Abstract
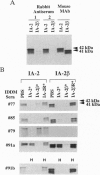
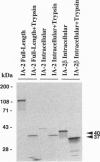
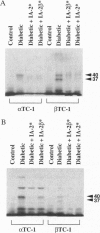
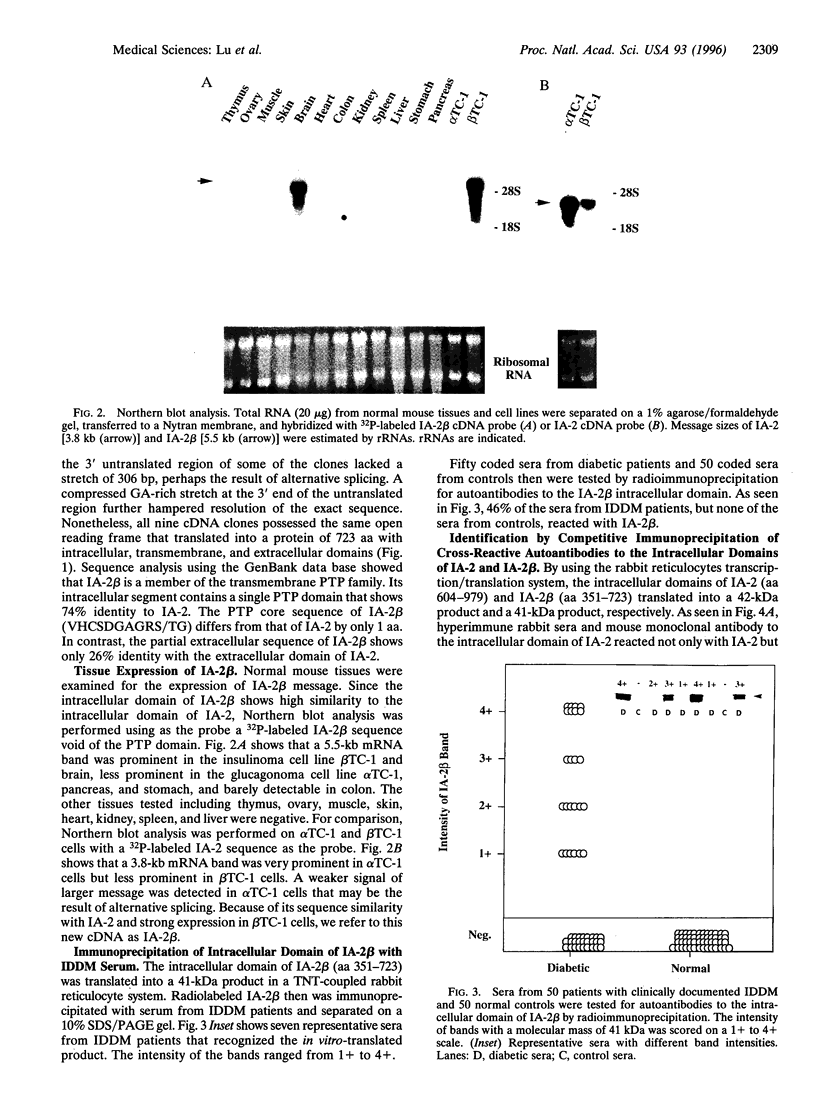
A novel cDNA, IA-2beta, was isolated from a mouse neonatal brain library. The predicted protein sequence revealed an extracellular domain, a transmembrane region, and an intracellular domain. The intracellular domain is 376 amino acids long and 74% identical to the intracellular domain of IA-2, a major autoantigen in insulin-dependent diabetes mellitus (IDDM). A partial sequence of the extracellular domain of IA-2beta indicates that it differs substantially (only 26% identical) from that of IA-2. Both molecules are expressed in islets and brain tissue. Forty-six percent (23 of 50) of the IDDM sera but none of the sera from normal controls (0 of 50) immunoprecipitated the intracellular domain of IA-2beta. Competitive inhibition experiments showed that IDDM sera have autoantibodies that recognize both common and distinct determinants on IA-2 and IA-2beta. Many IDDM sera are known to immunoprecipitate 37-kDa and 40-kDa tryptic fragments from islet cells, but the identity of the precursor protein(s) has remained elusive. The current study shows that treatment of recombinant IA-2beta and IA-2 with trypsin yields a 37-kDa fragment and a 40-kDa fragment, respectively, and that these fragments can be immunoprecipitated with diabetic sera. Absorption of diabetic sera with unlabeled recombinant IA-2 or IA-2beta, prior to incubation with radiolabeled 37-kDa and 40-kDa tryptic fragments derived from insulinoma or glucagonoma cells, blocks the immunoprecipitation of both of these radiolabeled tryptic fragments. We conclude that IA-2beta and IA-2 are the precursors of the 37-kDa and 40-kDa islet cell autoantigens, respectively, and that both IA-2 and IA-2beta are major autoantigens in IDDM.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baekkeskov S., Aanstoot H. J., Christgau S., Reetz A., Solimena M., Cascalho M., Folli F., Richter-Olesen H., De Camilli P., Camilli P. D. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990 Sep 13;347(6289):151–156. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- Baekkeskov S., Nielsen J. H., Marner B., Bilde T., Ludvigsson J., Lernmark A. Autoantibodies in newly diagnosed diabetic children immunoprecipitate human pancreatic islet cell proteins. Nature. 1982 Jul 8;298(5870):167–169. doi: 10.1038/298167a0. [DOI] [PubMed] [Google Scholar]
- Bottazzo G. F., Florin-Christensen A., Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974 Nov 30;2(7892):1279–1283. doi: 10.1016/s0140-6736(74)90140-8. [DOI] [PubMed] [Google Scholar]
- Castaño L., Russo E., Zhou L., Lipes M. A., Eisenbarth G. S. Identification and cloning of a granule autoantigen (carboxypeptidase-H) associated with type I diabetes. J Clin Endocrinol Metab. 1991 Dec;73(6):1197–1201. doi: 10.1210/jcem-73-6-1197. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Christie M. R., Genovese S., Cassidy D., Bosi E., Brown T. J., Lai M., Bonifacio E., Bottazzo G. F. Antibodies to islet 37k antigen, but not to glutamate decarboxylase, discriminate rapid progression to IDDM in endocrine autoimmunity. Diabetes. 1994 Oct;43(10):1254–1259. doi: 10.2337/diab.43.10.1254. [DOI] [PubMed] [Google Scholar]
- Christie M. R., Hollands J. A., Brown T. J., Michelsen B. K., Delovitch T. L. Detection of pancreatic islet 64,000 M(r) autoantigens in insulin-dependent diabetes distinct from glutamate decarboxylase. J Clin Invest. 1993 Jul;92(1):240–248. doi: 10.1172/JCI116556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie M. R., Tun R. Y., Lo S. S., Cassidy D., Brown T. J., Hollands J., Shattock M., Bottazzo G. F., Leslie R. D. Antibodies to GAD and tryptic fragments of islet 64K antigen as distinct markers for development of IDDM. Studies with identical twins. Diabetes. 1992 Jul;41(7):782–787. doi: 10.2337/diab.41.7.782. [DOI] [PubMed] [Google Scholar]
- Christie M. R., Vohra G., Champagne P., Daneman D., Delovitch T. L. Distinct antibody specificities to a 64-kD islet cell antigen in type 1 diabetes as revealed by trypsin treatment. J Exp Med. 1990 Sep 1;172(3):789–794. doi: 10.1084/jem.172.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan M. S., Lu J., Goto Y., Notkins A. L. Molecular cloning and identification of a receptor-type protein tyrosine phosphatase, IA-2, from human insulinoma. DNA Cell Biol. 1994 May;13(5):505–514. doi: 10.1089/dna.1994.13.505. [DOI] [PubMed] [Google Scholar]
- Lu J., Notkins A. L., Lan M. S. Isolation, sequence and expression of a novel mouse brain cDNA, mIA-2, and its relatedness to members of the protein tyrosine phosphatase family. Biochem Biophys Res Commun. 1994 Oct 28;204(2):930–936. doi: 10.1006/bbrc.1994.2549. [DOI] [PubMed] [Google Scholar]
- Ongagna J. C., Levy-Marchal C. Anti-37kDa antibodies are associated with the development of IDDM in individuals with islet cell antibodies. Diabetologia. 1995 Mar;38(3):370–375. doi: 10.1007/BF00400644. [DOI] [PubMed] [Google Scholar]
- Palmer J. P., Asplin C. M., Clemons P., Lyen K., Tatpati O., Raghu P. K., Paquette T. L. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science. 1983 Dec 23;222(4630):1337–1339. doi: 10.1126/science.6362005. [DOI] [PubMed] [Google Scholar]
- Payton M. A., Hawkes C. J., Christie M. R. Relationship of the 37,000- and 40,000-M(r) tryptic fragments of islet antigens in insulin-dependent diabetes to the protein tyrosine phosphatase-like molecule IA-2 (ICA512). J Clin Invest. 1995 Sep;96(3):1506–1511. doi: 10.1172/JCI118188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietropaolo M., Castaño L., Babu S., Buelow R., Kuo Y. L., Martin S., Martin A., Powers A. C., Prochazka M., Naggert J. Islet cell autoantigen 69 kD (ICA69). Molecular cloning and characterization of a novel diabetes-associated autoantigen. J Clin Invest. 1993 Jul;92(1):359–371. doi: 10.1172/JCI116574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin D. U., Pleasic S. M., Shapiro J. A., Yoo-Warren H., Oles J., Hicks J. M., Goldstein D. E., Rae P. M. Islet cell antigen 512 is a diabetes-specific islet autoantigen related to protein tyrosine phosphatases. J Immunol. 1994 Mar 15;152(6):3183–3188. [PubMed] [Google Scholar]