Abstract
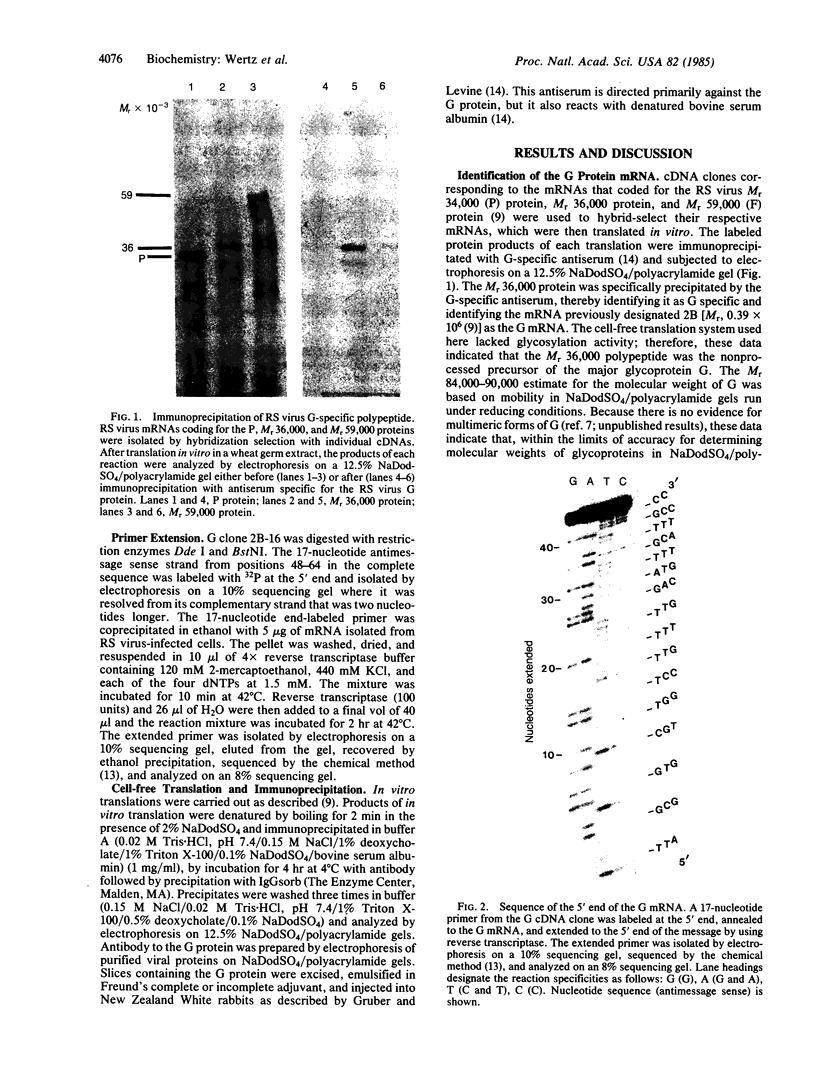
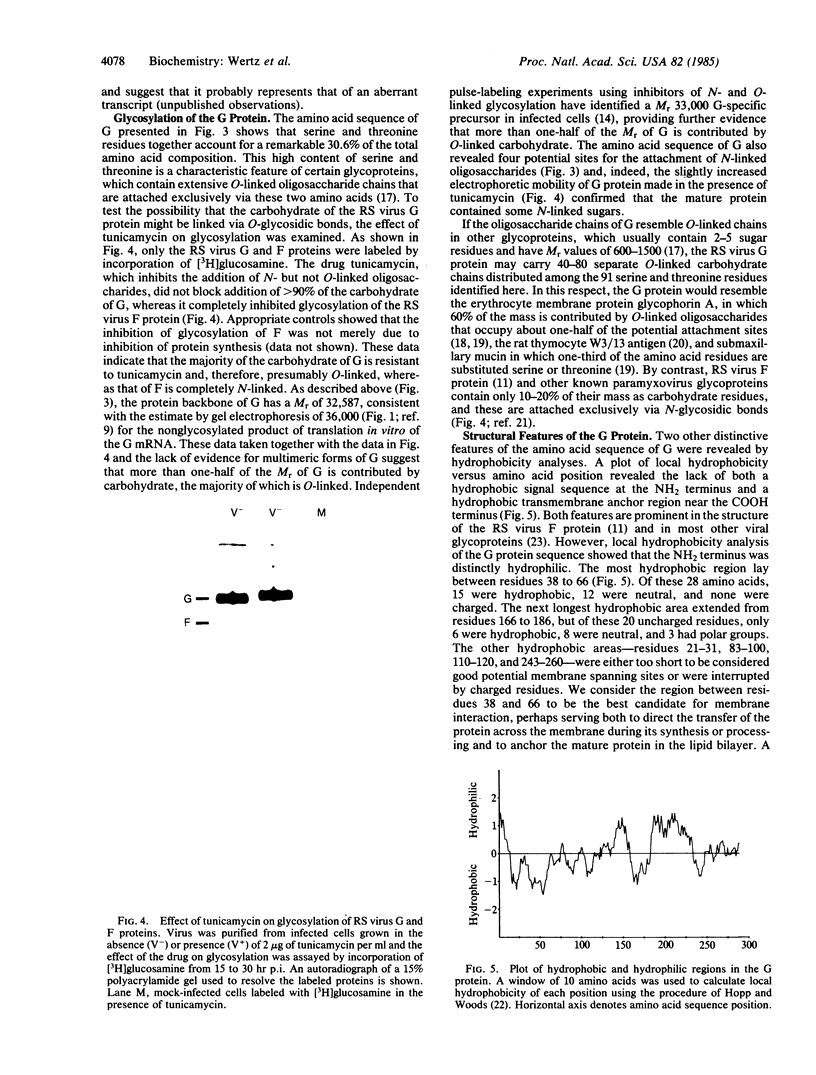
The major surface glycoprotein (G) of human respiratory syncytial (RS) virus has an estimated mature Mr of 84,000-90,000. Among a library of cDNA clones prepared from RS virus mRNAs, we identified clones that hybridized to a message that encoded a Mr 36,000 polypeptide that was specifically immunoprecipitated with anti-G antiserum. The amino acid sequence of the G protein backbone was determined by nucleotide sequence analysis of several of the cDNA clones. It contains a combination of structural features that make it unique among the known viral glycoproteins. The G mRNA is 918 nucleotides long and contains a single major open reading frame that encodes a polypeptide having 298 amino acid residues with a Mr of 32,587, a finding consistent with the Mr 36,000 estimate for the in vitro translation product of the G mRNA. This suggests that greater than 50% of the molecular weight of the mature glycoprotein may be contributed by carbohydrate. Glycosylation of G is largely resistant to tunicamycin, an inhibitor of the attachment of N-linked oligosaccharides, suggesting that the majority of the carbohydrate residues are attached via O-glycosidic bonds. In accordance with this, serine and threonine residues, the acceptor sites for O-linked oligosaccharides, comprise 30.6% of the total amino acid composition. There are also four potential acceptor sites for N-linked oligosaccharides. The amino acid sequence lacks both an NH2-terminal hydrophobic signal sequence and a COOH-terminal hydrophobic region. Instead, a strongly hydrophobic region is located between amino acid residues 38 and 66. This region may serve as both the signal to insert the nascent polypeptide through the membrane and as the membrane anchor site.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J., Niemann H., Smeekens S., Rottier P., Warren G. Sequence and topology of a model intracellular membrane protein, E1 glycoprotein, from a coronavirus. Nature. 1984 Apr 19;308(5961):751–752. doi: 10.1038/308751a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blok J., Air G. M., Laver W. G., Ward C. W., Lilley G. G., Woods E. F., Roxburgh C. M., Inglis A. S. Studies on the size, chemical composition, and partial sequence of the neuraminidase (NA) from type A influenza viruses show that the N-terminal region of the NA is not processed and serves to anchor the NA in the viral membrane. Virology. 1982 May;119(1):109–121. doi: 10.1016/0042-6822(82)90069-1. [DOI] [PubMed] [Google Scholar]
- Brown W. R., Barclay A. N., Sunderland C. A., Williams A. F. Identification of a glycophorin-like molecule at the cell surface of rat thymocytes. Nature. 1981 Feb 5;289(5797):456–460. doi: 10.1038/289456a0. [DOI] [PubMed] [Google Scholar]
- Choppin P. W., Scheid A. The role of viral glycoproteins in adsorption, penetration, and pathogenicity of viruses. Rev Infect Dis. 1980 Jan-Feb;2(1):40–61. doi: 10.1093/clinids/2.1.40. [DOI] [PubMed] [Google Scholar]
- Collins P. L., Huang Y. T., Wertz G. W. Identification of a tenth mRNA of respiratory syncytial virus and assignment of polypeptides to the 10 viral genes. J Virol. 1984 Feb;49(2):572–578. doi: 10.1128/jvi.49.2.572-578.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Huang Y. T., Wertz G. W. Nucleotide sequence of the gene encoding the fusion (F) glycoprotein of human respiratory syncytial virus. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7683–7687. doi: 10.1073/pnas.81.24.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Wertz G. W. cDNA cloning and transcriptional mapping of nine polyadenylylated RNAs encoded by the genome of human respiratory syncytial virus. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3208–3212. doi: 10.1073/pnas.80.11.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elango N., Venkatesan S. Amino acid sequence of human respiratory syncytial virus nucleocapsid protein. Nucleic Acids Res. 1983 Sep 10;11(17):5941–5951. doi: 10.1093/nar/11.17.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S., Winter G., Brownlee G. G. Structure of the neuraminidase gene in human influenza virus A/PR/8/34. Nature. 1981 Mar 19;290(5803):213–217. doi: 10.1038/290213a0. [DOI] [PubMed] [Google Scholar]
- Frank G., Brunner J., Hauser H., Wacker H., Semenza G., Zuber H. The hydrophobic anchor of small-intestinal sucrase--isomaltase: N-terminal sequence of isomaltase subunit. FEBS Lett. 1978 Dec 1;96(1):183–188. doi: 10.1016/0014-5793(78)81090-4. [DOI] [PubMed] [Google Scholar]
- Gruber C., Levine S. Respiratory syncytial virus polypeptides. III. The envelope-associated proteins. J Gen Virol. 1983 Apr;64(Pt 4):825–832. doi: 10.1099/0022-1317-64-4-825. [DOI] [PubMed] [Google Scholar]
- Hanover J. A., Lennarz W. J., Young J. D. Synthesis of N- and O-linked glycopeptides in oviduct membrane preparations. J Biol Chem. 1980 Jul 25;255(14):6713–6716. [PubMed] [Google Scholar]
- Hiebert S. W., Paterson R. G., Lamb R. A. Hemagglutinin-neuraminidase protein of the paramyxovirus simian virus 5: nucleotide sequence of the mRNA predicts an N-terminal membrane anchor. J Virol. 1985 Apr;54(1):1–6. doi: 10.1128/jvi.54.1.1-6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Rott R. Cotranslational and posttranslational processing of viral glycoproteins. Curr Top Microbiol Immunol. 1980;90:19–48. doi: 10.1007/978-3-642-67717-5_2. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreil G. Transfer of proteins across membranes. Annu Rev Biochem. 1981;50:317–348. doi: 10.1146/annurev.bi.50.070181.001533. [DOI] [PubMed] [Google Scholar]
- Land H., Grez M., Hauser H., Lindenmaier W., Schütz G. 5'-Terminal sequences of eucaryotic mRNA can be cloned with high efficiency. Nucleic Acids Res. 1981 May 25;9(10):2251–2266. doi: 10.1093/nar/9.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Peeples M., Levine S. Respiratory syncytial virus polypeptides: their location in the virion. Virology. 1979 May;95(1):137–145. doi: 10.1016/0042-6822(79)90408-2. [DOI] [PubMed] [Google Scholar]
- Richman A. V., Pedreira F. A., Tauraso N. M. Attempts to demonstrate hemagglutination and hemadsorption by respiratory syncytial virus. Appl Microbiol. 1971 Jun;21(6):1099–1100. doi: 10.1128/am.21.6.1099-1100.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. B., Winzler R. J. Structural studies on human erythrocyte glycoproteins. Alkali-labile oligosaccharides. J Biol Chem. 1969 Nov 10;244(21):5943–5946. [PubMed] [Google Scholar]
- Walsh E. E., Hruska J. Monoclonal antibodies to respiratory syncytial virus proteins: identification of the fusion protein. J Virol. 1983 Jul;47(1):171–177. doi: 10.1128/jvi.47.1.171-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]