Abstract
Copper chelation has been shown to favor the expansion of human hematopoietic stem/progenitor cells in vitro. To further understand the effects of copper modulation on defined subsets of stem cells versus progenitor cells, we extended the studies in a mouse system. We isolated mouse hematopoietic stem cells (HSCs) or hematopoietic progenitor cells (HPCs) and cultured them with or without the copper chelator tetraethylen-epentamine (TEPA) or CuCl2. Cytokine-stimulated HPC cultures treated with TEPA for 7 days generated about two to three times more total and erythroid colony-forming cells (CFCs) compared to control cultures. In contrast, CuCl2 treatment decreased the CFC numbers. Similar results were seen with HSC after 14, but not 7, days of culture. Transplant studies showed that HPCs cultured for 7 days in TEPA had about twofold higher short-term erythroid repopulation potential compared to control cultures, while CuCl2 decreased the erythroid potential of cultured HPCs compared to control cultures. HSCs cultured with TEPA for 7 days did not exhibit significantly higher repopulation potential in either leukocyte or erythrocyte lineages compared to control cultures in short-term or long-term assays. Based on JC-1 staining, the mitochondrial membrane potential of HPCs cultured with TEPA was lower relative to control cultures. Our data suggest that decreasing the cellular copper content with TEPA results in preferential expansion or maintenance of HPC that are biased for erythroid differentiation in vivo, but does not enhance the maintenance of HSC activity in culture.
Keywords: Hematopoietic stem cells, Progenitor cells, Adult stem cells, Bone marrow, Erythropoiesis
INTRODUCTION
Metal elements, such as iron (Fe), calcium (Ca), magnesium (Mg), zinc (Zn), and copper (Cu), are fundamental and essential for life. Cu is a cofactor for many cellular enzymes, such as cytochrome c oxidase, superoxide dismutase, tyrosinase, peptidylglycine alpha-amidating mono-oxygenase, and lysyl oxidase (17,43,51). Cu has also been shown to regulate gene expression, cellular protein functions, and cell differentiation (3,9,15,23,26,29,38,52). However, excess intracellular Cu is toxic due to its role in catalyzing the generation of reactive oxygen species (ROS) through Fenton chemistry (31). Specifically, Cu is essential for normal hematopoiesis in which mature blood cells are constantly replenished from hematopoietic stem cells in the bone marrow. In adult mammals, hematopoietic stem cells (HSCs) gradually lose their self-renewal capability and multipotency and restrict to specific lineages. HSC must replicate through self-renewing cell divisions to maintain the pool of HSCs. This process is tightly regulated by the interplay of extrinsic and intrinsic molecular mechanisms in order to both sustain blood production and maintain the HSC compartment (28).
In vitro studies showed that addition of Cu salts augmented the retinoic acid-induced differentiation of the human myeloid leukemia cell line, HL-60 (1). Lowering of the cellular Cu level by the Cu chelator tetraethylene-pentamine (TEPA) blocked 1,25-dihydroxyvitamin D3 or phorbol 12-myristate 13-acetate-induced U937 cell differentiation and this blocking was reversed by adding back Cu (22). The importance of Cu in regulating hematopoiesis is also inferred from inherited or acquired Cu deficiency in humans and mice. Cu deficiency due to genetic mutations or malnourishment in humans causes anemia, neutropenia, and thrombocytopenia due to the arrested differentiation at the hematopoietic progenitor cell level (6,13,14,16,19,36,44,46,58).
Interestingly, recent studies showed that the high-affinity copper chelator TEPA, together with early acting cytokines [thrombopoietin (TPO), stem cell factor (SCF), interleukin-6 (IL-6), and Flt3 ligand (FL)] can promote the expansion of both hematopoietic progenitor cells (HPCs) and hematopoietic stem cells (HSCs) from human cord blood-derived CD34+ cells in culture (39,41). The expansion of HPCs was shown by in vitro colony-forming assay and FACS analysis of the expression of cell surface markers. The expansion of the HSCs was shown by the repopulation of nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice with cultured cells (40,42). This finding has justified a clinical trial using TEPA together with cytokines for the ex vivo expansion of human HSC/HPCs prior to transplantation into patients (11,42). Although the expansion of HPCs is clearly established by both the in vitro and in vivo assays, the expansion of HSCs in these cultures is less convincing.
There are still many controversies about the faithfulness of the NOD/SCID mouse model in detection and quantification of human HSCs (7). Because the differences of the physiological environment of mouse and human and the differences of the life span and proliferation capacities of mouse and human hematopoietic cells, the detection of human cells in NOD/SCID mouse bone marrow 8 weeks after the transplantation does not prove the contribution from the human HSCs (20). As a result, it is unclear whether TEPA significantly promotes the self-renewal and expansion of HSCs in vitro.
The objective of the present study was to clarify these issues by extending the observations in the mouse system, where isolation and unequivocal assays for HSC and HPC are better defined. To separate mouse HSCs from HPCs for analysis of the effect of Cu modulation on each of these two populations, we used cell surface markers in combination with the mitochondrial dye rhodamine-123 to fractionate mouse bone marrow cells. The most primitive HSC and HPC reside in a population of bone marrow cells that does not express mature blood cell surface markers (lineage negative or Lin−) and expresses Sca-1 and c-Kit cell surface markers. These bone marrow cells are commonly referred to as the LSK population (37,49). We further divide the LSK population by rhodamine-123 staining. Rhodamine-123 (Rho) is a mitochondrial dye that stains cells based on their state of activation, in which the more metabolically active cells fluoresce brightly while more quiescent cells fluoresce dimly (2). Segregation of the LSK population using Rho has demonstrated that the LSKRholow population contains long-term HSC with a frequency of about 25%, while the LSKRhohi population lacks HSC but is highly enriched for multipotent and lineage-restricted progenitor cells (32,50). We cultured these two populations separately with early acting cytokines (SCF, TPO, IL-6, FL) and used TEPA or CuCl2 to modulate the Cu content within the cultured cells. We used in vitro colony-forming assays and in vivo mouse competitive repopulation assays to characterize and quantify the progenitor and stem cells within the cultures.
Our results show that TEPA promotes the expansion or maintenance of progenitor cells that are mainly erythroid lineage biased within the HPC culture. In contrast, TEPA did not significantly promote the expansion or maintenance of HSC in culture. The effects of TEPA on HPC correlate with its effect of reducing the mitochondrial membrane potential of cultured HPC.
MATERIALS AND METHODS
Mice
Mice carrying the Thy-1.1 and Ly-5.1 alleles on a C57BL background were generated in our breeding colony by mating the BKa.AK-Thy1a/Ka and B6.SJL-Ptprca Pep3b/BoyJ strains and selecting for cosegregation of Thy-1.1 (CD90a) and Ly-5.1 (CD45a). Breeding pairs of B6.Cg-Gpi1a Hbbd H1b/DehJ mice (18) were kindly provided by David Harrison (Jackson Laboratory, Bar Harbor, ME, USA). We refer to these mice as B6-Hbbd. GFP transgenic mice were provided by Dr. Masaru Okabe (Osaka University, Osaka, Japan). This transgenic strain was generated by pronuclear injection of fertilized eggs obtained from C57BL/6 matings as previously described (35) and are here referred to as B6-GFP. Mice carrying both GFP transgene and Hbbd allele were generated in our breeding colony by mating B6-Hbbd mice with B6-GFP mice and selected for cosegregation of GFP and Hbbd. We refer to these mice as B6-GFP/Hbbd. All animals were maintained in the animal resources center at the University of Utah under protocols approved by the institutional animal care and use committee.
Antibodies
Monoclonal antibodies against CD2 (Rm2.2), CD3 (KT3-1.1), CD5 (53-7.3), CD8 (53-6.7), CD11b (M1/70), Ly-6G (RB6-8C5), TER119, CD45R (B220; RA3-6B2), and CD19 (1D3) were purified from media of cultured hybridoma cell lines. Phycoerythrin (PE)-conjugated monoclonal antibodies to Sca-1 and CD19 were purchased from PharMingen (San Diego, CA, USA). Anti-c-kit mAb 3C11 and anti-Ly5.1 mAb A20.1 were purified and conjugated to Alexa Fluor 647 in our laboratory. PE-conjugated monoclonal antibodies to CD4, CD8, B220, Mac-1, and Gr-1 were purchased from eBioscience (San Diego, CA, USA) or purified and conjugated in our laboratory.
Cytokines
Recombinant mouse SCF was expressed in bacteria and purified from lysates as previously described (55). Recombinant mouse FL, IL-3, GM-CSF, and IL-6 were purchased from Peprotech (Rocky Hill, NJ, USA). The peptide mimetic of TPO was synthesized as previously described (8). Recombinant human erythropoietin (EPO) was purchased from Ortho Pharm. Corp. (Raritan, NJ, USA).
Isolation of Bone Marrow Hematopoietic Stem/Progenitor Cells (HSC/HPCs)
Mouse bone marrow cells were incubated in a cocktail of antibodies to CD2, CD3, CD5, CD8, CD11b, Ly- 6G, TER119, CD45R, and CD19. Lineage depletion was performed by two successive incubations in sheep anti-rat Ig-coupled magnetic beads (Dynal, Oslo, Norway). Lineage-negative (Lin−) cells were incubated with 0.2 μM rhodomine-123 (Molecular Probes, Eugene, OR, USA) in Hank’s balanced salt solution (HBSS) containing 5% fetal calf serum at 37°C for 20 min. After washing once, the cells were resuspended in fresh HBSS and incubated at 37°C for another 20 min to allow for efflux of the dye. After washing, the cells were stained with PE-conjugated anti-Sca-1 and AlexaFluor 647-conjugated anti-c-kit antibodies. Sorting was done using FACS Vantage or FACS Aria instruments (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA). We sorted Lin−Sca-1+c-kit+ (LSK) bone marrow cells as LSKRholow and LSKRhohi populations using Rho-stained bone marrow cells to determine the placement of the Rho gates. This usually resulted in LSKRholow cells comprising the lower 20% of the Rho distribution and LSKRhohi cells comprising the upper 50% of the Rho distribution. An aliquot of each sorted cell population was taken for reanalysis to evaluate purity and cell count.
In Vitro Liquid Culture of HSCs and HPCs
Purified LSKRholow and LSKRhohi cells were cultured in 24-well plates at 1000 cells/ml in α-MEM medium supplemented with 10% fetal bovine serum (FBS) (Hyclone), 2 mM l-glutamine, 100 μM 2-mercaptoethanol, 1% each sodium pyruvate and pen/strep stock solutions (Invitrogen Corp, Carlsbad, CA, USA), and the following mouse recombinant cytokines: SCF at 100 ng/ml, TPO mimetic at 5 nM, IL-6 at 10 ng/ml, and FL at 10 ng/ml. Cellular Cu content was modulated by supplementing the culture with either TEPA and/or CuCl2 (both obtained from Sigma-Aldrich, St. Louis, MO, USA) as indicated. At weekly intervals, cell cultures were demi-depopulated and supplemented with fresh medium, serum, cytokines, and other components as indicated. The cultures were incubated at 37°C in a humidified atmosphere of 5% CO2 in air. At various time points, cells were harvested and counted by hemocytometer following staining with trypan blue. The intracellular Cu content of cultured cells was determined using inductively coupled plasma-atomic emission spectrometry (ICP-AES) (Perkin Elmer Life Sciences) as described previously (30).
Colony-Forming Cell (CFC) Assay
Cells were plated in α-MEM-based methylcellulose containing 10% fetal bovine serum (FBS), 10% deionized bovine serum albumen (BSA), antibiotics, l-glutamine, and 2-mercaptoethanol. Colony growth and differentiation were stimulated with recombinant cytokines in combination as indicated, including SCF (100 ng/ml), G-CSF (10 ng/ml), IL-3 (10 ng/ml), IL-6 (20 ng/ml), TPO mimetic (5 nM), and Epo (4 U/ml). After 7 days of culture, quadruplicate plates were stained using benzidine hydrochloride and scored for total colonies and for colonies containing hemoglobinized erythroid lineage cells colonies based on benzidine staining.
Mouse Transplantation Assay
Radiation was delivered to B6-Hbbd or B6-GFP/Hbbd recipient mice in a split dose (2 × 6 Gy) with a 3-h interval between doses, using a Shepherd Mark I 137Cs source (JL Shepherd and Associates, Glendale, CA, USA) at a dose rate of 0.8 Gy/min. Cultured cells mixed with competitor BM cells were transplanted by the retro-orbital route under isoflurane anesthesia (IsoSol; Vedco Inc., St. Joseph, MO, USA). Peripheral blood samples were collected from the retro-orbital sinus under isoflurane anesthesia delivered using the E-Z Anesthesia system (Euthanex Corp., Palmer, PA, USA). Blood was collected in heparinized capillary tubes and mixed with acid citrate dextrose at a 10:1 ratio prior to determination of the complete blood count using a Serono System 9010+ CP hematology counter (Serono Diagnostics, Al-lentown, PA, USA). Samples were then mixed with an equal volume of 2% Dextran T500 (Amersham Biosciences, Piscataway, NJ, USA) in phosphate-buffered saline (PBS) and incubated at 37°C for 30 min. The upper layer, containing leukocytes, platelets, and residual erythrocytes, was collected for flow cytometry analysis, whereas the sedimented erythrocytes were used for Hbb analysis.
Determination of Hbb Variants by HPLC
A cation exchange protocol was developed to separate and quantitate Hbb variant alleles by HPLC (48). A stock solution of 100 mM 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) (Sigma-Aldrich) was prepared by dissolving 10 mg of DTNB in 250 μl of dimethyl sulfoxide and stored at −20°C. Erythrocytes obtained from heparinized blood were washed three times in dextrose-gelatin-veronal buffer and stored as packed pellets at 4°C for up to 2 weeks prior to analysis. Samples were derivatized by adding 10 μl of packed erythrocytes to 200 μl of 40 mM NaCl, 2 mM DTNB and incubating at room temperature for 30 min. Following centrifugation (12,000 × g for 2 min), samples were analyzed using a VARIANT hemoglobin testing system (Bio-Rad Laboratories, Hercules, CA, USA).
Statistical Analysis
All results are expressed as mean ± SEM. Statistical significance between any two conditions of interest was determined using the unpaired Student t-test with significance set at a value of p < 0.05, corrected where appropriate by the Bonferroni method.
RESULTS
Effects of TEPA and Cu Supplementation on Intracellular Cu Content of Cultured Progenitor Cells
Because previous studies using TEPA in hematopoietic cultures have focused on human cells, an initial dose–response experiment was performed in order to determine the optimal TEPA concentration in cultures of mouse HSC/HPC. We assayed total cell proliferation and CFC proliferation at each of the various concentrations of TEPA (5, 10, 20, 40, and 80 μM) in the presence of SCF, TPO mimetic, IL-6, and FL over 1–3 weeks. These experiments established a dose of 40 μM TEPA as optimal for mouse HSC/HPC proliferation in culture, based on CFC analysis (data not shown). We then determined the effect of supplementing TEPA and CuCl2 on intracellular Cu content of HPC cultures. We performed two independent measurements using inductively coupled plasma-optical emission spectrometry (ICP-OES) (30). LSKRhohi HPCs were cultured in serum and cytokine-containing medium for 7 days. Under these conditions, ICP-OES measurements indicated that cultured cells contained 7.6–12.7 ng Cu/107 cells. When the Cu chelator TEPA was added to the culture at 40 μM for 7 days, the cellular Cu content decreased to 2.4–2.8 ng/107 cells. TEPA-treated cultures showed only modest changes in other metals (Fe 30% decrease, Zn 8% decrease), suggesting that the TEPA effect is copper specific. Addition of 10 μM CuCl2 to the culture medium increased the cellular Cu content to 686.3–3088.5 ng/107 cells (Table 1). These results indicate that the cellular Cu content of HPC can be modulated by the addition of TEPA or CuCl2 to the culture medium.
Table 1.
Intracellular Cu Content of Cultured Hematopoietic Progenitor Cells Is Modulated by Cu Chelator and Cu Salt
| 40 μM TEPA | 10 μM CuCl2 | Control | |
|---|---|---|---|
| Cu (ng)/107 cells (experiment 1) | 2.4 | 686.3 | 7.6 |
| Cu (ng)/107 cells (experiment 2) | 2.8 | 3088.5 | 12.7 |
LSKRhohi HPCs were cultured with cytokines ± TEPA or CuCl2 for 7 days. The copper content of the cultured cells was determined by inductively coupled plasma-atomic emission spectrometry (ICP-AES).
Effects of TEPA and Cu Supplementation in HSC/HPC Cultures
We analyzed the progeny of HSC or HPC after 7 and 14 days of liquid culture by counting total cell numbers, and used the in vitro methylcellulose-based hematopoietic colony-forming assay to measure CFC activity in the cultures (Figs. 1 and 2). LSKRhohi HPC cultured with 40 or 80 μM TEPA plus FBS and cytokines for 7 days produced two to three times more total CFCs than control cultures maintained with FBS and cytokines alone (p = 0.003 and p = 0.0007) (Fig. 1A). Interestingly, in the presence of 80 μM TEPA the expansion of cell numbers was reduced relative to controls while the CFC numbers increased significantly (p = 0.0007) (Fig. 1A). In contrast, LSKRhohi HPC cultured with 10 μM CuCl2 plus FBS and cytokines for 7 days produced about half of the CFCs of control culture (p = 0.004) with a similar expansion of cell numbers. TEPA at 80 μM also increased the erythroid CFC number compared to control (p = 0.04) while 40 μM had no effect on erythroid CFCs (Fig. 1A). After 14 days of culture, LSKRhhi HPCs had decreased number of total cell numbers and CFC numbers compared to day 7 cultures, but cultures supplemented with 40 or 80 μM TEPA had much more total CFC and erythroid CFC number than cultures supplemented with CuCl2 or control cultures (Fig. 1B). In parallel cultures initiated with LSKRholow HSC, we observed no difference in total CFC number between cell cultures with TEPA, CuCl2, and control in 1 week of culture (Fig. 2A). Relative to control cultures, we observed an increase in erythroid CFC in cultures containing 40 μM TEPA (p = 0.0001) and a decrease in erythroid CFC in cultures containing supplementary Cu (Fig. 2A). After 2 weeks of culture, LSKRholow cells cultured with 40 μM TEPA generated about 30% more total CFCs compared to control cultures containing cytokines only, while treatment with 10 μM CuCl2 decreased the total CFCs in culture compared to control (Fig. 2B).
Figure 1.
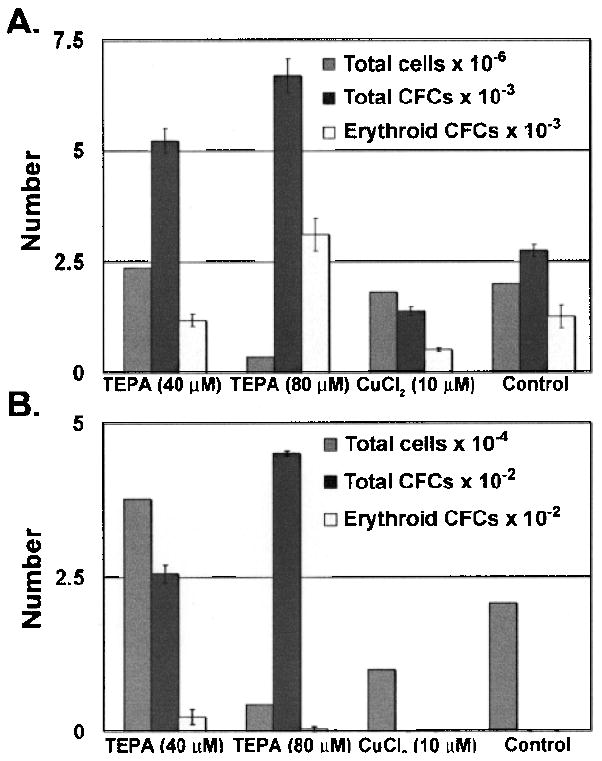
The effects of TEPA or Cu supplementation on HPC cultures. LSKRhohi HPCs (1000 cells) were cultured with cytokines ± TEPA/Cu at indicated concentrations for 7 days (A) or 14 days (B). The total cell number and total CFC and erythroid CFC number were determined at the end of culture.
Figure 2.
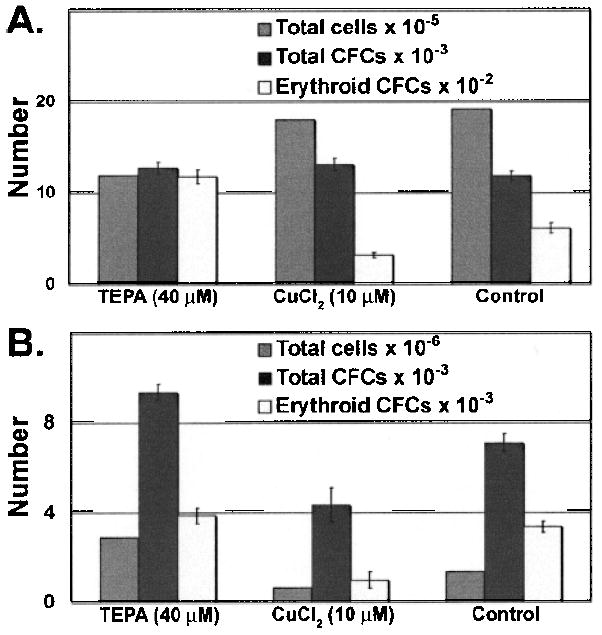
The effects of TEPA or Cu supplementation on HSC cultures. LSKRholow HSCs (1000 cells) were cultured with cytokines ± TEPA/Cu at indicated concentrations for 7 days (A) or 14 days (B). The total cell number and total CFC and erythroid CFC number were determined at the end of culture.
We examined the morphology of the cells in cultures established from LSKRhohi HPCs by May-Grunwald-Giemsa staining of cytospins. Cultures supplemented with 40 μM TEPA contained cells with a uniform and blast-like morphology, while control cultures contained more cells with morphological characteristics of differentiation (Fig. 3A, B). Supplementation with 10 μM CuCl2 increased the portion of cells with differentiated cell morphology, while adding TEPA to CuCl2-supplemented culture reversed the effect of Cu (Fig. 3C, D).
Figure 3.
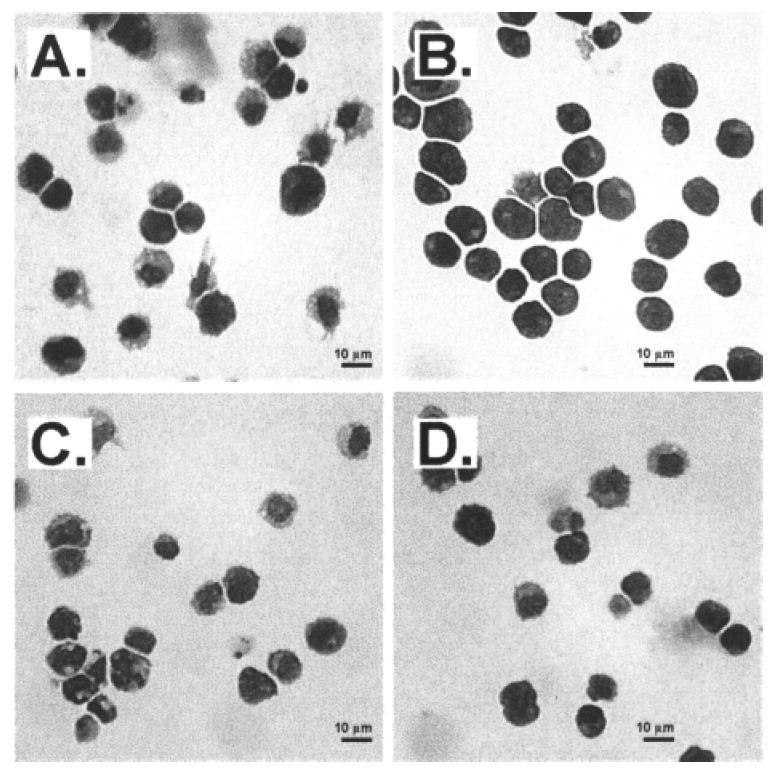
The effects of TEPA or Cu supplementation on the morphology of HPC cultures. LSKRhohi HPCs were cultured with cytokines ± TEPA/Cu at the indicated concentrations for 7 days. Cultured cells were cytospun into slides and stained with May-Grunwald-Giemsa. The culture conditions for cells shown were: (A) control; (B) 40 μM TEPA; (C) 10 μM CuCl2; (D) 40 μM TEPA 10 + μM CuCl2. Scale bar: 10 μm.
Effects of TEPA and Cu Supplementation on In Vivo Short-Term Trilineage Repopulating Activity of Cultured HPCs
To evaluate the in vivo repopulation activity of cultured cells in the three major blood lineages, we harvested 1-week cultures of LSKRhohi HPCs and transplanted these cells together with a fixed number of competitor bone marrow cells into lethally irradiated congenic recipient mice. Cultured cells were tracked based on expression of the Ly5.1 allele on the cell surface of white blood cells and the hemoglobin-β single (Hbbs) variant in red blood cells and lack of GFP expression on platelets (48). Competitor bone marrow and recipient mice cells expressed the Ly5.2 allele, the hemoglobin-β diffuse (Hbbd) variant, and GFP. This transplant model allows us to track the in vivo contribution of cultured cells into the leukocyte, platelet, and erythrocyte lineages. We transplanted cultured cells obtained from a 7-day culture of 1000 LSKRhohi cells, together with 105 freshly obtained competitor bone marrow cells into lethally irradiated mice and followed the relative contributions of cultured cells and competitor BM cells into the leukocyte, platelet, and erythrocyte lineages in the peripheral blood of recipient mice over time. LSKRhohi cells cultured with 40 μM TEPA generated about twofold higher percentage of reconstitution of the erythroid lineage compared to LSKRhohi cells cultured with cytokines alone, in spite of a lower degree of cellularity in the cultures expanded under this condition (Fig. 4A, B). The red blood cell count of the recipient mice was similar among the groups (data not shown). This enhancement of erythroid engraftment persisted over the time course of progenitor cell activity in the recipients, and was limited to the erythroid lineage as these cultures had about 30% of the leukocyte lineage repopulation activity of LSKRhohi cells cultured with cytokines alone. The effect of TEPA was reversed by addition of CuCl2 into the culture medium. TEPA and Cu had no obvious effects on platelet lineage repopulation activity of cultured LSKRhohi cells (data not shown). The contribution of cultured cells to the peripheral blood of recipient mice was limited to 9 weeks posttransplantation, consistent with the absence of stem cells in the LSKRhohi population and its cultured progeny. Collectively, our results suggest that TEPA preferentially promotes the maintenance or expansion of erythroid-biased progenitor cells in cultures established from primitive progenitor cells.
Figure 4.
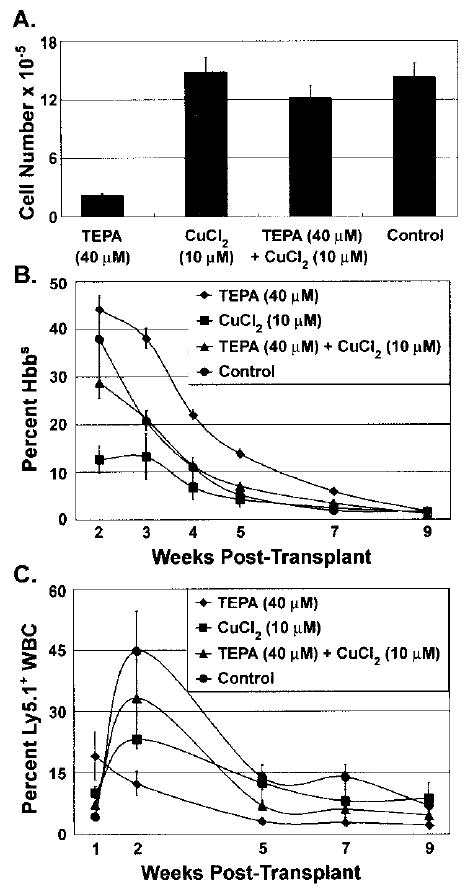
The effects of TEPA or Cu supplementation on the short-term erythrocyte and leukocyte repopulation potential of HPC cultures. LSKRhohi HPCs (1000 cells) were cultured with cytokines ± TEPA/Cu at the indicated concentrations for 7 days. The total cell numbers were determined at the end of culture (A). The total cells were mixed with 105 whole BM cells and injected intravenously into lethally irradiated mice. The percentage contribution of cultured cells to peripheral blood erythrocytes (B) and leukocytes (C) were determined at the various times posttransplantation.
Effects of TEPA and CuCl2 Supplementation on the Short-Term and Long-Term Repopulation Capacity of Cultured HSCs
To evaluate the effects of TEPA on expansion of HSC number and function, we tested the activity of cultured LSKRholow HSC in the long-term competitive repopulation assay. The 7-day cultured cells derived from 103 LSKRholow BM cells were cotransplanted with 106 fresh competitor BM cells into lethally irradiated recipient mice. The relative contribution of cultured cells and competitor cells into peripheral blood leukocyte and erythrocyte lineages were analyzed using Ly5 and Hbb variants at 4, 8, 15, and 20 weeks posttransplantation. At 4 weeks, LSKRhlow HSCs cultured with 40 μM TEPA had significantly less repopulation capacity in the leukocyte lineage than control cultures (p = 0.02) or cultures supplemented with 10 μM CuCl2 (p = 0.002) (Fig. 5A). At 15 weeks posttransplantation, there was no significant difference of leukocyte and erythrocyte repopulation potential of cultured cells under different conditions (Fig. 5B). At 5 months posttransplantation, HSCs cultured with 40 μM TEPA showed a slight increase in the repopulation in leukocyte and erythrocyte lineages, but this increase was not statistically significant (p = 0.38) (Fig. 5A, C). Based on these data, we conclude that TEPA does not significantly increase the maintenance or expansion of short-term or long-term HSCs in culture.
Figure 5.
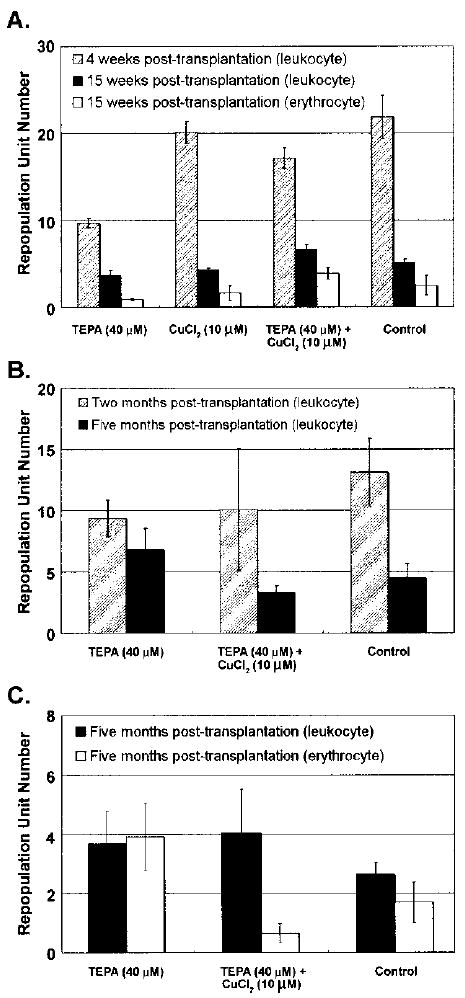
The effect of TEPA or Cu supplementation on the short-term and long-term repopulation potential of HSC cell cultures. LSKRholow HSC [1000 cells, equaling approximately 100 repopulation unit (RU)] were cultured under the indicated conditions for 7 days and then transplanted together with 106 competitor BM cells into lethally irradiated recipients. The peripheral blood was sampled at various times and the contribution of cultured cells to leukocyte and erythrocyte pools was determined by flow cytometry and HPLC. RU number was calculated based on 1RU = 105 competitor BM cells. Results from three independent experiments are shown in (A), (B), and (C).
Effects of TEPA and Cu Supplementation on the Mitochondrial Membrane Potential of Cultured Progenitor Cells
To gain some insights into the mechanisms of TEPA’s effect on LSKRhohi progenitor cells and their progenies in culture, we measured the mitochondrial membrane potential of day 7 cultures of LSKRhohi cells. We used the cationic dye JC-1 that indicates increased mitochondrial polarization by shifting its fluorescence emission from green (525 nm) to red (575 nm). JC-1 staining of LSKRhohi bone marrow cells cultured for 7 days showed that 40 μM TEPA increased the number of cells that have relatively low mitochondrial membrane potential and decreased the number of cells that have relatively high mitochondrial membrane potential compared to control cultures containing only cytokines (Fig. 6). The effect of TEPA on mitochondrial membrane potential of cultured cells was reversed by adding a low amount of exogenous Cu into TEPA-supplemented cultures, suggesting reduction of Cu concentration within the cell is responsible for the effect of TEPA (Fig. 6).
Figure 6.
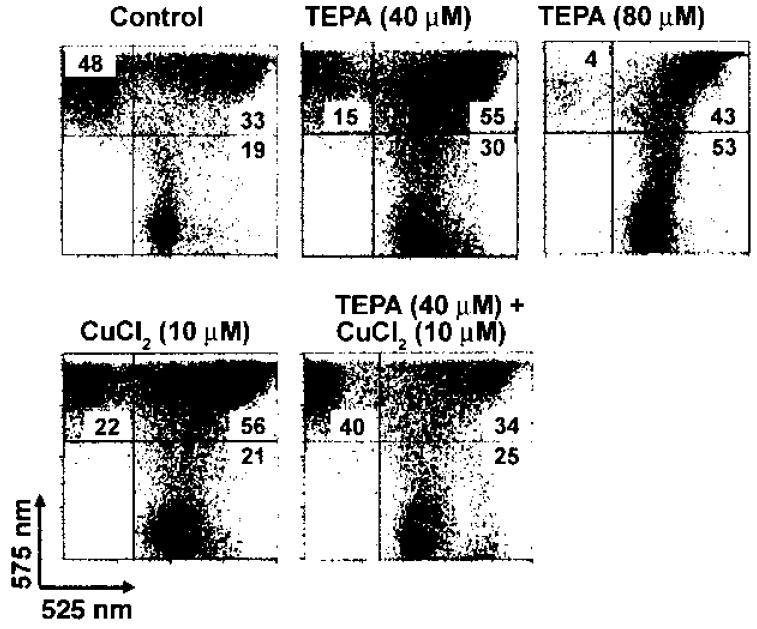
The effect of TEPA or Cu supplementation on the mitochondrial membrane potential of cultured HPC. LSKRhohi cells were cultured under the indicated conditions for 7 days, stained with JC-1, and analyzed by flow cytometry. Fluorescence emission at 525 nm indicates cells with low relative mitochondrial membrane potential, while emission at 575 nm indicates high relative mitochondrial membrane potential. The percentages of cells in each quadrant are shown in the figure.
DISCUSSION
Copper has long been known to be essential for normal hematopoiesis in mammals from both in vitro and in vivo studies. However, the effects of fine-tuning of intracellular copper content on the regulation of HSC/HPCs self-renewal and differentiation are relatively unknown. Recently, some interesting findings suggested that reducing the intracellular Cu content by Cu chelation in human HSC/HPCs is beneficial for their expansion or maintenance in culture. While this finding is very intriguing and promising, it is unclear whether copper chelation expands or maintains the activities HSC, HPC or both, due to the difficulty of faithfully separating and measuring human stem and progenitor cell activities. HSC are strikingly different from HPC in that HSC can repopulate the hematopoietic system for a life-long period, while HPC can only function for a limited time. The in vivo repopulation potential of human HSC/HPCs can be estimated using xenotransplantation models, usually using the NOD/SCID mouse as a transplant recipient. The NOD/SCID mouse is a widely used model for assessing human HSC/HPCs. However, it is still uncertain how well it is able to faithfully measure and quantify human HSC/HPCs, and the utility of the model in predicting human engraftment is controversial.
To better address this issue and to gain some insights into the mechanisms of Cu regulation on HSC/HPC fate decisions, we performed experiments using the more defined mouse model system. Using cell surface markers and mitochondrial dye Rho, we isolated HSCs and HPCs as separate populations from mouse bone marrow (27, 50). When these two populations were cultured separately in medium containing FBS and cytokines, they responded differently to Cu modulation. We performed in vitro CFC assays to quantify the progenitor cell content in the day 7 and day 14 HPC and HSC cultures. TEPA supplementation at 40 and 80 μM increased the total and erythroid CFC number in the HPC culture by two- to threefold compared to control cultures after 7 days of culture, and the relative increase was even more striking after 14 days of culture, although the overall CFC number was decreased from day 7. In contrast, the HSC cultures showed no difference in total CFC number due to TEPA or Cu supplementation after 7 days of culture. There were slight increases in total CFC numbers in HSC cultures supplemented with TEPA compared to controls and cultures supplemented with Cu for 14 days.
These in vitro CFC assay data suggest that Cu chelation has a positive effect on progenitor cell expansion or maintenance. We also assessed the in vivo repopulation potential of the cultured HPCs or HSCs by competitive repopulation assays. When the cultured cells competed with a fixed number of normal competitor BM cells, the HPCs cultured with 40 μM TEPA showed a twofold higher short-term erythroid repopulation potential compared to control culture cells. In contrast, the leukocyte and platelet lineage repopulation from cultured cells was decreased or unchanged by TEPA treatment. Cu addition had the opposite effect, and Cu reversed most effects of TEPA, indicating that intracellular Cu reduction is largely responsible for TEPA’s activities in our experiments. We transplanted cultures established from HSCs into lethally irradiated hosts in a competitive repopulation setting, and followed the contribution of cultured cells to the peripheral blood of hosts periodically over a time frame up to 5 months posttransplantation. At 4 weeks posttransplantation, HSC cultures treated with TEPA showed a decreased leukocyte repopulation potential compared control cultures. At 3–5 months post-transplantation, HSC cultures treated with TEPA showed a slightly increased repopulation potential in both leukocyte and erythroid lineages, but this increase was not statistically significant. These results suggest that TEPA does not promote the expansion or maintenance of HSCs in cytokine-supplemented cultures. Interestingly, we did not see a similar effect of TEPA on short-term erythroid-biased progenitors in LSKRhlow HSC culture as in LSKRhhi HPC culture. This suggests that TEPA acts on a very specific population of progenitor cells that are contained in the LSKRhhi cell population or their progenies but not contained significantly in LSKRhlow cells and their cultured progenies. Further studies are needed to identify and characterize this cell population.
Cu is a cofactor of a key enzyme in the mitochondrial electron transport chain, cytochrome c oxidase (CCO). Reducing Cu content in cells has been shown to result in decreased activity of CCO and mitochondrial membrane potential in myogenic cells (5). To investigate whether Cu chelation decreases the mitochondrial membrane potential of cultured HPCs, we stained day 7 HPC cultures with JC-1, a cationic dye that indicates mitochondrial polarization by shifting its fluorescence emission from green to red due to concentration-dependent formation of red fluorescent J-aggregates (47). HPC cultured with 40 and 80 μM TEPA contained a higher number of cells with low mitochondrial potential compared to control cultures. These cells may represent erythroid-biased progenitor cells. Currently, we do not know whether reducing the mitochondrial membrane potential of these cells is responsible for the enhanced generation or maintenance of erythroid-biased progenitor cells in culture. Further studies are required to answer this question.
Reducing cellular Cu content by TEPA may attenuate CCO activity, and this may in turn lead to a switch in metabolic flux towards glycolysis. Metabolic influences may be one mechanism by which HPCs regulate the choice between proliferation and differentiation (4). Mammalian cultures with impaired CCO maturation do show a shift toward glycolysis. TEPA treatment can also reduce the reactive oxygen species (ROS) in some cell types, including HPCs (45). Reducing ROS level has been shown to be essential for the maintenance of HSCs in bone marrow (24,25,54), and control of ROS level is also essential for normal erythropoiesis (33). Currently, we do not know if TEPA reduces the ROS level in HSC/HPC in our culture system. Our results suggest it is very important to separate HSC activity from HPC activity and look for the repopulation potential in all blood lineages when testing the effects of changing Cu or ROS levels on HSCs and HPCs.
There are several mechanisms that can possibly explain the effect of TEPA in hematopoietic cultures. One possibility is the increased survival of progenitor cells, especially erythroid progenitor cells in TEPA-supplemented culture. Secondly, the inhibition of differentiation of progenitor cells into mature blood cells in TEPA-supplemented cultures may explain our results. TEPA has been shown to have antidifferentiation effects on some established hematopoietic progenitor cell lines, and whether it has similar effects on primary hematopoietic progenitor cells should be investigated. Third, increased proliferation of erythroid progenitor cells in the TEPA-supplemented culture is possibly involved in the selective increases in this lineage in both in vitro and in vivo settings. We are currently investigating whether one or a combination of these mechanisms accounts for TEPA’s effects on hematopoietic progenitor cells.
Although we did not see a positive effect of TEPA on HSC expansion or maintenance in our culture system, we cannot rule out the possibility that TEPA may have positive effects on stem cell maintenance and expansion in the context of other culture environments or with human cells. It will be very interesting to investigate the effect of TEPA on HSCs together with supportive stromal cells (21,34). A variety of transcription factors and signaling molecules, such as Wnt and Notch proteins (12), fibroblast growth factor-1 (10), insulin-like growth factor-2 (57), and angiopoietin-like proteins (56), have been shown to promote the self-renewal of HSCs in culture, and inhibition of apoptosis using caspase inhibitors can help maintain HSC function in culture (53). The specific effect of TEPA on erythroid lineage progenitor cells in cultures of HPCs is very intriguing; this effect may be exploited in clinical situations where rapid erythroid repopulation is desired. Furthermore, it will be interesting to study whether Cu modulation has similar effects on the progenitor cells from other tissues.
Acknowledgments
This project was supported by funding from a program project grant (P30DK072437) and by individual grants to G.J.S. (R01DK057899) and D.R.W. (R01ES03817). Additional funding was provided by the Brian Rooney Fund of the Lymphoma Foundation.
References
- 1.Bae B, Percival SS. Retinoic acid-induced HL-60 cell differentiation is augmented by copper supplementation. J Nutr. 1993;123:997–1002. doi: 10.1093/jn/123.6.997. [DOI] [PubMed] [Google Scholar]
- 2.Bertoncello I, Hodgson GS, Bradley TR. Multiparameter analysis of transplantable hemopoietic stem cells. II. Stem cells of long-term bone marrow-reconstituted recipients. Exp Hematol. 1988;16:245–249. [PubMed] [Google Scholar]
- 3.Birkaya B, Aletta JM. NGF promotes copper accumulation required for optimum neurite outgrowth and protein methylation. J Neurobiol. 2005;63:49–61. doi: 10.1002/neu.20114. [DOI] [PubMed] [Google Scholar]
- 4.Chen CT, Shih YR, Kuo TK, Lee OK, Wei YH. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2008;26:960–968. doi: 10.1634/stemcells.2007-0509. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Medeiros DM, Jennings D. Mitochondrial membrane potential is reduced in copper-deficient C2C12 cells in the absence of apoptosis. Biol Trace Elem Res. 2005;106:51–64. doi: 10.1385/BTER:106:1:051. [DOI] [PubMed] [Google Scholar]
- 6.Cordano A, Placko RP, Graham GG. Hypocupremia and neutropenia in copper deficiency. Blood. 1966;28:280–283. [PubMed] [Google Scholar]
- 7.Coulombel L. Identification of hematopoietic stem/progenitor cells: Strength and drawbacks of functional assays. Oncogene. 2004;23:7210–7222. doi: 10.1038/sj.onc.1207941. [DOI] [PubMed] [Google Scholar]
- 8.Cwirla SE, Balasubramanian P, Duffin DJ, Wagstrom CR, Gates CM, Singer SC, Davis AM, Tansik RL, Mattheakis LC, Boytos CM, Schatz PJ, Baccanari DP, Wrighton NC, Barrett RW, Dower WJ. Peptide agonist of the thrombopoietin receptor as potent as the natural cytokine. Science. 1997;276:1696–1699. doi: 10.1126/science.276.5319.1696. [DOI] [PubMed] [Google Scholar]
- 9.Dahl SL, Rucker RB, Niklason LE. Effects of copper and cross-linking on the extracellular matrix of tissue-engineered arteries. Cell Transplant. 2005;14:367–374. doi: 10.3727/000000005783982936. [DOI] [PubMed] [Google Scholar]
- 10.de Haan G, Weersing E, Dontje B, van Os R, Bystrykh LV, Vellenga E, Miller G. In vitro generation of long-term repopulating hematopoietic stem cells by fibroblast growth factor-1. Dev Cell. 2003;4:241–251. doi: 10.1016/s1534-5807(03)00018-2. [DOI] [PubMed] [Google Scholar]
- 11.de Lima M, McMannis J, Gee A, Komanduri K, Couriel D, Andersson BS, Hosing C, Khouri I, Jones R, Champlin R, Karandish S, Sadeghi T, Peled T, Grynspan F, Daniely Y, Nagler A, Shpall EJ. Transplantation of ex vivo expanded cord blood cells using the copper chelator tetraethylenepentamine: A phase I//II clinical trial. Bone Marrow Transplant. 2008;41:771–778. doi: 10.1038/sj.bmt.1705979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, Yoon K, Cook JM, Willert K, Gaiano N, Reya T. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005;6:314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- 13.Fuhrman MP, Herrmann V, Masidonski P, Eby C. Pancytopenia after removal of copper from total parenteral nutrition. J Parenter Enteral Nutr. 2000;24:361–366. doi: 10.1177/0148607100024006361. [DOI] [PubMed] [Google Scholar]
- 14.Goyens P, Brasseur D, Cadranel S. Copper deficiency in infants with active celiac disease. J Pediatr Gastroenterol Nutr. 1985;4:677–680. doi: 10.1097/00005176-198508000-00033. [DOI] [PubMed] [Google Scholar]
- 15.Hainaut P, Rolley N, Davies M, Milner J. Modulation by copper of p53 conformation and sequence-specific DNA binding: Role for Cu(II)/Cu(I) redox mechanism. Oncogene. 1995;10:27–32. [PubMed] [Google Scholar]
- 16.Halfdanarson TR, Kumar N, Li CY, Phyliky RL, Hogan WJ. Hematological manifestations of copper deficiency: A retrospective review. Eur J Haematol. 2008;80:523–531. doi: 10.1111/j.1600-0609.2008.01050.x. [DOI] [PubMed] [Google Scholar]
- 17.Harris ED. Cellular copper transport and metabolism. Annu Rev Nutr. 2000;20:291–310. doi: 10.1146/annurev.nutr.20.1.291. [DOI] [PubMed] [Google Scholar]
- 18.Harrison DE, Astle CM, Lerner C. Number and continuous proliferative pattern of transplanted primitive immunohematopoietic stem cells. Proc Natl Acad Sci USA. 1988;85:822–826. doi: 10.1073/pnas.85.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirase N, Abe Y, Sadamura S, Yufu Y, Muta K, Umemura T, Nishimura J, Nawata H, Ideguchi H. Anemia and neutropenia in a case of copper deficiency: Role of copper in normal hematopoiesis. Acta Haematol. 1992;87:195–197. doi: 10.1159/000204758. [DOI] [PubMed] [Google Scholar]
- 20.Horn PA, Kiem HP. Expansion of SCID repopulating cells does not prove expansion of hematopoietic stem cells. Blood. 2006;108:771–772. doi: 10.1182/blood-2006-02-002618. [DOI] [PubMed] [Google Scholar]
- 21.Huang GP, Pan ZJ, Jia BB, Zheng Q, Xie CG, Gu JH, McNiece IK, Wang JF. Ex vivo expansion and transplantation of hematopoietic stem/progenitor cells supported by mesenchymal stem cells from human umbilical cord blood. Cell Transplant. 2007;16:579–585. doi: 10.3727/000000007783465073. [DOI] [PubMed] [Google Scholar]
- 22.Huang ZL, Failla ML, Reeves PG. Differentiation of human U937 promonocytic cells is impaired by moderate copper deficiency. Exp Biol Med. 2001;226:222–228. [PubMed] [Google Scholar]
- 23.Iseki A, Kambe F, Okumura K, Hayakawa T, Seo H. Regulation of thyroid follicular cell function by intra- cellular redox-active copper. Endocrinology. 2000;141:4373–4382. doi: 10.1210/endo.141.12.7835. [DOI] [PubMed] [Google Scholar]
- 24.Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, Nomiyama K, Hosokawa K, Sakurada K, Nakagata N, Ikeda Y, Mak TW, Suda T. Regulation of oxidative stress by ATM is required for self- renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 25.Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, Suda T. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 26.Kang J, Lin C, Chen J, Liu Q. Copper induces histone hypoacetylation through directly inhibiting histone acetyltransferase activity. Chem Biol Interact. 2004;148:115–123. doi: 10.1016/j.cbi.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Kim M, Cooper DD, Hayes SF, Spangrude GJ. Rhodamine-123 staining in hematopoietic stem cells of young mice indicates mitochondrial activation rather than dye efflux. Blood. 1998;91:4106–4117. [PubMed] [Google Scholar]
- 28.Kondo M, Wagers AJ, Manz MG, Prohaska SS, Scherer DC, Beilhack GF, Shizuru JA, Weissman IL. Biology of hematopoietic stem cells and progenitors: Implications for clinical application. Annu Rev Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- 29.Kudrin AV. Trace elements in regulation of NF-kappaB activity. J Trace Elem Med Biol. 2000;14:129–142. doi: 10.1016/s0946-672x(00)80001-2. [DOI] [PubMed] [Google Scholar]
- 30.Leary SC, Cobine PA, Kaufman BA, Guercin GH, Mattman A, Palaty J, Lockitch G, Winge DR, Rustin P, Horvath R, Shoubridge EA. The human cytochrome c oxidase assembly factors SCO1 and SCO2 have regulatory roles in the maintenance of cellular copper homeostasis. Cell Metab. 2007;5:9–20. doi: 10.1016/j.cmet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Leary SC, Winge DR. The Janus face of copper: its expanding roles in biology and the pathophysiology of disease. Meeting on Copper and Related Metals in Biology. EMBO Rep. 2007;8:224–227. doi: 10.1038/sj.embor.7400915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li CL, Johnson GR. Rhodamine123 reveals heterogeneity within murine Lin−, Sca-1+ hemopoietic stem cells. J Exp Med. 1992;175:1443–1447. doi: 10.1084/jem.175.6.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marinkovic D, Zhang X, Yalcin S, Luciano JP, Brugnara C, Huber T, Ghaffari S. Foxo3 is required for the regulation of oxidative stress in erythropoiesis. J Clin Invest. 2007;117:2133–2144. doi: 10.1172/JCI31807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monga SP, Tang Y, Candotti F, Rashid A, Wildner O, Mishra B, Iqbal S, Mishra L. Expansion of hepatic and hematopoietic stem cells utilizing mouse embryonic liver explants. Cell Transplant. 2001;10:81–89. [PubMed] [Google Scholar]
- 35.Nakanishi T, Kuroiwa A, Yamada S, Isotani A, Yamashita A, Tairaka A, Hayashi T, Takagi T, Ikawa M, Matsuda Y, Okabe M. FISH analysis of 142 EGFP transgene integration sites into the mouse genome. Genomics. 2002;80:564–574. doi: 10.1006/geno.2002.7008. [DOI] [PubMed] [Google Scholar]
- 36.Naveh Y, Hazani A, Berant M. Copper deficiency with cow’s milk diet. Pediatrics. 1981;68:397–400. [PubMed] [Google Scholar]
- 37.Okada S, Nakauchi H, Nagayoshi K, Nishikawa S, Miura Y, Suda T. In vivo and in vitro stem cell function of c-kit- and Sca-1-positive murine hematopoietic cells. Blood. 1992;80:3044–3050. [PubMed] [Google Scholar]
- 38.Ostrakhovitch EA, Lordnejad MR, Schliess F, Sies H, Klotz LO. Copper ions strongly activate the phosphoinositide-3-kinase/Akt pathway independent of the generation of reactive oxygen species. Arch Biochem Biophys. 2002;397:232–239. doi: 10.1006/abbi.2001.2559. [DOI] [PubMed] [Google Scholar]
- 39.Peled T, Glukhman E, Hasson N, Adi S, Assor H, Yudin D, Landor C, Mandel J, Landau E, Prus E, Nagler A, Fibach E. Chelatable cellular copper modulates differentiation and self-renewal of cord blood-derived hematopoietic progenitor cells. Exp Hematol. 2005;33:1092–1100. doi: 10.1016/j.exphem.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 40.Peled T, Landau E, Mandel J, Glukhman E, Goudsmid NR, Nagler A, Fibach E. Linear polyamine copper chelator tetraethylenepentamine augments long-term ex vivo expansion of cord blood-derived CD34+ ells and increases their engraftment potential in NOD/SCID mice. Exp Hematol. 2004;32:547–555. doi: 10.1016/j.exphem.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Peled T, Landau E, Prus E, Treves AJ, Nagler A, Fibach E. Cellular copper content modulates differentiation and self-renewal in cultures of cord blood-derived CD34+ cells. Br J Haematol. 2002;116:655–661. doi: 10.1046/j.0007-1048.2001.03316.x. [DOI] [PubMed] [Google Scholar]
- 42.Peled T, Mandel J, Goudsmid RN, Landor C, Hasson N, Harati D, Austin M, Hasson A, Fibach E, Shpall EJ, Nagler A. Pre-clinical development of cord blood-derived progenitor cell graft expanded ex vivo with cytokines and the polyamine copper chelator tetraethylenepentamine. Cytotherapy. 2004;6:344–355. doi: 10.1080/14653240410004916. [DOI] [PubMed] [Google Scholar]
- 43.Pena MM, Lee J, Thiele DJ. A delicate balance: homeostatic control of copper uptake and distribution. J Nutr. 1999;129:1251–1260. doi: 10.1093/jn/129.7.1251. [DOI] [PubMed] [Google Scholar]
- 44.Porea TJ, Belmont JW, Mahoney DH., Jr Zinc-induced anemia and neutropenia in an adolescent. J Pediatr. 2000;136:688–690. doi: 10.1067/mpd.2000.103355. [DOI] [PubMed] [Google Scholar]
- 45.Prus E, Fibach E. The effect of the copper chelator tetraethylenepentamine on reactive oxygen species generation by human hematopoietic progenitor cells. Stem Cells Dev. 2007;16:1053–1056. doi: 10.1089/scd.2007.0052. [DOI] [PubMed] [Google Scholar]
- 46.Simon SR, Branda RF, Tindle BF, Burns SL. Copper deficiency and sideroblastic anemia associated with zinc ingestion. Am J Hematol. 1988;28:181–183. doi: 10.1002/ajh.2830280310. [DOI] [PubMed] [Google Scholar]
- 47.Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith TW, Steele GD, Jr, Chen LB. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci USA. 1991;88:3671–3675. doi: 10.1073/pnas.88.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spangrude GJ, Cho S, Guedelhoefer O, Vanwoerkom RC, Fleming WH. Mouse models of hematopoietic engraftment: Limitations of transgenic green fluorescent protein strains and a high-performance liquid chromatography approach to analysis of erythroid chimerism. Stem Cells. 2006;24:2045–2051. doi: 10.1634/stemcells.2006-0013. [DOI] [PubMed] [Google Scholar]
- 49.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 50.Spangrude GJ, Johnson GR. Resting and activated subsets of mouse multipotent hematopoietic stem cells. Proc Natl Acad Sci USA. 1990;87:7433–7437. doi: 10.1073/pnas.87.19.7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uauy R, Olivares M, Gonzalez M. Essentiality of copper in humans. Am J Clin Nutr. 1998;67:952S–959S. doi: 10.1093/ajcn/67.5.952S. [DOI] [PubMed] [Google Scholar]
- 52.Vanacore RM, Eskew JD, Morales PJ, Sung L, Smith A. Role for copper in transient oxidation and nuclear translocation of MTF-1, but not of NF-kappa B, by the heme-hemopexin transport system. Antioxid Redox Signal. 2000;2:739–752. doi: 10.1089/ars.2000.2.4-739. [DOI] [PubMed] [Google Scholar]
- 53.Wiesmann A, Searles AE, Pierce LJ, Spangrude GJ. Effects of caspase inhibitors on hematopoietic engraftment after short-term culture. Cell Transplant. 2002;11:351–358. [PubMed] [Google Scholar]
- 54.Yalcin S, Zhang X, Luciano JP, Kumar Mungamuri S, Marinkovic D, Vercherat C, Sarkar A, Grisotto M, Taneja R, Ghaffari S. Foxo3 is essential for the regulation of ATM and oxidative stress-mediated homeostasis of hematopoietic stem cells. J Biol Chem. 2008;283:25692–25705. doi: 10.1074/jbc.M800517200. [DOI] [PubMed] [Google Scholar]
- 55.Yee NS, Langen H, Besmer P. Mechanism of kit ligand, phorbol ester, and calcium-induced down-regulation of c-kit receptors in mast cells. J Biol Chem. 1993;268:14189–14201. [PubMed] [Google Scholar]
- 56.Zhang CC, Kaba M, Ge G, Xie K, Tong W, Hug C, Lodish HF. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat Med. 2006;12:240–245. doi: 10.1038/nm1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang CC, Lodish HF. Insulin-like growth factor 2 expressed in a novel fetal liver cell population is a growth factor for hematopoietic stem cells. Blood. 2004;103:2513–2521. doi: 10.1182/blood-2003-08-2955. [DOI] [PubMed] [Google Scholar]
- 58.Zidar BL, Shadduck RK, Zeigler Z, Winkelstein A. Observations on the anemia and neutropenia of human copper deficiency. Am J Hematol. 1977;3:177–185. doi: 10.1002/ajh.2830030209. [DOI] [PubMed] [Google Scholar]


