Abstract
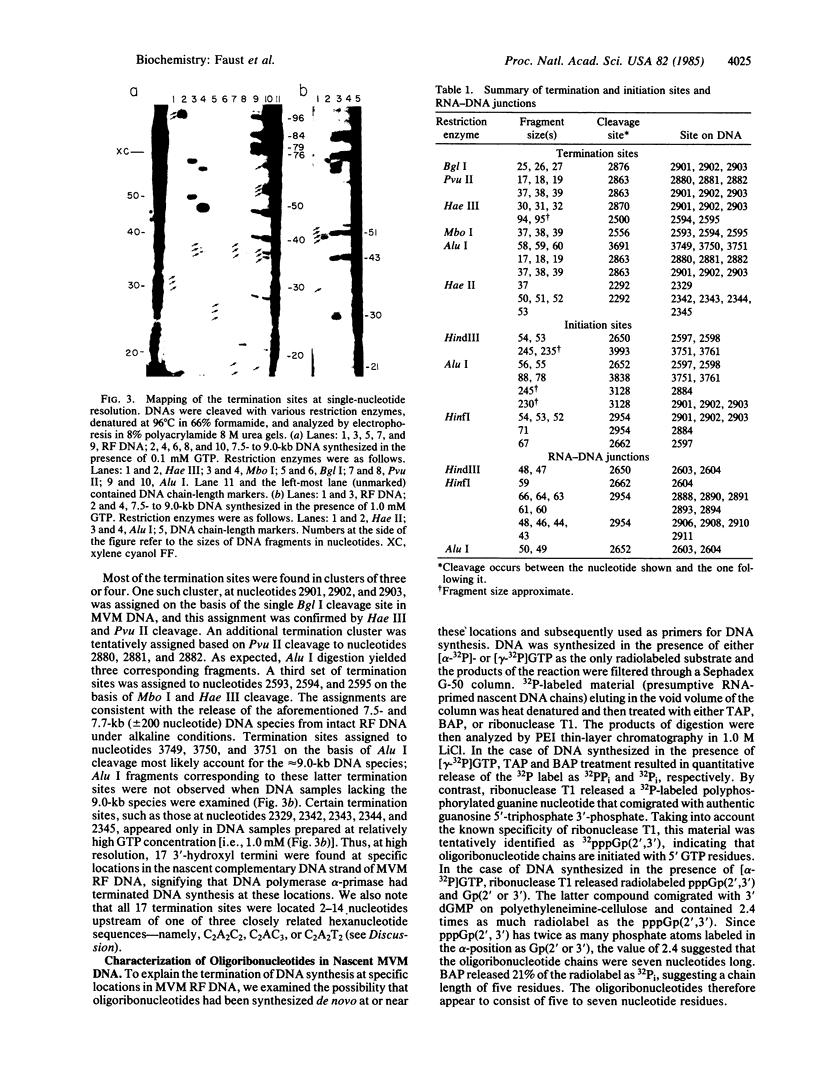
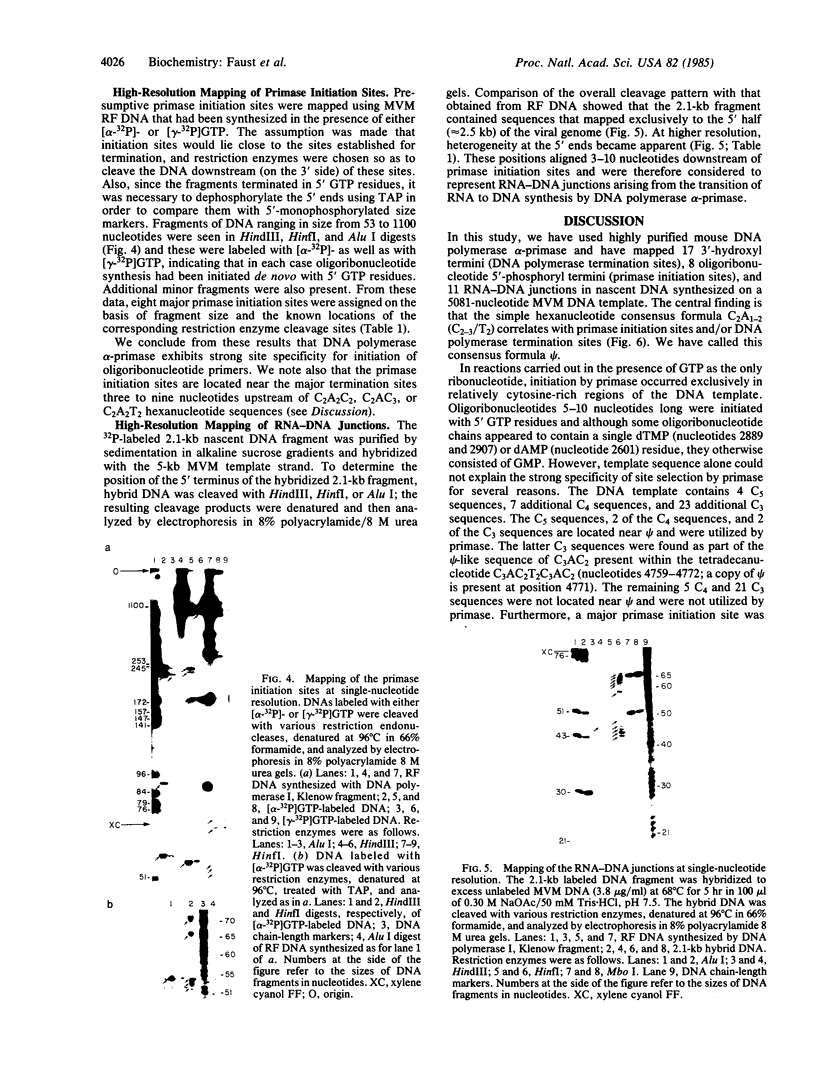
The distribution of termination and initiation sites in a 5081-nucleotide minute virus of mice DNA template being copied by a highly purified mouse DNA polymerase alpha-DNA primase complex in the presence of GTP has been examined. The 3'-hydroxyl termini (17 in all) were clustered at six sites that were located 2-14 nucleotides upstream of C2A2C2, C2AC3, or C2A2T2 sequences. When either [alpha-32P]- or [gamma-32P]GTP was included in the DNA polymerase reaction mixtures, nascent DNA became radiolabeled. Analysis of the 32P-labeled material following treatment of the DNA with tobacco acid pyrophosphatase, bacterial alkaline phosphatase, or ribonuclease T1 revealed the presence of oligoribonucleotide chains averaging 5-7 nucleotides long and beginning with 5' GTP residues. Eight presumptive DNA primase initiation sites were located opposite C4 or C5 sequences 3-9 nucleotides upstream of one of the three closely related hexanucleotides C2A2C2, C2AC3, and C2A2T2. RNA-DNA junctions were found 3-10 nucleotides downstream of DNA primase initiation sites. The results indicate that hexanucleotides having the general formula C2A1-2(C2-3/T2), herein referred to as psi, are involved in promoting termination of DNA synthesis and/or de novo initiation of RNA-primed DNA chains by DNA polymerase alpha-primase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astell C. R., Thomson M., Merchlinsky M., Ward D. C. The complete DNA sequence of minute virus of mice, an autonomous parvovirus. Nucleic Acids Res. 1983 Feb 25;11(4):999–1018. doi: 10.1093/nar/11.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. H. Telomeres: do the ends justify the means? Cell. 1984 May;37(1):7–8. doi: 10.1016/0092-8674(84)90295-2. [DOI] [PubMed] [Google Scholar]
- Conaway R. C., Lehman I. R. A DNA primase activity associated with DNA polymerase alpha from Drosophila melanogaster embryos. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2523–2527. doi: 10.1073/pnas.79.8.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conaway R. C., Lehman I. R. Synthesis by the DNA primase of Drosophila melanogaster of a primer with a unique chain length. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4585–4588. doi: 10.1073/pnas.79.15.4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust E. A., Gloor G. Characterization of a metastable, partially replicated dimeric intermediate of minute virus of mice. J Virol. 1984 Feb;49(2):621–625. doi: 10.1128/jvi.49.2.621-625.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust E. A., Gloor G., MacIntyre M. F., Nagy R. ATP(GTP)-dependent conversion of MVM parvovirus single-stranded DNA to its replicative form by a purified 10 S species of mouse DNA polymerase alpha. Biochim Biophys Acta. 1984 Apr 5;781(3):216–224. doi: 10.1016/0167-4781(84)90086-1. [DOI] [PubMed] [Google Scholar]
- Faust E. A., Rankin C. D. In vitro conversion of MVM parvovirus single-stranded DNA to the replicative form by DNA polymerase alpha from Ehrlich ascites tumour cells. Nucleic Acids Res. 1982 Jul 24;10(14):4181–4201. doi: 10.1093/nar/10.14.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust E. A., Ward D. C. Incomplete genomes of the parvovirus minute virus of mice: selective conservation of genome termini, including the origin for DNA replication. J Virol. 1979 Oct;32(1):276–292. doi: 10.1128/jvi.32.1.276-292.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt N., Dinsart C., Spadari S., Pedrali-Noy G., Rommelaere J. Interrelation between viral and cellular DNA synthesis in mouse cells infected with the parvovirus minute virus of mice. J Gen Virol. 1983 Sep;64(Pt 9):1991–1998. doi: 10.1099/0022-1317-64-9-1991. [DOI] [PubMed] [Google Scholar]
- Hay R. T., DePamphilis M. L. Initiation of SV40 DNA replication in vivo: location and structure of 5' ends of DNA synthesized in the ori region. Cell. 1982 Apr;28(4):767–779. doi: 10.1016/0092-8674(82)90056-3. [DOI] [PubMed] [Google Scholar]
- Hay R. T., Hendrickson E. A., DePamphilis M. L. Sequence specificity for the initiation of RNA-primed simian virus 40 DNA synthesis in vivo. J Mol Biol. 1984 May 15;175(2):131–157. doi: 10.1016/0022-2836(84)90471-6. [DOI] [PubMed] [Google Scholar]
- Holmes A. M., Johnston I. R. Molecular asymmetry of rat liver cytoplasmic DNA polymerase. FEBS Lett. 1973 Jan 1;29(1):1–6. doi: 10.1016/0014-5793(73)80002-x. [DOI] [PubMed] [Google Scholar]
- Kaguni L. S., Rossignol J. M., Conaway R. C., Lehman I. R. Isolation of an intact DNA polymerase-primase from embryos of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2221–2225. doi: 10.1073/pnas.80.8.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann G., Bar-Shavit R., DePamphilis M. L. Okazaki pieces grow opposite to the replication fork direction during simian virus 40 DNA replication. Nucleic Acids Res. 1978 Jul;5(7):2535–2545. doi: 10.1093/nar/5.7.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozu T., Yagura T., Seno T. De novo DNA synthesis by a novel mouse DNA polymerase associated with primase activity. Nature. 1982 Jul 8;298(5870):180–182. doi: 10.1038/298180a0. [DOI] [PubMed] [Google Scholar]
- Mosbaugh D. W., Linn S. Gap-filling DNA synthesis by HeLa DNA polymerase alpha in an in vitro base excision DNA repair scheme. J Biol Chem. 1984 Aug 25;259(16):10247–10251. [PubMed] [Google Scholar]
- Närkhammar-Meuth M., Kowalski J., Denhardt D. T. Both strands of polyoma DNA are replicated discontinuously with ribonucleotide primers in vivo. J Virol. 1981 Jul;39(1):21–30. doi: 10.1128/jvi.39.1.21-30.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd, Klaassen B. DNA sequence of the 5' terminus containing the replication origin of parvovirus replicative form DNA. J Virol. 1982 Mar;41(3):990–999. doi: 10.1128/jvi.41.3.990-999.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng B. Y., Ahlem C. N. A DNA primase from mouse cells. Purification and partial characterization. J Biol Chem. 1983 Aug 25;258(16):9845–9849. [PubMed] [Google Scholar]
- Tseng B. Y., Ahlem C. N. DNA primase activity from human lymphocytes. Synthesis of oligoribonucleotides that prime DNA synthesis. J Biol Chem. 1982 Jul 10;257(13):7280–7283. [PubMed] [Google Scholar]
- Tseng B. Y., Ahlem C. N. Mouse primase initiation sites in the origin region of simian virus 40. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2342–2346. doi: 10.1073/pnas.81.8.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl A. F., Kowalski S. P., Harwell L. W., Lord E. M., Bambara R. A. Immunoaffinity purification and properties of a high molecular weight calf thymus DNA alpha-polymerase. Biochemistry. 1984 Apr 24;23(9):1895–1899. doi: 10.1021/bi00304a001. [DOI] [PubMed] [Google Scholar]
- Wang T. S., Hu S. Z., Korn D. DNA primase from KB cells. Characterization of a primase activity tightly associated with immunoaffinity-purified DNA polymerase-alpha. J Biol Chem. 1984 Feb 10;259(3):1854–1865. [PubMed] [Google Scholar]
- Yagura T., Kozu T., Seno T., Saneyoshi M., Hiraga S., Nagano H. Novel form of DNA polymerase alpha associated with DNA primase activity of vertebrates. Detection with mouse stimulating factor. J Biol Chem. 1983 Nov 10;258(21):13070–13075. [PubMed] [Google Scholar]
- Yagura T., Tanaka S., Kozu T., Seno T., Korn D. Tight association of DNA primase with a subspecies of mouse DNA polymerase alpha. J Biol Chem. 1983 Jun 10;258(11):6698–6700. [PubMed] [Google Scholar]