972;124(1):72–75. [PubMed] [Google Scholar]
- Beacham W. S., Konishi A., Hunt C. C. Observations of the microcirculatory bed in rat mesocecum using differential interference constrast microscopy in vivo and electron microscopy. Am J Anat. 1976 Aug;146(4):385–425. doi: 10.1002/aja.1001460404. [DOI] [PubMed] [Google Scholar]
- Cranston H. A., Boylan C. W., Carroll G. L., Sutera S. P., Williamson J. R., Gluzman I. Y., Krogstad D. J. Plasmodium falciparum maturation abolishes physiologic red cell deformability. Science. 1984 Jan 27;223(4634):400–403. doi: 10.1126/science.6362007. [DOI] [PubMed] [Google Scholar]
- David P. H., Hommel M., Miller L. H., Udeinya I. J., Oligino L. D. Parasite sequestration in Plasmodium falciparum malaria: spleen and antibody modulation of cytoadherence of infected erythrocytes. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5075–5079. doi: 10.1073/pnas.80.16.5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J. B. Concentration from continuous culture of erythrocytes infected with trophozoites and schizonts of Plasmodium falciparum. Am J Trop Med Hyg. 1978 Nov;27(6):1274–1276. doi: 10.4269/ajtmh.1978.27.1274. [DOI] [PubMed] [Google Scholar]
- Jensen J. B., Trager W. Plasmodium falciparum in culture: use of outdated erthrocytes and description of the candle jar method. J Parasitol. 1977 Oct;63(5):883–886. [PubMed] [Google Scholar]
- Lee M. V., Ambrus J. L., DeSouza J. M., Lee R. V. Diminished red blood cell deformability in uncomplicated human malaria. A preliminary report. J Med. 1982;13(5-6):479–485. [PubMed] [Google Scholar]
- Leech J. H., Aley S. B., Miller L. H., Howard R. J. Plasmodium falciparum malaria: cytoadherence of infected erythrocytes to endothelial cells and associated changes in the erythrocyte membrane. Prog Clin Biol Res. 1984;155:63–77. [PubMed] [Google Scholar]
- Luse S. A., Miller L. H. Plasmodium falciparum malaria. Ultrastructure of parasitized erythrocytes in cardiac vessels. Am J Trop Med Hyg. 1971 Sep;20(5):655–660. [PubMed] [Google Scholar]
- Miller L. H. Distribution of mature trophozoites and schizonts of Plasmodium falciparum in the organs of Aotus trivirgatus, the night monkey. Am J Trop Med Hyg. 1969 Nov;18(6):860–865. doi: 10.4269/ajtmh.1969.18.860. [DOI] [PubMed] [Google Scholar]
- Rudzinska M. A., Trager W. The fine structure of trophozoites and gametocytes in Plasmodium coatneyi. J Protozool. 1968 Feb;15(1):73–88. doi: 10.1111/j.1550-7408.1968.tb02091.x. [DOI] [PubMed] [Google Scholar]
- Schmidt J. A., Udeinya I. J., Leech J. H., Hay R. J., Aikawa M., Barnwell J., Green I., Miller L. H. Plasmodium falciparum malaria. An amelanotic melanoma cell line bears receptors for the knob ligand on infected erythrocytes. J Clin Invest. 1982 Aug;70(2):379–386. doi: 10.1172/JCI110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Rudzinska M. A., Bradbury P. C. The fine structure of Plasmodium falciparum and its host erythrocytes in natural malarial infections in man. Bull World Health Organ. 1966;35(6):883–885. [PMC free article] [PubMed] [Google Scholar]
- Trager W., Tershakovec M., Lyandvert L., Stanley H., Lanners N., Gubert E. Clones of the malaria parasite Plasmodium falciparum obtained by microscopic selection: their characterization with regard to knobs, chloroquine sensitivity, and formation of gametocytes. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6527–6530. doi: 10.1073/pnas.78.10.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udeinya I. J., Schmidt J. A., Aikawa M., Miller L. H., Green I. Falciparum malaria-infected erythrocytes specifically bind to cultured human endothelial cells. Science. 1981 Jul 31;213(4507):555–557. doi: 10.1126/science.7017935. [DOI] [PubMed] [Google Scholar]
- Wyler D. J., Quinn T. C., Chen L. T. Relationship of alterations in splenic clearance function and microcirculation to host defense in acute rodent malaria. J Clin Invest. 1981 May;67(5):1400–1404. doi: 10.1172/JCI110168. [DOI] [PMC free article] [PubMed] [Google Scholar]