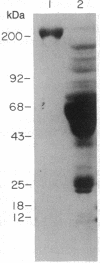
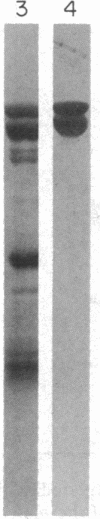
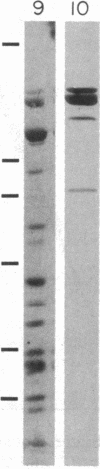
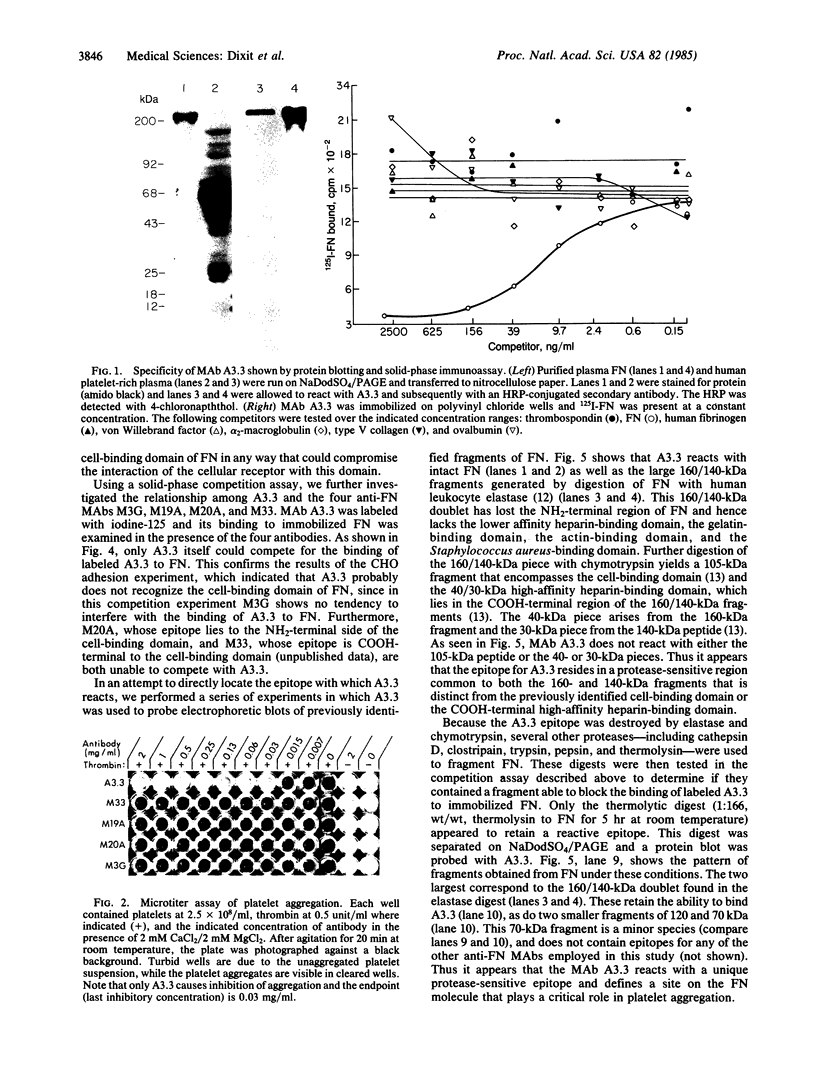
Abstract
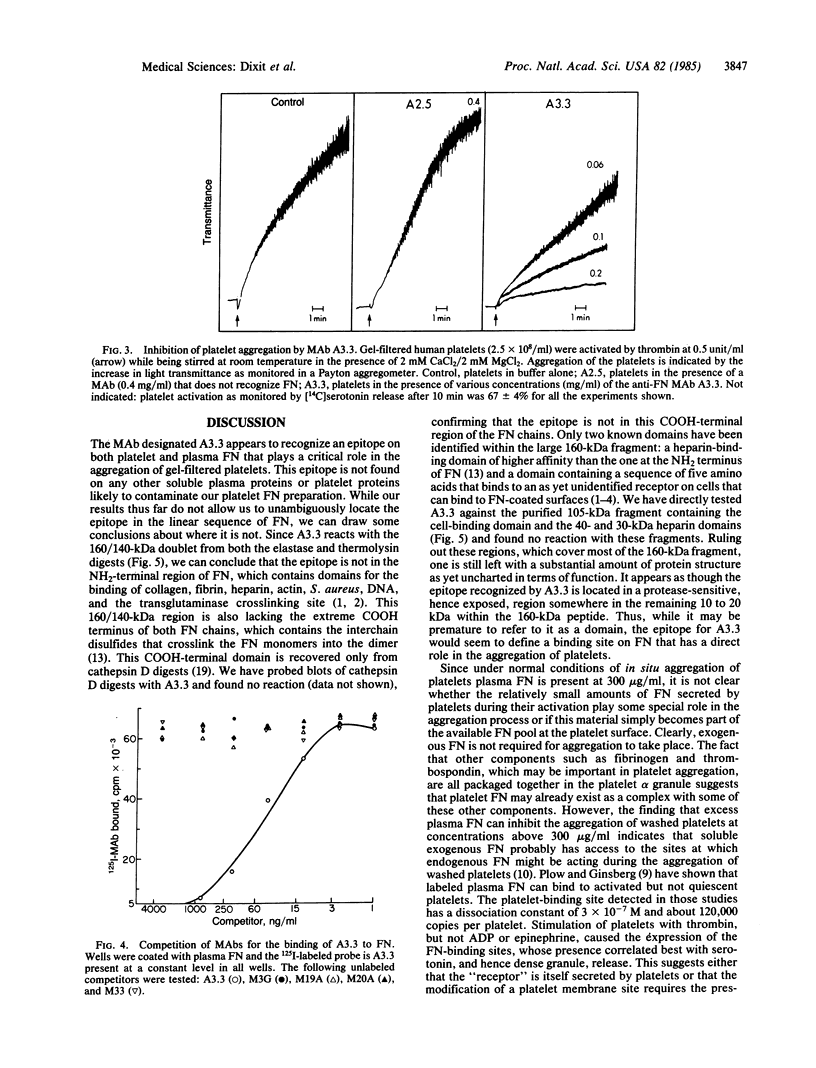
A monoclonal antibody (A3.3) has been generated against human platelet fibronectin (FN). A3.3 reacts with human plasma FN but with no other plasma proteins. A3.3 was found to inhibit thrombin- or ionophore A23187-stimulated aggregation of gel-filtered platelets in a concentration-dependent manner in both an aggregometer assay and a sensitive well plate aggregation assay. The antibody does not block secretion of serotonin. Four other anti-FN monoclonal antibodies that recognize different epitopes on FN than A3.3 does have no effect on platelet aggregation. A3.3 does not block the adhesion of CHO cells to FN-coated surfaces, indicating that it does not bind to the identified cell-binding domain of FN. A3.3 reacts with a 160/140-kDa doublet, known to contain the cell-binding domain, that is produced by digestion of FN with elastase or thermolysin. However, the antibody does not react with lower molecular weight species that also contain the cell-binding domain or with any of the other identified domains of FN. The A3.3 epitope is extremely protease sensitive and the smallest fragment found in any digest that retains reactivity with A3.3 is a 70-kDa peptide produced in low yield by mild thermolytic cleavage of FN. These data suggest that A3.3 defines a functional site present on both the platelet and plasma FN molecule that has a direct role in platelet aggregation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensusan H. B., Koh T. L., Henry K. G., Murray B. A., Culp L. A. Evidence that fibronectin is the collagen receptor on platelet membranes. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5864–5868. doi: 10.1073/pnas.75.12.5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit V. M., Grant G. A., Frazier W. A., Santoro S. A. Isolation of the fibrinogen-binding region of platelet thrombospondin. Biochem Biophys Res Commun. 1984 Mar 30;119(3):1075–1081. doi: 10.1016/0006-291x(84)90884-2. [DOI] [PubMed] [Google Scholar]
- Dixit V. M., Grant G. A., Santoro S. A., Frazier W. A. Isolation and characterization of a heparin-binding domain from the amino terminus of platelet thrombospondin. J Biol Chem. 1984 Aug 25;259(16):10100–10105. [PubMed] [Google Scholar]
- Gartner T. K., Williams D. C., Minion F. C., Phillips D. R. Thrombin-induced platelet aggregation is mediated by a platelet plasma membrane-bound lectin. Science. 1978 Jun 16;200(4347):1281–1283. doi: 10.1126/science.663608. [DOI] [PubMed] [Google Scholar]
- Ginsberg M. H., Painter R. G., Forsyth J., Birdwell C., Plow E. F. Thrombin increases expression of fibronectin antigen on the platelet surface. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1049–1053. doi: 10.1073/pnas.77.2.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg M. H., Wencel J. D., White J. G., Plow E. F. Binding of fibronectin to alpha-granule-deficient platelets. J Cell Biol. 1983 Aug;97(2):571–573. doi: 10.1083/jcb.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Yamada K. M. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982 Nov;95(2 Pt 1):369–377. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham K. C., Brew S. A., Broekelmann T. J., McDonald J. A. Thermal stability of human plasma fibronectin and its constituent domains. J Biol Chem. 1984 Oct 10;259(19):11901–11907. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lahav J., Schwartz M. A., Hynes R. O. Analysis of platelet adhesion with a radioactive chemical crosslinking reagent: interaction of thrombospondin with fibronectin and collagen. Cell. 1982 Nov;31(1):253–262. doi: 10.1016/0092-8674(82)90425-1. [DOI] [PubMed] [Google Scholar]
- Lawler J. W., Slayter H. S., Coligan J. E. Isolation and characterization of a high molecular weight glycoprotein from human blood platelets. J Biol Chem. 1978 Dec 10;253(23):8609–8616. [PubMed] [Google Scholar]
- Leung L. L., Nachman R. L. Complex formation of platelet thrombospondin with fibrinogen. J Clin Invest. 1982 Sep;70(3):542–549. doi: 10.1172/JCI110646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. A., Broekelmann T. J., Kelley D. G., Villiger B. Gelatin-binding domain-specific anti-human plasma fibronectin Fab' inhibits fibronectin-mediated gelatin binding but not cell spreading. J Biol Chem. 1981 Jun 10;256(11):5583–5587. [PubMed] [Google Scholar]
- McDonald J. A., Kelley D. G. Degradation of fibronectin by human leukocyte elastase. Release of biologically active fragments. J Biol Chem. 1980 Sep 25;255(18):8848–8858. [PubMed] [Google Scholar]
- McEver R. P., Bennett E. M., Martin M. N. Identification of two structurally and functionally distinct sites on human platelet membrane glycoprotein IIb-IIIa using monoclonal antibodies. J Biol Chem. 1983 Apr 25;258(8):5269–5275. [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Plow E. F., Birdwell C., Ginsberg M. H. Identification and quantitation of platelet-associated fibronectin antigen. J Clin Invest. 1979 Mar;63(3):540–543. doi: 10.1172/JCI109334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow E. F., Ginsberg M. H. Specific and saturable binding of plasma fibronectin to thrombin-stimulated human platelets. J Biol Chem. 1981 Sep 25;256(18):9477–9482. [PubMed] [Google Scholar]
- Richter H., Hörmann H. Early and late cathepsin D-derived fragments of fibronectin containing the C-terminal interchain disulfide cross-link. Hoppe Seylers Z Physiol Chem. 1982 Apr;363(4):351–364. doi: 10.1515/bchm2.1982.363.1.351. [DOI] [PubMed] [Google Scholar]
- Santoro S. A., Cowan J. F. Adsorption of von Willebrand factor by fibrillar collagen--implications concerning the adhesion of platelets to collagen. Coll Relat Res. 1982 Jan;2(1):31–43. doi: 10.1016/s0174-173x(82)80039-3. [DOI] [PubMed] [Google Scholar]
- Santoro S. A., Cunningham L. W. Fibronectin and the multiple interaction model for platelet-collagen adhesion. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2644–2648. doi: 10.1073/pnas.76.6.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro S. A. Inhibition of platelet aggregation by fibronectin. Biochem Biophys Res Commun. 1983 Oct 14;116(1):135–140. doi: 10.1016/0006-291x(83)90391-1. [DOI] [PubMed] [Google Scholar]
- Sekiguchi K., Hakomori S. Domain structure of human plasma fibronectin. Differences and similarities between human and hamster fibronectins. J Biol Chem. 1983 Mar 25;258(6):3967–3973. [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Kennedy D. W. Dualistic nature of adhesive protein function: fibronectin and its biologically active peptide fragments can autoinhibit fibronectin function. J Cell Biol. 1984 Jul;99(1 Pt 1):29–36. doi: 10.1083/jcb.99.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]