Abstract
TPL-2 expression is required for efficient polarization of naïve T cells to Th1 effector cells in vitro, and for Th1-mediated immune responses. In the present study, we investigated the potential role of TPL-2 in Th17 cells. TPL-2 was found to be dispensable for Th17 cell differentiation in vitro, and for the initial priming of Th17 cells in experimental autoimmune encephalomyelitis (EAE), a Th17 cell-mediated disease model for multiple sclerosis. Nevertheless, TPL-2-deficient mice were protected from EAE, which correlated with reduced immune cell infiltration, demyelination and axonal damage in the CNS. Adoptive transfer experiments demonstrated that there was no T cell-intrinsic function for TPL-2 in EAE, and that TPL-2 signaling was not required in radiation-sensitive hematopoietic cells. Rather, TPL-2 signaling in radiation-resistant stromal cells promoted the effector phase of the disease. Importantly, using a newly generated mouse strain expressing a kinase-inactive form of TPL-2, we demonstrated that stimulation of EAE was dependent on TPL-2’s catalytic activity, and not its adaptor function to stabilize the associated ubiquitin-binding protein ABIN-2. Our data therefore raise the possibility that small molecule inhibitors of TPL-2 may be beneficial in multiple sclerosis therapy.
INTRODUCTION
TPL-2, also known as COT and MAP3K8, is expressed in both hematopoietic and non-hematopoietic cells, and functions as a MAP 3-kinase that activates the MAP 2-kinases MEK-1/2 (1). During innate immune responses, TPL-2 expression is required for activating ERK-1/2 MAP kinases in myeloid cells by Toll-like receptors (TLRs), as well as the receptors for TNF and IL-1β (1-4). TPL-2 is critical for the production of TNF during TLR4-induced acute inflammatory responses, and is also required for the development of TNF-induced Crohn’s-like inflammatory bowel disease (2, 5). Consequently, TPL-2 is considered an attractive drug target for TNF-dependent inflammatory diseases (6).
In contrast to its positive role in inflammation driven by innate immune cells, TPL-2 can negatively regulate Th2 cell-driven inflammation. When re-challenged with ovalbumin intranasally, ovalbumin-sensitized Map3k8−/− mice express higher levels of immunoglobulin E, and develop more severe broncheoalveolar inflammation (7). This augmented inflammation occurs as a consequence of a default increase in Th2 effector cells, resulting from impaired Th1 differentiation of CD4+ T cells in Map3k8−/− mice (8), possibly through the removal of IFNγ-mediated Th2 inhibition (9). However, it has not been established that TPL-2-mediated inhibition of lung inflammation in the ovalbumin lung allergy model actually results from a T cell-intrinsic signaling function. In contrast, the protective role for TPL-2 in promoting Th1 effector cell differentiation during the immune response to Toxoplasma gondii, a Th1 cell-inducing parasite, has been directly demonstrated to be mediated by TPL-2 signaling in T cells (8).
Th17 cells, a subset of effector CD4+ T cells characterized by the production of the cytokine IL-17 (10), are important in immune responses to bacteria and fungi. In addition, aberrant Th17 cell activation is causally linked to the development of several human autoimmune diseases. In the present study, we investigated the potential requirement for TPL-2 in cytokine-induced Th17 cell differentiation in vitro, and also the role of TPL-2 in experimental autoimmune encephalomyelitis (EAE), in which Th17 cells have an important pathogenic role (11). EAE is an animal model for multiple sclerosis (MS), which is induced by immunization with myelin antigens, such as myelin oligodendrocyte glycoprotein (MOG) (12). We found that TPL-2 was dispensable for cytokine-induced differentiation of naïve T cells to the Th17 cell lineage in vitro. Furthermore, TPL-2 was not required for the initial activation and expansion of MOG-specific Th17 cells in the periphery during EAE. Generation of interferon γ (IFNγ)-producing Th1 cells, which may also play a role in EAE (11), was also independent of TPL-2 expression. Nevertheless, TPL-2 was demonstrated to regulate both the onset and severity of EAE. Cell transfer experiments established that TPL-2 regulated the effector phase of EAE in the CNS, functioning in radiation-resistant stromal cells. Importantly, using a newly generated Map3k8 knock-in mouse strain, we also showed that the development of EAE was dependent on TPL-2 catalytic activity, whilst ruling out any potential function of TPL-2 as scaffolding protein in the disease process. Our data therefore suggest that small molecule inhibitors of TPL-2 might be beneficial therapeutically in MS, the most common inflammatory demyelinating disease of the CNS (13).
Materials and Methods
Mice
Map3k8−/− (2), Tnip2−/− (14) and Ifnar−/− mice were fully backcrossed to C57BL/6. Map3k8−/−Rag1−/− and Ifnar−/−Map3k8−/− were generated by intercrossing the appropriate single knockout mice. For the generation of Map3k8D270A/D270A mice a P1-derived artificial chromosome clone (519-N17), including the Map3k8 gene was isolated from an RPC121 P1-derived artificial chromosome library (obtained from the UK Human Genome Mapping Project Resource Centre, Hinxton) by hybridization with an 1.5-kilobase (kb) probe encompassing full-length rat Map3k8 cDNA. A 6.6 kb ClaI-BamHI fragment containing exon 5, which encodes D270, was isolated and subcloned into pBluescript SK+/− (Invitrogen) to create pSK-LA (‘left arm’). From this plasmid, a 3.2 kb PstI fragment was subcloned into pBluescript SK+/− to create pSK-LA.PstI. PCR was used to mutate the sequence encoding D270 to alanine. To facilitate screening for the mutation, a BssSI site was introduced next to the 3′ of the D270A mutation without altering with the coding sequence. The D270A-containing PstI fragment was subsequently reinserted into pSK-LA, to create pSK-LA.D270A and from here a 5.2 kb HpaI fragment containing the mutation was cloned into a unique XhoI cloning site of the pLox-AP1 vector, to produce the pLox-AP1-LA (left arm). The pLox-AP1 vector contains neor flanked by two loxP sites and the herpes simplex virus thymidine kinase gene, both driven by the PGK1 promoter. A 4.7 kb BamHI-SalI fragment was isolated and subcloned into pBluescript SK+/− to create pSK-RA (‘right arm’). From this plasmid, a 4.4 kb HpaI-Bsu36I fragment was subcloned into pLox-AP1-LA to create the pLox-AP1-Tpl2D270A targeting vector (Supplementary Figure 4D). The vector was linearized with NotI and transfected into ES cells (carried out by PolyGene AG, Switzerland). C57BL/6 (CD45.2+, wild type), CD45.1 C57BL/6, CD45.1 Rag1−/−, Tcra−/− and μMT−/− mice were bred in specific pathogen-free animal facilities of the National Institute for Medical Research (London, UK) in accordance with the UK Home Office regulations. 6–10 week old male mice were used for all experiments.
Antibodies
Antibodies to TPL-2, IκBα, ERK-1, ERK-2, actin were purchased from Santa Cruz Biotechnology, whilst p-p105 (Ser933), p-p38 and p-ERK (Thr185/Tyr187) antibodies were obtained from Invitrogen. Tubulin mAb was kindly provided by Keith Gull (University of Oxford).
A number of fluorescently labelled antibodies for flow cytometry were used against: GMSCF-PE; Gr1-FITC; CD25-PE; TCRβ-PECy5; TCRγδ-PE; Streptavidin-PerCP; Streptavidin-PE were purchased from BD Pharmingen. IL-17A-APC; IFNγ-FITC; CD4-FITC, -PE; F4/80-APC, -PE; Gr1-biotinylated; CD25-APC; CD44-AF450, -FITC; CD45.2-FITC, -AF450; CD45.1-biotinylated; CD11c-PE; CD11b-PE, -biotinylated; MHCII-biotinylated were obtained from eBioscience. CD4-PerCP; CD19-Pacific Blue were purchased from BioLegend. CD4-PE/Texas Red; CD8-PE/Texas Red were obtained from Invitrogen.
Induction and assessment of EAE
Active EAE was induced by immunizing subcutaneously with 250μg of MOG35-55 peptide (Cambridge Research Biochemicals), emulsified in complete Freund’s adjuvant (CFA), containing 250μg heat-killed Mycobacterium tuberculosis (H37RA; Difco Laboratories). Mice received 200ng pertussis toxin (Calbiochem) intraperitoneally on day 0 and 2 days post-immunization. For passive EAE experiments, Map3k8−/− or WT mice were injected intravenously with 30 × 106 Th17-polarized MOG35-55 specific T cells and intraperitoneally with Pertussis toxin (day 0 and 2). To generate Th17 cell populations, drain lymph node cells from Map3k8−/− or WT mice were collected 10 days after immunization with MOG35-55 peptide plus CFA. Cells were cultured for 3 days (5 × 106 cells/ml; 24-well-plates) in Iscove’s modified Dulbecco medium (Sigma) supplemented with 10 % (vol/vol) heat-inactivated FCS (Labtech), 5mM L-glutamine, 50μM β-mercaptoethanol and antibiotics (all Gibco) in the presence of 10μg/ml MOG35-55 peptide under Th17-polarizing conditions: 20ng/ml rmIL-23 (R&D) plus 20ng/ml rmIL-1β (Invitrogen), or Th1-polarizing conditions: 20ng/ml rmIL-12 (R&D) and 1μg/ml αIL-23p19 (eBioscience). Clinical signs of disease were assigned daily: 0, no symptoms of disease; 1, loss of tail tonicity; 2, hind limb weakness/impaired gait; 3, partial hind limb paralysis; 4, complete hind limb paralysis; 5, forelimb paralysis or moribund.
Generation of bone marrow radiation chimeras
Bone marrow cells from Map3k8−/− or WT control mice were depleted of T cells with biotinylated TCRβ mAb (H57-597: BD Phamingen) and streptavidin-labelled magnetic beads (Dynal, Invitrogen). 5 – 10 × 106 cells were then transferred by intravenous injection into lethally irradiated (twice 400 rads) Rag1−/− or Tpl2−/− Rag1−/− hosts. For mixed bone marrow chimeras, WT or Map3k8−/− bone marrow cells were mixed with Tcra−/− or μMT−/− bone marrow cells at a ratio of 1:4, prior to injection into sub-lethally irradiated (500 rads) Rag1−/− mice. EAE was induced 6-8 weeks after cell transfer.
Isolation of CNS cells
Brains and spinal cords were mechanically homogenized and passed through a 70μm cell strainer (BD Pharmingen). Cells were centrifuged at 400 × g for 5 min at 4°C, and lymphocytes separated on a 36.5% Percoll gradient (GE Healthcare) prior to flow cytometric staining.
Histological analysis
Spinal cords were removed and fixed in 4% formalin. Paraffin embedded sections were stained, as previously described (15), with luxol fast blue (LFB), anti-CD3 (Serotec), anti-Mac-3, anti-B220 (both BD Pharmingen) and amyloid precursor protein (APP) (Chemicon).
T cell recall assay
Lymph node cells were isolated from mice 9 days after immunization with MOG35-55 peptide. Cells were cultured for 24 h in complete medium (2 × 106 cells/ml; 96-well-flat-bottomed plates) containing 10μg/ml MOG35-55 peptide, and supernatants collected for quantification of IL-17 and IFNγ by ELISA. For proliferation assays, lymph node cells were labelled with carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes) as described previously (16), and cultured for a total of 72h.
ELISA quantitation of cytokines
Levels of IL-17 and IFNγ were quantified by ELISA according to the manufacturer’s protocol (eBioscience).
Real-time quantitative PCR
Spinal cord tissue was collected in RNAlater stabilisation buffer (Qiagen) 15 days after MOG35-55 peptide/CFA immunization. Total RNA was isolated from spinal cords, cultured T cells, and primary cultures of microglia and astrocytes (RNeasy kit, Qiagen). After treatment with DNAase I (Invitrogen), cDNA was synthesised (1μg RNA; SuperScript First Strand Synthesis System, Invitrogen), and expression of mRNA determined using an Applied Biosystems ABI Prism 7000 Sequence Detection System and commercial FAM labelled probes (Applied Biosystems). Gene expression is displayed in arbitrary units relative to Hprt mRNA (encoding hypoxanthine guanine phosphoribosyl transferase).
Protein Analyses
Purified BMDM, BMDC and T cells were serum-starved for 12 h (1% FCS) to reduce basal ERK activation. BMDM and BMDC were stimulated with 1μg/ml heat-inactivated Mtb (Difco Laboratories), while CD4+ T cells were stimulated with soluble anti-CD3 (1 μg/ml; BD Pharmingen) plus anti-CD28 (1 μg/ml; BD Pharmingen). Cultured primary microglia and astrocytes were stimulated with LPS (100 ng/ml; Enzo), murine recombinant TNF (50 ng/ml, R&D), IFNγ (100 ng/ml; R&D), IL-1β (20 ng/ml; Peprotech) and IL-17A (100 ng/ml; R&D), alone or in the indicated combinations. Cells were washed once in PBS before lysis in buffer A (50 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 50 mM NaF, 1 mM Na3VO4, 100 nM okadaic acid; Calbiochem, 2 mM Na4P2O7 plus protease inhibitors) containing 1% Nonidet-P40, 0.5% deoxycholate and 0.1% SDS. Centrifuged lysates were mixed with an equal volume of 2× Laemmli sample buffer, resolved by SDS-PAGE, and immunoblotted. Protein concentration in lysates was determined by Bradford assay (Bio-Rad).
Flow cytometry
Single-cell suspensions were obtained from LN, spleen, brain or spinal cords of mice via gentle homogenisation through nylon mesh filters (70μM, BD Pharmingen). Cell concentrations were determined using a Casy Counter (Scharfe Instrument Systems). Erythrocytes in spleen samples were lysed prior to staining.
For analysis of surface markers, cells were stained with the indicated antibodies in PBS (2% (wt/vol) BSA). For intracellular cytokine staining, cells were restimulated for 4 h with PdBU (0.5μg/ml; Sigma), Ionomycin (0.5μg/ml; Sigma) and Brefeldin A (1μg/ml; GolgiPlug; BD Pharmingen), or with MOG35-55 peptide for 12 h, adding Brefeldin A for the last 4 h of culture. Cells were stained for surface antigens as indicated, fixed for 15 min in 4 % (vol/vol) paraformaldehyde (Sigma) and permeabilized with 0.1 % (vol/vol) Nonidet-P40 for 4 min. Intracellular antibodies were added in PBS containing 0.01% (vol/vol) sodium azide and 24G2 cell supernatant to block Fc receptor binding. Four- and seven-colour cytometric staining was analyzed on FACSCalibur and Cyan instruments (Becton Dickinson), respectively. Data analysis was performed with FlowJo V8.5 software (TreeStar).
Cell culture and purification
Macrophages and myeloid DC were generated from BM stem cells as described previously (17), with purities of ≥95% for BMDM (F4/80+) and BMDC (CD11c+) cell populations. For biochemical analyses, CD4+ T cells were purified (≥95% CD4+) from single-cell suspensions prepared from LN by negative selection as described (16). For the isolation of naïve T cells, CD4+ T cells were prepared from pooled lymph nodes and spleens by negative selection, as described above. Cells were then stained with anti-CD4 (RM45, BD Biosciences), anti-CD25 (PC61.5; eBioscience) and anti-CD44 (IM7; BD Biosciences), and CD4+CD44loCD25− naïve cells isolated to purities of over 98% on a MoFlo cytometer (Dako Cytomation). Naïve T cells were differentiated into Th17 cells as described (18, 19).
Mixed glial cultures were prepared from 1-2 day old mice using a published protocol (20). In brief, brains were dissected and meninges were removed. Brains were mechanically homogenized and passed through a 70μm cell strainer (BD Pharmingen). The resulting cell suspension was cultured in DMEM (Invitrogen) supplemented with 10% heat-inactivated FCS, antibiotics and 20% L929 cell supernatant, with medium changes every 3-4 days. After 10 – 14 days, the floating and loosely adherent microglial cells were harvested by vigorous shaking of culture flasks (250 r.p.m., 3h, 37 °C), before being re-plated at 2.0 × 105 in 24-well-plates and stimulated the following day. Adherent astrocytes were trypsinized, seeded at densities of 1.0 × 106 per 6-well, rested overnight in 1% serum and stimulated the following day. Microglial cell populations were ≥98 % CD11b+, while astrocyte cell populations were ≥85% GFAP+ (glial fibrillary acidic protein-staining), as determined by flow cytometry.
Statistics
Data are presented as means ± s.e.m. For analysis of clinical scores, a two-way analysis of variance (ANOVA) with Bonferroni correction was applied. An unpaired Student’s t-test with 2-tailed p-values was used for statistical analysis of in vitro assays. A non-parametric Mann-Whitney U-test was used for statistical analyses of flow cytometric data. All statistical analyses were calculated using Graph Pad Prism 5 software. P-values of less than 0.05 were considered significant.
Results
TPL-2 is not essential for in vitro generation of Th17 polarized cells
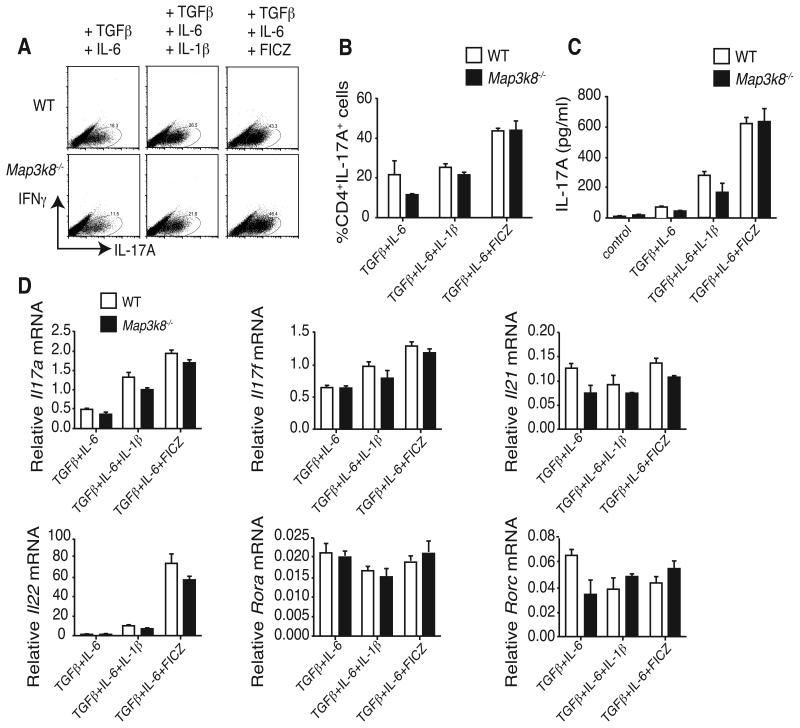
TPL-2 is required for efficient polarization of naïve CD4+ T cells to interferon γ (IFNγ)-producing Th1 cells in vitro, and in vivo after T. gondii infection (8). To initially determine whether TPL-2 was required for polarization to the Th17 cell lineage, CD25− CD44lo CD4+ T cells from wild type (WT) and Map3k8−/− mice were cultured under Th17-polarizing conditions (19). Intracellular staining revealed that TPL-2 deficiency did not significantly reduce the fraction of IL-17A+ cells (Fig. 1A and B). The amount of IL-17A in culture supernatants was also equivalent between WT and Map3k8−/− cells (Fig. 1C). Similarly, TPL-2 expression was not required for production of IL-17A+ cells or IL-17 protein in culture supernatants when either IL-1β or the Aryl Hydrocarbon Receptor (AhR) ligand 6-formylindolo[3,2-b]carbazole (FICZ) (18, 21) were added to the culture medium (Fig. 1A – D).
Figure 1. TPL-2 is not required for in vitro differentiation of Th17 cells.
(A - C) CD25− CD44hiCD4+ T cells (naïve CD4+ T cells) were isolated from WT (Map3k8+/+) and Map3k8−/− mice by FACS sorting, and cultured in triplicates for 4 days with anti-CD3/anti-CD28 plus the indicated Th17 polarizing cytokines and the AhR ligand FICZ. (A and B) IFNγ and IL-17A expression in CD4+ T cells was determined by intracellular staining and flow cytometry (mean ± s.e.m.). (C) ELISA of IL-17A production by CD4+ T cells (mean + s.e.m.). (D) Expression of the indicated mRNAs in Th17-polarized cell populations was determined by qRT-PCR, presented relative to Hprt1 mRNA (mean ± s.e.m.). Data are representative for three independent experiments.
Quantitative real-time PCR was used to determine whether TPL-2 regulated the induction of other Th17 signature cytokines and transcription factors (22). Steady-state levels of Il17a, Il17f and Il21 mRNAs were equivalent in Map3k8−/− and WT CD4+ T cells, in each of the conditions tested (Fig. 1D). The abundance of Il22 mRNA in IL-1β or FICZ containing cultures was also similar between Map3k8−/− and WT CD4+ cell cultures (18). The orphan nuclear receptor ROR-γt, which is selectively expressed in Th17 cells, induces the development of Th17 cells, in conjunction with ROR-α (23, 24). Expression of mRNAs encoding ROR-γt and ROR-α was comparable in Map3k8−/− and WT CD4+ T cells (Fig. 1D). TPL-2, therefore, was not essential for in vitro differentiation of CD4+ T cells to the Th17 cell lineage.
TPL-2 regulates the onset and severity of EAE
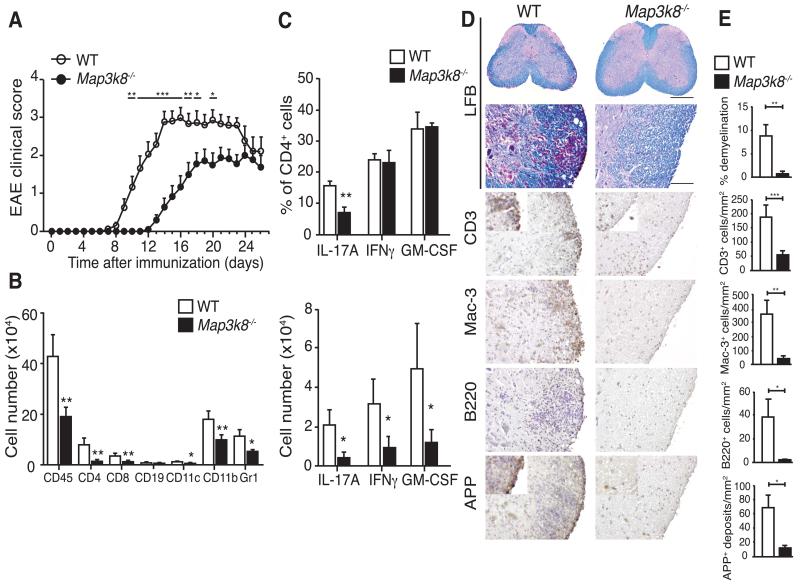
To investigate the physiological role of TPL-2 in Th17 cell development and function, we tested the susceptibility of Map3k8−/− mice to EAE, a Th17 cell-mediated animal model for multiple sclerosis (11). Map3k8−/− mice and control C57BL/6 WT mice were immunized with MOG35-55, and disease progression monitored (13). Despite 100% incidence, Map3k8−/− mice showed a delay in the onset and a reduced disease severity relative to controls (Fig. 2A and Table 1). Accordingly, flow cytometric analyses at the peak of disease revealed significantly fewer CD45+ hematopoietic cells, including CD4+ and CD8+ T cells, CD19+ B cells, dendritic cells (DC), CD45+CD11b+ macrophages and neutrophils, in the spinal cords of Map3k8−/− mice compared to WT mice (Fig. 2B).
Figure 2. TPL-2 regulates the onset and severity of EAE.
(A) Mean clinical scores of WT and Map3k8−/− mice (n = 19/WT; n=18/Map3k8−/−) at various times after immunization with MOG33-55/CFA. Data presented are combined from two independent experiments (n = 9-10/genotype/experiment). (B) Immune cell infiltration in the spinal cords of WT and Map3k8−/− mice (n = 8/genotype) at the peak of disease was determined by flow cytometry using the indicated markers (mean ± s.e.m.) (C) Intracellular staining for infiltrated IL-17A-, IFNγ– and GM-CSF-expressing CD4+ T cells in the spinal cords of WT and Map3k8−/− mice (n=8/genotype) on d12 after MOG33-55/CFA immunization. In D and E, EAE was induced in WT and Map3k8−/− mice, and spinal cords removed at the peak of disease. (D) Spinal cord sections were stained with luxol fast blue (LFB) to monitor demyelination (scale bar represents 200μm in the first panel, and 50μm in the lower panels). Immune cell infiltration was revealed by antibody staining: CD3 for T cells, Mac-3 for macrophages and B220 for B cells. Axonal damage was visualized by staining for amyloid precursor protein (APP). (E) Quantitation of demyelination, immune infiltration and axonal damage (mean ± s.e.m.). Data in B and C are compiled from 2 independent experiments (n=4/genotype/experiment). Data in D and E are representative of two independent experiments. * p ≤ 0.05; ** p≤0.01; *** p≤0.001.
Table 1.
Clinical features of MOG35-55–induced EAE
| Incidence | Mean maximum score (Mean ± S.D.) | Mean day of onset (Mean ± S.D.) | |
|---|---|---|---|
| WT | 19/19 (100%) | 3.5 ± 0.9 | 10.52 ± 2.1 |
| Map3k8 −/− | 18/18 (100%) | 2.7 ± 0.9 | 15.07 ± 2.5 |
Intracellular staining showed similar proportions of CD4+ T cells expressing IFNγ and GM-CSF in the spinal cord between WT and Map3k8−/− mice (Fig. 2C, upper panel). However, the fraction of CD4+ cells producing IL-17A was significantly decreased by approximately 50% in the absence of TPL-2. Consistent with the overall decrease in CD4+ T cell number, the total numbers of CD4+ T cells expressing each of these encephalitogenic cytokines were significantly reduced in Map3k8−/− mice (Fig. 2C, lower panel). The proportions of γδT cells expressing IL-17A and IFNγ in the CNS were unaffected by TPL-2 deficiency, and total γδT cell numbers were not statistically significantly different from WT (Supplemental Fig. 1A). Immunohistochemistry confirmed reduced infiltration of T and B cells, and macrophages in the spinal cords of Map3k8−/− mice, accompanied by significantly reduced degrees of demyelination and neuronal damage (Fig. 2D and E).
A number of pro-inflammatory cytokines and chemokines are produced in the spinal cord by infiltrating reactivated CD4+ T cells, and activated CNS-resident cells (25, 26). Quantitative RT-PCR demonstrated significantly reduced amounts of mRNAs encoding the majority of these proteins in the spinal cords of Map3k8−/−mice at the peak of disease (Supplemental Fig. 1B). In contrast, steady-state levels of Il12a and Il12b mRNAs were increased, consistent with earlier in vitro studies (27).
Together these data indicate that TPL-2 deficiency protected mice from EAE by limiting CNS inflammation, thereby reducing demyelination and axonal damage.
TPL-2 is not required for T cell priming during EAE
The disease course of EAE can be considered to occur in two stages: a priming phase, in which immunization leads to activation and expansion of peripheral myelin-specific T cells, and an effector phase in which infiltrating inflammatory cells cause CNS damage. The priming phase involves the stimulation and expansion of myelin-responsive T cells by activated antigen-presenting DC in lymph nodes. The production of IL-1β, IL-6 and IL-23 by DC, and other innate immune cells, is critical for the initial induction of Th17 cells during an immune response (10), and signaling via each of these cytokines is essential for EAE induction (21, 25). Since TPL-2 has an established signaling function in DC, we initially investigated the effect of TPL-2 deficiency on the induction of Th17 polarizing cytokines by bone marrow-derived DC (BMDC) in response to heat-inactivated Mycobacterium tuberculosis (MtbHI).
Immunoblotting of cell lysates revealed that TPL-2 deficiency blocked the early activation of ERK by MtbHI, while a second wave of ERK-1/2 phosphorylation was TPL-2-independent (Supplemental Fig. 2A). p38 activation was also reduced in Map3k8−/− BMDC, similar to the reported effects of TPL-2 deficiency after LPS stimulation (27). Despite these decreases in MAP kinase activation, qRT-PCR revealed that MtbHI was still able to induce Il6 and Il23p19 mRNAs to similar levels in Map3k8−/− and WT BMDC (Supplemental Fig. 2B). TPL-2 deficiency reduced the induction of Il1b mRNA by approximately 40%, while the induction of Il12b mRNA was increased. TPL-2 deficiency had similar effects on MtbHI induction of ERK-1/2 activation and cytokines expression in BM-derived macrophages (BMDM) (Supplemental Fig. 3A and B). These data indicated that TPL-2 was not essential for the induction of Th17 polarizing cytokines by MtbHI in BMDC and BMDM, although MtbHI activation of ERK was largely dependent on TPL-2-expression in both cell types. Consistently, Map3k8−/− BMDC were able to induce IL-17A production by CD4+ T cells to the same extent as WT BMDC in an in vitro Th17 differentiation assay (Supplemental Fig. 2C and D) (19).
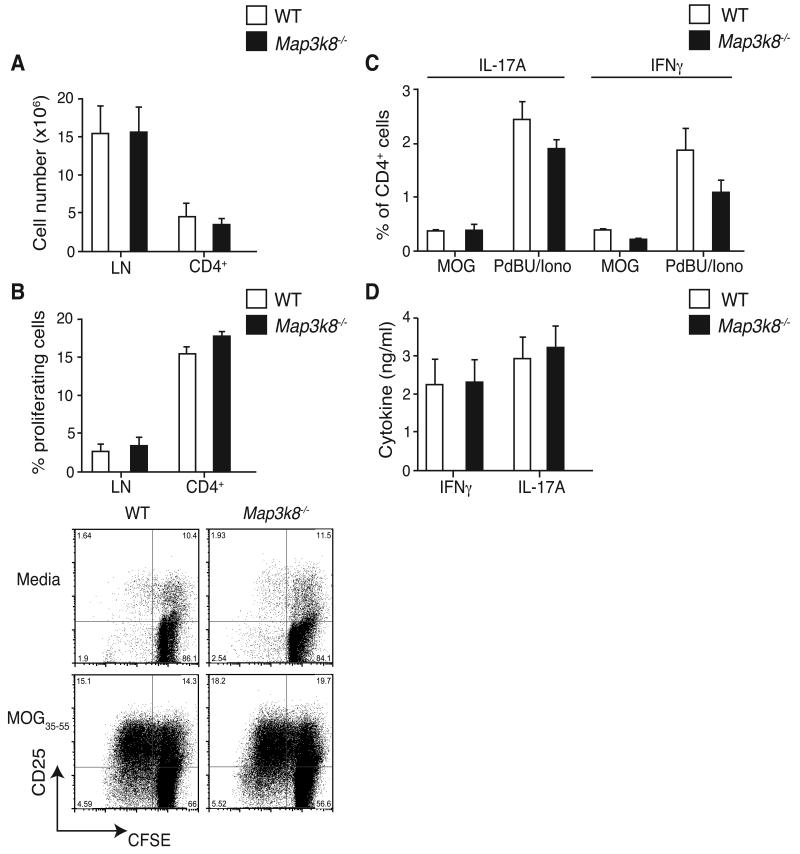
Our in vitro experiments suggested that TPL-2 expressed in antigen-presenting DC and in responding CD4+ T cells might not be essential for initial generation of Th17 cells in EAE. To investigate this, the in vivo priming and differentiation of Th17 cells was examined in WT and Map3k8−/− mice during the early stages of EAE. Analysis of draining lymph nodes (dLN) isolated from mice nine days after MOG35-55 immunization revealed that both the total cellularity and CD4+ T cell numbers were unaffected by TPL-2 deficiency (Fig. 3A). This suggested that the activation and expansion of MOG35-55-specific Map3k8−/− CD4+ T cells occurred normally. To quantify the antigen-specific T cell response, dLN cells were labeled with carboxyfluoresceine diacetate succinimidyl ester (CFSE) and restimulated in vitro with MOG35-55. Consistent with normal priming in EAE, a similar fraction of MOG35-55-specific CD4+ T cells divided within the WT and Map3k8−/− LN cell populations (Fig. 3B).
Figure 3. TPL-2 is not required for T cell priming in EAE.
WT and Map3k8−/− mice were immunized with MOG33-55/CFA, and draining LN isolated on d9. (A) CD4+ T cell numbers were determined by flow cytometry (mean ± s.e.m.; n = 3). (B) Flow cytometric analysis of the proliferation of CFSE-labelled CD4+ T cells cultured for 3d with MOG33-55 peptide (mean ± s.e.m.; n = 3). (C) Frequencies of IL-17A- and IFNγ–expressing CD4+ T cells were determined by flow cytometry, after restimulation with PdBu/ionomycin or MOG33-55 peptide (mean ± s.e.m.; n = 3). (D) ELISA of IFNγ and IL-17A production by LN cells restimulated with MOG33-55 peptide (mean ± s.e.m.; n = 3). Data are representative of at least three independent experiments.
Although T cell activation and expansion in EAE were unaffected by TPL-2 deficiency, it was possible that the ability of responding T cells to differentiate into Th17 and/or Th1 cells following MOG35-55 immunization was impaired. However, restimulation of dLN cells with either MOG35-55 peptide or PdBU and ionomycin revealed a similar fraction of IFNγ- and IL-17A-producing CD4+ T cells, as well as a similar production of both cytokines by MOG35-55-specific CD4+ T cells from immunized WT and Map3k8−/− mice (Fig. 3C and D).
In vivo experiments therefore indicated that CD4+ T cell activation, expansion and differentiation into Th1 and Th17 effector cells occurred independently of TPL-2. These results were consistent with the ability of Map3k8−/− CD4+ T cells to activate ERK-1/2 (Supplemental Fig. 3C) and proliferate normally in response to CD3/CD28 crosslinking (16), and for Map3k8−/− BMDC to induce differentiation of CD4+ T cells to the Th17 cell lineage in vitro to a similar extent as WT BMDC (Supplemental Fig. 2C).
TPL-2 does not function within T cells to promote EAE
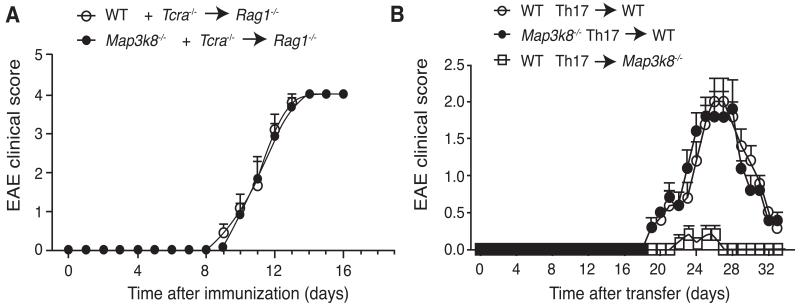
Defective T cell priming did not explain the reduced susceptibility of Map3k8−/− mice to EAE. Nevertheless, it remained possible that TPL-2 had a T cell-intrinsic function at a later stage in EAE pathogenesis. This was investigated by mixing BM cells from Tcra−/− mice with WT or Map3k8−/− BM cells (ratio 4:1), before transferring them into Rag1−/− hosts. In the resulting chimeras, all of the T cells developed from either WT or TPL-2-deficient donor BM, whilst the majority (80%) of the other hematopoetic cells was derived from the TPL-2-sufficient Tcra−/− BM. Upon induction, the onset and severity of EAE were essentially identical in both sets of mixed BM chimeras (Fig. 4A), indicating that there was no T cell-intrinsic function for TPL-2 in EAE pathogenesis.
Figure 4. TPL-2 signaling in T cells is not required for EAE development.
(A) BM cells from WT and Map3k8−/− mice were mixed in a 1:4 ratio with BM cells from Tcra−/− mice, and transferred into Rag1−/− hosts. After 8 weeks, EAE was induced by immunization with MOG33-55/CFA, and clinical scores determined (mean ± s.e.m.; n = 6). (B) Draining LN cells from WT and Map3k8−/−mice were isolated 9d after immunization with MOG33-55/CFA, and restimulated in vitro with MOG33-55 peptide in the presence of recombinant IL-1β and 1L-23 for 5d. These Th17-polarized cells were then transferred into naïve WT and Map3k8−/− mice. Graph represents the average clinical score after Th17 cell transfer (mean ± s.e.m.; n = 5). Data in A and B are representative of two independent experiments.
The potential function of TPL-2 in T cells was also investigated in a passive EAE model, in which encephalitogenic myelin-responsive T cells were transferred into naïve recipients (13). Draining LN cells were isolated from Map3k8−/− mice nine days after MOG35-55 immunization, and then cultured with MOG35-55 peptide, IL-1β and IL-23 to expand MOG35-55-specific Th17 cells (28). Intracellular cytokine staining indicated similar polarization efficiency within WT and Map3k8−/− CD4+ T cell populations (data not shown). Following intravenous injection, the onset and extent of EAE induced by transferred Map3k8−/− and WT Th17-polarized cells were very similar (Fig. 4B), consistent with the results of active EAE in mixed BM chimeras.
CD20 antibody depletion has demonstrated that B cells promote EAE disease progression via production of IL-6 (29, 30), in addition to their late-acting regulatory role (31, 32). TPL-2 is known to regulate ERK-1/2 activation in B cells following CD40 and TLR stimulation (3, 33), raising the possibility that the protection of Map3k8−/− mice from EAE might be due to TPL-2 signaling in B cells. To investigate this, chimeric mice were generated in which BM from μMT−/− mice was mixed with Map3k8−/− BM cells (ratio 4:1), and injected into lethally irradiated Rag1−/− recipients. The onset and severity of EAE were similar between mice lacking TPL-2 expression in B cells and WT controls (Supplemental Fig. 4A). Therefore, the protection from EAE observed in Map3k8−/− mice was not due to the absence of TPL-2 in T or B cells.
TPL-2 signaling in radiation-resistant cells promotes the effector phase of EAE
Since the priming phase of EAE was normal in Map3k8−/− mice, this suggested that TPL-2 functioned during the effector phase of the disease. To investigate this, the ability of WT MOG35-55 peptide-primed Th17 cells to induce passive EAE in WT and Map3k8−/− recipient mice was determined. A delay in onset of clinical symptoms and a reduction in disease severity were observed upon transfer of encephalitogenic Th17-polarized cells into Map3k8−/− mice (Fig. 4B). TPL-2-deficient mice were also protected from EAE induced by WT MOG35-55 peptide-primed Th1 cells, suggesting the protection from disease did not involve a Th-cell specific factor (Supplemental Fig. 4B and C).
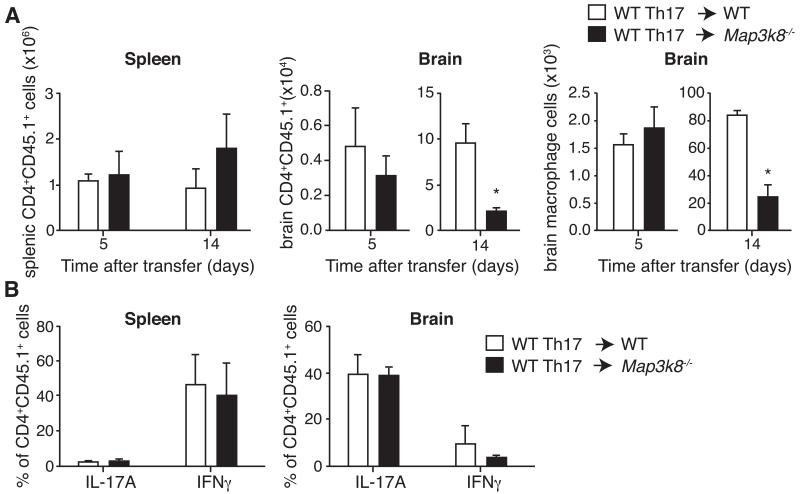
The effector phase of EAE involves two waves of immune cell infiltration into the CNS (34-36). Firstly, ‘pioneer’ MOG35-55-specific CD4+ T cells enter the brain via the choroid plexus. Inflammatory cytokines produced by re-activated CD4+ T cells then induce recruitment of a second wave of infiltrating immune cells. We investigated the recruitment of immune cells to the CNS in passive EAE. CD45.1+ MOG35-55 peptide-primed Th17 cells generated from WT mice were transferred to CD45.2+ WT or Map3k8−/− mice. Similar numbers of CD45.1+ CD4+ T cells were detected in the spleens and brains of WT and Map3k8−/− mice early after adoptive transfer (Fig. 5A, left and middle panels). However, at the peak of the disease, significantly more CD45.1+ CD4+ T cells accumulated in the brains of WT relative to Map3k8−/− mice (Fig. 5A, middle panel). Moreover, consistent with a defect in secondary immune cell infiltration into Map3k8−/− brains, fewer CD45+F4/80+ macrophages were detected 14 days after Th17 cell transfer (Fig. 5A, right panel). The number of CD45.1+ CD4+ T cells in the spleen at these later time points was similar between the two recipient CD45.2+ genotypes, demonstrating that host TPL-2 expression was not required for CD4+ T cell survival (Fig. 5A, left panel). Furthermore, transferred CD4+ T cells in the CNS of Map3k8−/− hosts produced similar fractions of IFNγ and IL-17 to WT hosts (Fig. 5B). ‘Pioneer’ Th17 cells, therefore, could infiltrate the CNS of Map3k8−/− mice, but failed to optimally trigger a secondary infiltration of immune cells, the major mediators of myelin damage.
Figure 5. TPL-2 regulates the effector phase of EAE.
(A) MOG33-55-specific Th17 cells were generated from CD45.1+ WT mice, and then transferred into CD45.2+ WT and Map3k8−/− mice as in Figure 6C. Numbers of CD45.1+ CD4+ T cells in the spleen and brain were determined by flow cytometry at the indicated time points after Th17 cell transfer (mean ± s.e.m.; n = 3). Macrophage numbers in the brain after transfer were determined by F4/80 staining. (B) Intracellular staining for IL-17A- and IFNγ-expression in transferred Th17 polarized CD45.1+ CD4+ T cells in the spleen and brain on d14 after transfer (mean ± s.e.m., n=3). Data are representative of at least two independent experiments. * p ≤ 0.05
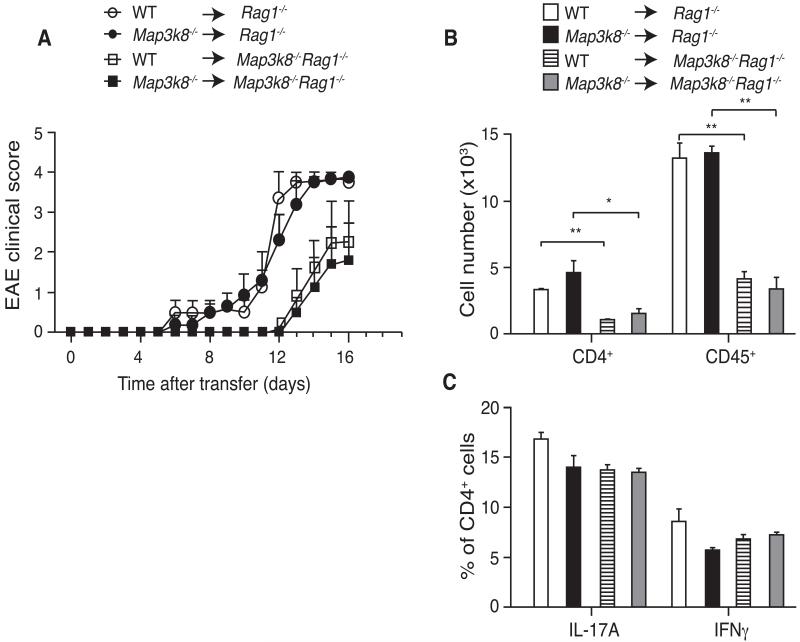
Although transfer of encephalitogenic Th17 cells demonstrated that the TPL-2 deficient host environment was protective in passive EAE (Fig. 4B), it did not reveal whether TPL-2 promoted EAE by signaling in hematopoietic cells other than T or B cells. This was investigated by generating BM chimeras, via transferring WT or Map3k8−/− BM cells into lethally irradiated Rag1−/− or Rag1−/−Map3k8−/− recipients. In these chimeras, all lymphocytes, and more than 99% of MHC Class II+ cells, were of donor-origin (data not shown). Upon EAE induction, deletion of TPL-2 in radiation-sensitive cells (Map3k8−/− > Rag1−/− compared with Map3k8+/+ > Rag1−/−) was not able to protect against disease (Fig. 6A). However, transferring WT BM cells into Rag1−/− hosts that additionally lacked TPL-2 resulted in delayed EAE onset. Furthermore, lack of TPL-2 expression in the radiation-resistant compartment resulted in a reduction in immune infiltrates in the spinal cord (Fig. 6B), but did not affect polarization of CD4+ T cells to produce IL-17A and IFNγ (Fig. 6C). Therefore, TPL-2 promoted the effector phase of EAE by signaling in radiation-resistant non-hematopoietic cells, which induced the secondary infiltration of immune cells into the CNS parenchyma.
Figure 6. TPL-2 signaling in radiation-resistant cells promotes EAE.
(A) BM cells from WT and Map3k8−/− mice were adoptively transferred into lethally irradiated Rag1−/− or Map3k8−/−Rag1−/− hosts, and EAE induced 8 weeks later. Mean clinical scores are shown (mean ± s.e.m; n = 4-5). (B and C) EAE was induced in chimeric mice generated as in A. (B) Total numbers of CD4+ and CD45+ cells in the spinal cords 12d after MOG33-55 peptide plus CFA immunization were determined by flow cytometry (mean ± s.e.m., n=4). (C) Intracellular staining for IL-17A- and IFNγ–expressing CD4+ T cells in the spinal cord at peak of disease (mean ± s.e.m.; n = 4). Data are representative for three independent experiments. * p ≤ 0.05; ** p≤0.01.
TPL-2 signals in both microglia and astrocytes
CNS-resident microglia and astrocytes have been reported previously to play pivotal roles in EAE disease manifestation (20, 37, 38). These radiation-resistant cell types produce a number of pro-inflammatory cytokines and chemokines during EAE, which promote the secondary influx of hematopoietic cells into the CNS (34, 36, 39). Defective activation of one or both of these cell types, therefore, could account for the protection of Map3k8−/− mice from EAE development. We explored this possibility by determining whether TPL-2 deficiency affected cytokine and chemokine induction in primary in vitro-derived microglia and astrocytes.
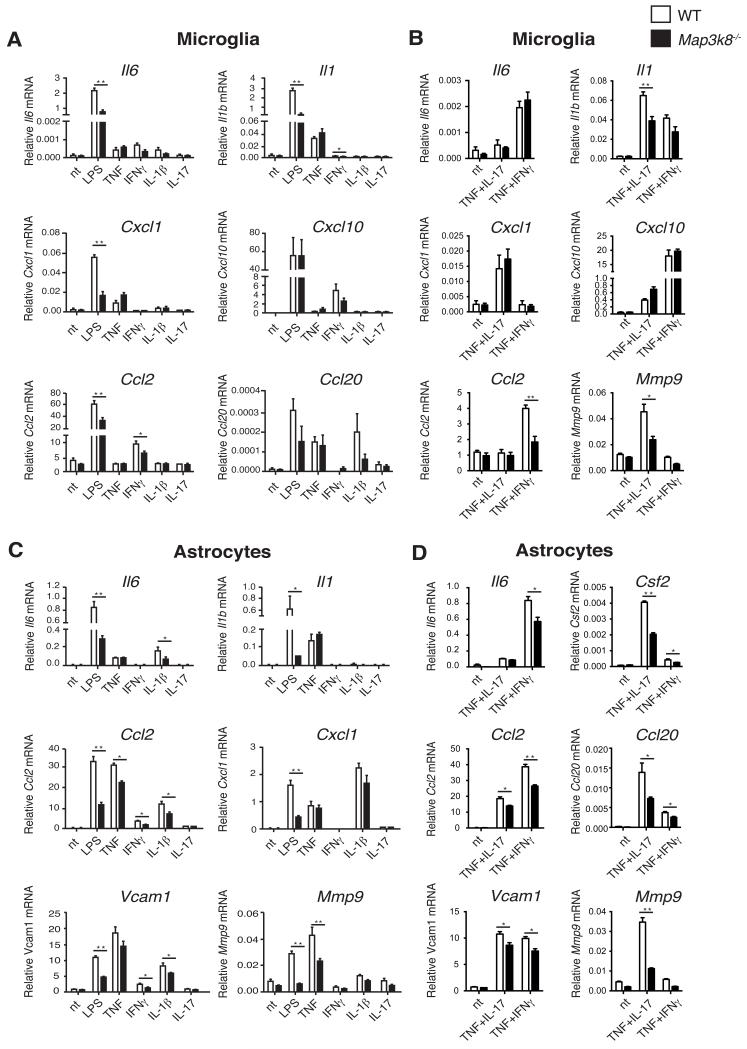
Map3k8−/− microglia expressed significantly less Il6, Il1, Cxcl1, Ccl2 and Ccl20 mRNAs in response to LPS stimulation than their WT counterparts (Fig. 7A). In addition, induction of Il1 and Ccl2 mRNAs was reduced by TPL-2 deficiency following stimulation with IFNγ, or TNF plus IFNγ (Fig. 7 A and B). LPS upregulation of mRNAs encoding IL-6, IL-1β, CCL2, CXCL1, VCAM1 and MMP9 was also significantly reduced in Map3k8−/− astrocytes compared to WT control cells (Fig. 7C). Furthermore, IL-1β induction of Il6, Ccl2 and Vcam1 mRNAs, as well as TNF induction of Ccl2 and Mmp9 mRNAs, were reduced by TPL-2 deficiency in astrocytes (Fig. 7C). Defective TNF induction of Ccl2, Vcam1 and Mmp9 mRNAs in Map3k8−/− astrocytes was not rescued by co-stimulation with IL-17A (Fig. 7D). In line with earlier studies, stimulation of astrocytes with IL-17A alone had minimal effect on the expression of mRNAs encoding pro-inflammatory cytokines or chemokines in astrocytes (20, 40).
Figure 7. TPL-2 regulates pro-inflammatory gene expression in primary microglia and astrocytes.
Quantitative RT-PCR of mRNA expression in primary microglia (A, B) and astrocytes (C, D) from WT and Map3k8−/− mice, normalized to Hprt mRNA (mean ± s.e.m.). Cells were either left untreated (nt) or stimulated with the indicated agonists for 6h. Data are compiled from three (A, C) or two (B, D) independent experiments. * p ≤ 0.05, ** p≤0.01.
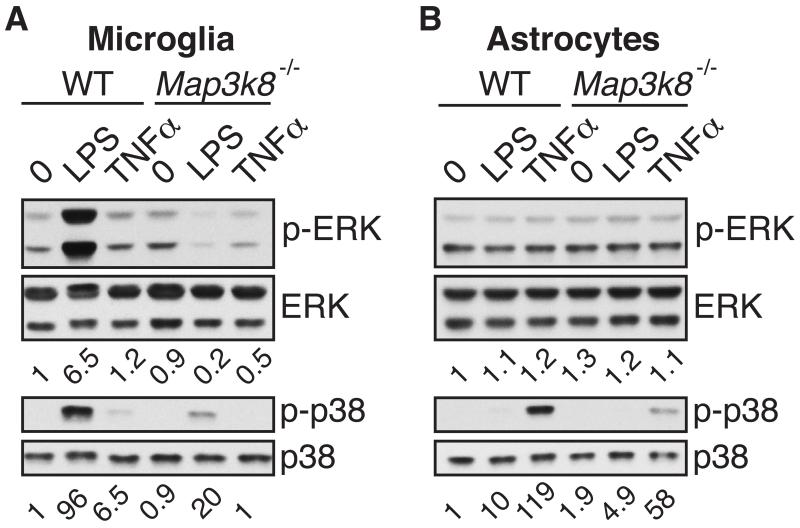
We next analysed the activation of MAP kinase signaling pathways in microglia and astrocytes to gain insight into how TPL-2 deficiency might impair pro-inflammatory gene expression in these cell types. In line with earlier studies using primary macrophages and dendritic cells (2, 3, 27), ERK-1/2 and p38α phosphorylation following LPS or TNF stimulation of microglia was reduced by TPL-2 deficiency (Fig. 8A). Analysis of NF-κB1 p105 phosphorylation (data not shown) indicated that this decrease did not result from impaired activation of the IKK complex (41). Phosphorylation of ERK-1/2 and p38α was not induced by stimulation with either IFNγ or IL-17A (data not shown).
Figure 8. TPL-2 is required for optimal MAP kinase activation in microglia and astrocytes.
Lysates of microglia (A) and astrocytes (B), generated from WT and Map3k8−/− mice and stimulated for the indicated time points with LPS or TNF, were immunoblotted. Phosphorylated protein bands were quantified by laser densitometry using the Quantity One software package, and are presented as relative density normalized to the respective total protein. Data are representative for 3 – 4 independent experiments with similar results.
ERK-1 and 2 were basally phosphorylated at high levels in astrocytes, and this was not detectably altered by stimulation with LPS or TNF (Fig. 8B). However, LPS and TNF clearly induced p38α phosphorylation, and this was partially decreased by TPL-2 deficiency following stimulation with either agonist. Addition of IFNγ or IL-17A did not alter p38α phosphorylation in astrocytes (data not shown).
In conclusion, our biochemical analyses demonstrated that TPL-2 contributed to p38α activation in both microglia and astrocytes after stimulation with LPS and TNF, and was also required for optimal activation of ERK-1/2 in microglia by these agonists. Together with the qRT-PCR analyses of cytokine and chemokine mRNA expression (Fig. 7), these data were consistent with the hypothesis that TPL-2 signaling in both microglia and astrocytes could contribute to EAE disease development.
TPL-2 kinase activity is required for development of EAE
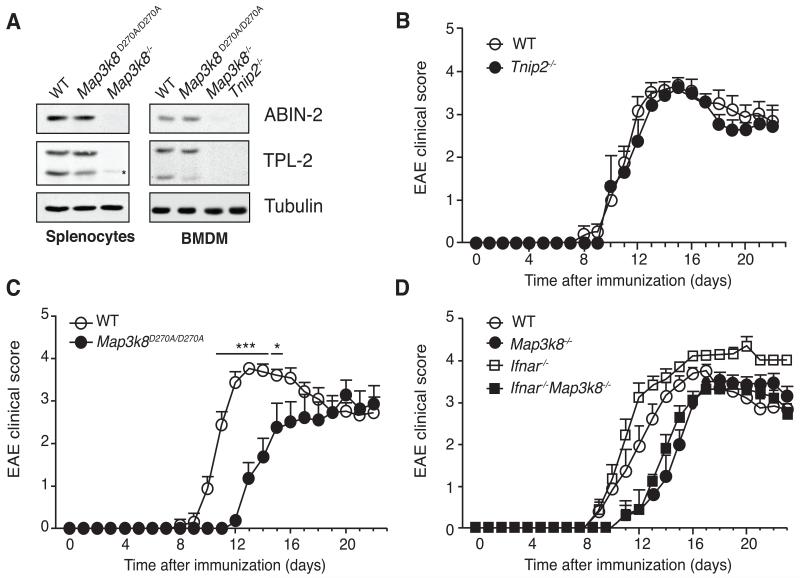
TPL-2 is associated with the ubiquitin-binding protein ABIN-2, which is required to maintain TPL-2 stability (42). ABIN-2-deficient mice have reduced steady-state levels of TPL-2 in multiple cell type compared to WT mice (14). In a reciprocal fashion, analysis of cells from Map3k8−/− mice revealed that TPL-2 is required to maintain steady-state expression of ABIN-2, and both splenocytes and BMDM from Map3k8−/− mice have substantially reduced amounts of ABIN-2 compared to WT (Fig. 9A). This suggested that the resistance of Map3k8−/− mice to EAE might result from reduced ABIN-2 expression. However, EAE disease severity was similar between Tnip2−/− (which completely lack ABIN-2 expression) and WT mice (Fig. 9B). These data indicated ABIN-2 was not required for EAE development, and suggested that the low levels of TPL-2 expressed in Tnip2−/− mice were sufficient to promote maximal disease.
Figure 9. TPL-2 kinase activity is required to induce EAE.
(A) Lysates were prepared from splenocytes (left panel) and BMDM (right panel) generated from WT, Map3k8−/−, Map3k8D270A/D270A and TNIP2−/− mice, and immunoblotted for the indicated antigens. (B) Mean clinical scores of WT and Tnip2−/− mice (mean ± s.e.m.; n = 7 - 8). Data are compiled from 2 different experiments (n = 3-4/genotype/experiment). (C) Mean clinical scores of WT, Map3k8D270A/D270A and Map3k8−/− mice mice at various times after immunization with MOG33-55/CFA (mean ± s.e.m.; n = 8 - 9). Data are compiled from 2 different experiments (n = 3-4/genotype/experiment). * p ≤ 0.05; *** p≤0.001. (D) Mean clinical scores of WT, Map3k8−/−, Ifnar−/− and Map3k8−/−Ifnar−/− mice after immunization with MOG33-55/CFA (mean ± s.e.m.; n = 9 - 10). Data are compiled from 2 different experiments (n = 4-5/genotype/experiment).
Together our analyses of Map3k8−/− and Tnip2−/− mice demonstrated that TPL-2 expression was required for efficient EAE development. However, these experiments did not establish whether this reflected a role for TPL-2 catalytic activity, and it remained possible that TPL-2 promoted disease by functioning as an adaptor protein. To distinguish these possibilities, we generated a novel knock-in mouse strain expressing mutant TPL-2D270A (Supplemental Fig. 4D), which is catalytically inactive (43). In contrast to Map3k8−/− cells, Map3k8D270A/D270A cells expressed similar amounts of ABIN-2 as WT cells (Fig. 7A). Compared to WT controls, EAE development was delayed and reduced in severity in Map3k8D270A/D270A mice (Fig. 9C), similar to Map3k8−/− mice. TPL-2 catalytic activity was therefore required to promote EAE, and this was independent of effects on the steady-state expression of ABIN-2 protein, or any potential adaptor function of TPL-2.
TPL-2 promotes EAE independently of type I interferon signaling
Development of EAE is exacerbated in mice deficient in type I interferon receptor (IFNAR), with markedly higher inflammation and demyelination in the CNS compared to WT controls (44). TPL-2/ERK-1/2 signaling negatively regulates TLR induction of IFN-β (27), raising the possibility that TPL-2 deficiency might reduce EAE disease severity by augmenting IFN-β production. We investigated this possibility genetically by generating Map3k8−/− Ifnar−/− mice, lacking expression of both TPL-2 and the receptor for type I IFNs, IFNAR.
As reported previously (44), IFNAR-deficiency did not affect EAE disease onset, but increased the severity and duration of the effector phase (Fig. 9D). However, the onset of EAE and maximal clinical scores were similar in Map3k8−/− and Map3k8−/− Ifnar−/− mice. The inhibitory effects of TPL-2 deficiency on EAE development, therefore, were independent of type I IFN signaling.
Discussion
We investigated the potential role of the MAP 3-kinase TPL-2 in Th17 cell generation and function. Our results show that TPL-2 was dispensable for the generation of Th17 cells in vitro, and during the priming phase of EAE. Nevertheless, TPL-2 regulated both the onset and severity of EAE, functioning in the effector phase of this Th17 cell-mediated autoimmune disease model. Importantly, the effects of TPL-2 in EAE required its catalytic activity, suggesting that small molecule inhibitors of TPL-2 might be therapeutically beneficial in multiple sclerosis (MS). IFN-β is widely used for treatment of relapsing-remitting MS (45). TPL-2 promoted EAE independently of type I IFN signaling, raising the possibility that administration of a TPL-2 inhibitor in combination with the established drug IFN-β might be more effective for MS therapy.
Earlier pharmacological studies with the MEK-1/2 inhibitor U0126 have suggested that ERK-1/2 activation is required for induction of the Th17 polarizing cytokines IL-1β and IL-23 in DC following stimulation with MtbHI (28). MtbHI can potentially activate multiple pattern recognition receptors on DC, including TLR-2, TLR-4, TLR-9, NOD-2 and C-type lectins (46, 47). TPL-2 is required for activation of ERK-1/2 in DC following TLR2, TLR4 or TLR9 stimulation (4, 27), and also contributes to the activation of ERK in macrophages following NOD-1 and NOD-2 stimulation, but is dispensable for activation of ERK-1/2 by the C-type lectin Dectin-1 (4). Consistent with this, stimulation of ERK phosphorylation by MtbHI was largely dependent on TPL-2 expression in BMDC. However, TPL-2 deficiency only partially reduced MtbHI induction of IL-1β, and did not impair induction of mRNAs encoding IL-6, IL-12p35 or IL-23p19 in these cells, which were able to induce normal Th17 cell differentiation in vitro. In line with these data, the generation of Th17 cells in the draining LN during the priming phase of EAE was unaffected by TPL-2 deficiency. It is likely that low levels of TPL-2-independent ERK-1/2 activation explain the ability of Map3k8−/− BMDC to induce mRNAs encoding IL-1β and IL-23p19.
Experiments with lethally irradiated BM chimeras demonstrated that TPL-2 functioned in a radiation-resistant cell population to promote EAE, implying that TPL-2 signaling was not required in T cells, or antigen presenting cells (DC, macrophages). These data are consistent with in vitro experiments showing that TPL-2 expression was dispensable for Th17 cell differentiation induced with recombinant cytokines, or for MtbHI induction of Th17 polarizing cytokines in BMDC and BMDM. Furthermore, mixed BM chimera experiments demonstrated that TPL-2 expression in either T or B cells was not required for EAE development. Instead, passive EAE experiments using MOG35-55-specific Th17 cells revealed that TPL-2 signaling in host cells regulated the effector phase of disease in the CNS. A similar requirement for host TPL-2 was found after adoptive transfer of WT MOG35-55 -specific Th1 cells. Therefore, TPL-2 did not have a Th17-specific function in the effector phase of EAE.
After priming in lymph nodes, antigen-specific Th17 cells traffic through the choroid plexus into the subarachnoid space, where they are reactivated (35). As a consequence of productive T cell-APC interactions, Th17 cell cytokines and chemokines are then produced, which activate parenchymal vasculature. This promotes a secondary wave of leukocyte infiltration, leading to inflammatory CNS damage and EAE onset (34). Passive EAE experiments indicated that the initial trafficking of Th17 cells into the brain was not affected by TPL-2 deficiency. Rather that TPL-2 was required for the second wave of leukocyte recruitment into the CNS. The expression of several chemokines known to be involved in regulating the migration of leukocytes into the CNS was significantly reduced in intact Map3k8−/− mice during EAE, including CCL2, CCL3, CCL5 and CXCL10, which have each been implicated to have positive roles in EAE induction (34). The protective effect of TPL-2 deficiency in EAE, therefore, may result from impaired upregulation of these chemokines in CNS-resident cells.
An important outstanding question is the identity of the radiation-resistant non-hematopoietic cell type in which TPL-2 signals to promote EAE. In vitro experiments indicated that TPL-2 was required for optimal ERK-1/2 and p38α activation in microglia, resident myeloid-lineage cells in the brain and spinal cord (48). Microglia can contribute to EAE disease initiation by presenting antigens to naïve T cells, and are also a potent source of inflammatory cytokines and chemokines (37, 39). In addition, our in vitro experiments revealed that TPL-2 signaling contributes to the activation of p38α in astrocytes, the most abundant cells of the brain which also produce cytokines and chemokines, and have an important role in regulating the recruitment and function of T cells in the CNS (39). TPL-2 expression was found to be required for optimal expression of pro-inflammatory cytokines and chemokines in both microglia and astrocytes, possibly due to TPL-2’s contribution to MAP kinase activation in these cell types. Earlier studies with knockout mice have shown the importance of the affected cytokines and chemokines for EAE disease development (34, 39). Our data raise the possibility that the reduced secondary infiltration of inflammatory cells into the CNS in Map3k8−/− mice compared to WT controls during EAE results from the decreased expression of cytokines and chemokines by microglia and astrocytes.
Genetic deletion of IKK2 or NEMO in neuroectodermal-derived cells ameliorates EAE in mice, which correlates with decreased expression of proinflammatory cytokines and chemokines by CNS-resident cells (38). Recently, it has also been shown that conditional deletion of Tak1, which functions upsteam of the IKK complex, in either astrocytes or microglia also ameliorates EAE development (49). The IKK complex is a critical positive regulator of the TPL-2 signaling pathway, inducing proteolysis of its inhibitor NF-κB1 p105 (50, 51) and directly phosphorylating a key regulatory serine in the TPL-2 C-terminus (52). The protective effects of IKK2/NEMO deficiency in astrocytes or TAK1 deficiency in astrocytes and microglia, therefore, may be mediated in part by blockade of TPL-2 signaling. The generation of conditional knockout mouse strains lacking TPL-2 expression in astrocytes or microglia will be essential to determine whether TPL-2 signaling in either or both these cell types is important for development of EAE.
Earlier studies of Map3k8−/− mice identified TPL-2 as a potential drug target in septic shock, and inflammatory bowel disease (2, 5). Interestingly, Mapk1 was recently identified as a disease susceptibility gene locus in multiple sclerosis (53). Mapk1 encodes ERK2, a major downstream target of TPL-2 signaling. These data, together with the present study, suggest that blockade of TPL-2 catalytic activity might also be therapeutically beneficial in multiple sclerosis.
Supplementary Material
Acknowledgements
This work was supported by the U.K. Medical Research Grants U117584209 (to S.C.L.), U117565642 (to A.O.), and U117512792 (to B.S.) and Arthritis Research U.K. Grant 19431 (to S.C.L.), as well as by European Commission programs Inflammation and Cancer Research in Europe (Contract 223151), Mechanisms to Attack Steering Effectors of Rheumatoid Syndromes with Innovated Therapy Choices (Contract 223404), and Innovative Medicines Initiative Joint Undertaking “Be the Cure” (Contract 115142) (all to G.K.).
References
- 1.Gantke T, Sriskantharajah S, Ley SC. Regulation and function of TPL-2, an IκB kinase-regulated MAP kinase kinase kinase. Cell Res. 2010;21:131–145. doi: 10.1038/cr.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dumitru CD, Ceci JD, Tsatsanis C, Kontoyiannis D, Stamatakis K, Lin J-H, Patriotis C, Jenkins NA, Copeland NG, Kollias G, Tsichlis PN. TNFα induction by LPS is regulated post-transcriptionally via a TPL2/ERK-dependent pathway. Cell. 2000;103:1071–1083. doi: 10.1016/s0092-8674(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 3.Eliopoulos AG, Wang C-C, Dumitru CD, Tsichlis PN. TPL-2 transduces CD40 and TNF signals that activate ERK and regulates IgE induction by CD40. EMBO J. 2003;22:3855–3864. doi: 10.1093/emboj/cdg386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mielke LA, Elkins KL, Wei L, Starr R, Tsichlis PN, O’Shea J, Watford WT. Tumor progression locus 2 (Map3k8) is critical for host defense against Listeria monocytogenes and IL-1 production. J. Immunol. 2009;183:7984–7993. doi: 10.4049/jimmunol.0901336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kontoyiannis D, Boulougouris G, Manoloukos M, Armaka M, Apostolaki M, Pizarro TT, Kotlyarov A, Forster I, Flavell RA, Gaestel M, Tsichlis PN, Cominelli F, Kollias G. Genetic dissection of the cellular pathways and signaling mechanisms in modeled tumor necrosis factor-induced Crohn’s-like inflammatory bowel disease. J Exp Med. 2002;196:1563–1574. doi: 10.1084/jem.20020281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George D, Salmeron A. Cot/TPL-2 protein kinase as a target for the treatment of inflammatory disease. Curr. Topics. Med. Chem. 2009;9:611–622. doi: 10.2174/156802609789007345. [DOI] [PubMed] [Google Scholar]
- 7.Watford WT, Wang C-C, Tsatsanis C, Mielke LA, Eliopoulos AG, Daskalakis C, Charles N, Odom S, Rivera J, O’Shea J, Tsichlis PN. Ablation of tumor progression locus 2 promotes a type 2 Th cell response in ovalbumin-immunized mice. J. Immunol. 2009;184:105–113. doi: 10.4049/jimmunol.0803730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watford WT, Hissong BD, Durant LR, Yamane H, Muul LM, Kanno Y, Tato CM, Ramos HL, Berger AE, Mielke L, Pesu M, Solomon B, Frucht DM, Paul WE, Sher A, Jankovic D, Tsichlis PN, O’Shea JJ. Tpl2 kinase regulates T cell interferon-γ production and host resistance to Toxoplasma gondii. J. Exp. Med. 2008;205:2803–2812. doi: 10.1084/jem.20081461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glimcher LH, Murphy KM. Lineage commitment in the immune system: the T helper lymphocyte grows up. Genes Dev. 2000;14:1693–1711. [PubMed] [Google Scholar]
- 10.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 11.Becher B, Segal BM. Th17 cytokines in autoimmune neuro-inflammation. Curr Opin Immunol. 2011;23:707–712. doi: 10.1016/j.coi.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnamoorthy G, Wekerle H. EAE: an immunologist’s magic eye. Eur. J. Immunol. 2009;39:1991–2058. doi: 10.1002/eji.200939568. [DOI] [PubMed] [Google Scholar]
- 13.Denic A, Johnson AJ, Bieber AJ, Warrington AE, Rodriguez M, Pirko I. The relevance of animal models in multiple sclerosis research. Pathophys. 2010;18:21–29. doi: 10.1016/j.pathophys.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papoutsopoulou S, Symons A, Tharmalingham T, Belich MP, Kaiser F, Kioussis D, O’Garra A, Tybulewicz V, Ley SC. ABIN-2 is required for optimal activation of the TPL-2/ERK MAP kinase pathway in innate immune responses. Nature Immunol. 2006;7:606–615. doi: 10.1038/ni1334. [DOI] [PubMed] [Google Scholar]
- 15.Dann A, Poeck H, Croxford AL, Gaupp S, Kierdorf K, Knust M, Pfeifer D, Maihoefer C, Endres S, Kalinke U, Meuth SG, Wiendl H, Knobeloch KP, Akira S, Waisman A, Hartmann G, Prinz M. Cytosolic RIG-I-like helicases act as negative regulators of sterile inflammation in the CNS. Nat. Neurosci. 2011;15:98–106. doi: 10.1038/nn.2964. [DOI] [PubMed] [Google Scholar]
- 16.Sriskantharajah S, Belich MP, Papoutsopoulou S, Janzen J, Tybulewicz V, Seddon B, Ley SC. Proteolysis of NF-κB1 p105 is essential for T cell antigen receptor-induced proliferation. Nat. Immunol. 2009;10:38–47. doi: 10.1038/ni.1685. [DOI] [PubMed] [Google Scholar]
- 17.Boonstra A, Asselin-Paturel C, Gilliet M, Crain C, Trinchieri G, Liu YJ, O’Garra A. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential toll-like receptor ligation. J. Exp. Med. 2003;197:101–109. doi: 10.1084/jem.20021908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veldhoen M, Hirota K, Westendorft AM, Buer J, Dumoutier L, Renauld J-C, Stockinger B. The aryl hydrocarbon receptor links TH17-cell mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 19.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Kang Z, Altuntas CZ, Gulen MF, Liu C, Giltiay N, Qin H, Liu L, Qian W, Ransohoff RM, Bergmann C, Stohlman S, Tuohy VK, Li X. Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity. 2010;32:414–425. doi: 10.1016/j.immuni.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early TH17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of TH17 cells. Nat. Rev. Immunol. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORγT directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 24.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGeachy MJ, Anderton SM. Cytokines in the induction and resolution of experimental autoimmune encephalomyelitis. Cytokine. 2005;32:81–84. doi: 10.1016/j.cyto.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Wilson EH, Weninger W, Hunter CA. Trafficking of immune cells in the central nervous system. J. Clin. Invest. 2010;120:1368–1378. doi: 10.1172/JCI41911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaiser F, Cook D, Papoutsopoulou S, Rajsbaum R, Wu X, Yang HT, Grant S, Ricciardi-Castagnoli P, Tsichlis PN, Ley SC, O’Garra A. TPL-2 negatively regulates interferon-beta production in macrophages and myeloid dendritic cells. J. Exp. Med. 2009;206:1863–1871. doi: 10.1084/jem.20091059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brereton CF, Sutton CE, Lalor SJ, Lavelle EC, Mills KHG. Inhibition of ERK MAPK suppresses IL-23- and IL-1-driven IL-17 production and attenuates autoimmune disease. J. Immunol. 2009;183:1715–1723. doi: 10.4049/jimmunol.0803851. [DOI] [PubMed] [Google Scholar]
- 29.Barr TA, Shen P, Brown S, Lampropoulou V, Roch T, Lawrie S, Fan B, O’Connor RA, Anderton SM, Bar-Or A, Fillatreau S, Gray D. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J. Exp. Med. 2012;209:1001–1010. doi: 10.1084/jem.20111675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsushita T, Yanaba K, Bouaziz J-D, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J. Clin. Invest. 2008;118:3420–3420. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderton SM, Fillatreau S. Activated B cells in autoimmune diseases: the case for a regulatory role. Nat. Clin. Pract. Rheumatol. 2008;4:657–666. doi: 10.1038/ncprheum0950. [DOI] [PubMed] [Google Scholar]
- 32.Fillatreau S, Sweenie MJ, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 33.Banerjee A, Gugasyan R, McMahon M, Gerondakis S. Diverse Toll-like receptors utilize Tpl2 to activate extracellular signal-regulated kinase (ERK) in hemopoietic cells. Proc Natl Acad Sci U S A. 2006;103:3274–3279. doi: 10.1073/pnas.0511113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prendergast CT, Anderton SM. Immune cell entry to the central nervous system-current understanding and prospective therapeutic targets. Drug Targets. 2009;9:315–327. doi: 10.2174/187153009789839219. [DOI] [PubMed] [Google Scholar]
- 35.Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A, Engelhardt B, Sallusto F. C-C chemokine receptor 6-regulated entry of Th17 cells into the CNS through the chorid plexus is required for the initiation of EAE. Nat. Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 36.Zepp J, Wu L, Li X. IL-17 receptor signaling and T helper 17-mediated autoimmune demyelinating disease. Trends Immunol. 2011;32:232–239. doi: 10.1016/j.it.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heppner FL, Greter M, Marino D, Falsig J, Raivich G, Hovelmeyer N, Waisman A, Rulicke T, Prinz M, Priller J, Becher B, Aguzzi A. Experimental autoimmune encephalomyelitis repressed by micoglial paralysis. Nat. Med. 2005;11:146–152. doi: 10.1038/nm1177. [DOI] [PubMed] [Google Scholar]
- 38.van Loo G, De Lorenzi R, Schmidt H, Huth M, Mildner A, Schmidt-Supprian M, Lassmann H, Prinz M, Pasparakis M. Inhibition of transcription factor NF-kB in the central nervous system ameliorates autoimmune encephalomyelitis in mice. Nat. Immunol. 2006;7:954–961. doi: 10.1038/ni1372. [DOI] [PubMed] [Google Scholar]
- 39.Chastain EML, Duncan AS, Rodgers JM, Miller SD. The role of antigen presenting cells in multiple sclerosis. Biochim. Biophys. Acta. 2011;1812:265–274. doi: 10.1016/j.bbadis.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Deckert M, Xuan NT, Nishanth G, Just S, Waisman A, Naumann M, Schluter D. Astrocytic A20 ameliorates experimental autoimmune encephalomyelitis by inhibiting NF-κB and STAT1-dependent. Acta Neuropathol. 2013 doi: 10.1007/s00401-013-1183-9. DOI 10.1007/s00401-013-1183-9. [DOI] [PubMed] [Google Scholar]
- 41.Yang HT, Papoutsopoulou M, Belich M, Brender C, Janzen J, Gantke T, Handley M, Ley SC. Coordinate regulation of TPL-2 and NF-κB signaling in macrophages by NF-κB1 p105. Mol. Cell. Biol. 2012;32:3438–3451. doi: 10.1128/MCB.00564-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lang V, Symons A, Watton SJ, Janzen J, Soneji Y, Beinke S, Howell S, Ley SC. ABIN-2 forms a ternary complex with TPL-2 and NF-κB1 p105 and is essential for TPL-2 protein stability. Mol. Cell. Biol. 2004;24:5235–5248. doi: 10.1128/MCB.24.12.5235-5248.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salmeron A, Ahmad TB, Carlile GW, Pappin D, Narsimhan RP, Ley SC. Activation of MEK-1 and SEK-1 by Tpl-2 proto-oncoprotein, a novel MAP kinase kinase kinase. EMBO J. 1996;15:817–826. [PMC free article] [PubMed] [Google Scholar]
- 44.Prinz M, Schmidt H, Mildner A, Knobeloch K-P, Hanisch U-K, Raasch J, Merkler D, Detje C, Gutcher I, Mages J, Lang R, Martin R, Gold R, Becher B, Bruck W, Kalinke U. Distinct and nonredundant in vivo functions of IFNAR on myeloid cells limit autoimmunity in the central nervous system. Immunity. 2008;28:675–686. doi: 10.1016/j.immuni.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 45.Prinz M, Knobeloch K-P. Type I interferons as ambiguous modulators of chronic inflammation in the central nervous system. Frontiers Immunol. 2012;3:1–9. doi: 10.3389/fimmu.2012.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kleinnijenhuis J, Oosting M, Joosten LAB, Netea MG, van Crevel R. innate immune recognition of Mycobacterium tuberculosis. Clin. Devel. Immunol. 2011 doi: 10.1155/2011/405310. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marta M. Toll-like receptors in multiple sclerosis mouse experimental models. Ann. N.Y. Acad. Sci. 2009;1173:458–462. doi: 10.1111/j.1749-6632.2009.04849.x. [DOI] [PubMed] [Google Scholar]
- 48.Prinz M, Priller J, Sisodia SS, Ransohoff RM. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat. Neurosci. 2011;14:1227–1235. doi: 10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- 49.Goldmann T, Wieghofer P, Muller PF, Wolf Y, Varol D, Yona S, Brendecke SM, Kierdorf K, Staszewski O, Datta M, Luedde T, Heikenwalder M, Jung S, Prinz M. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat. Neurosci. 2013;16 doi: 10.1038/nn.3531. doi:10.1038/nn.3531. [DOI] [PubMed] [Google Scholar]
- 50.Beinke S, Robinson MJ, Salmeron A, Hugunin M, Allen H, Ley SC. Lipopolysaccharide activation of the TPL-2/MEK/Extracellular signal-regulated kinase mitogen-activated protein kinase cascade is regulated by IκB kinase-induced proteolysis of NF-κB1 p105. Mol. Cell. Biol. 2004;24:9658–9667. doi: 10.1128/MCB.24.21.9658-9667.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waterfield M, Jin W, Reiley W, Zhang MY, Sun S-C. IKKβ is an essential component of the TPL-2 signaling pathway. Mol. Cell Biol. 2004;24:6040–6048. doi: 10.1128/MCB.24.13.6040-6048.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roget K, Ben-Addi A, Mambole-Dema A, Gantke T, Janzen J, Yang H-T, Shpiro N, Morrice N, Abbott D, Ley SC. IKK2 regulates TPL-2 activation of ERK-1/2 MAP kinases by direct phosphorylation of TPL-2 serine 400. Mol. Cell. Biol. 2012;32:4684–4690. doi: 10.1128/MCB.01065-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Consortium, I. M. S. G. W. T. C. C. C. 2 Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214–219. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.