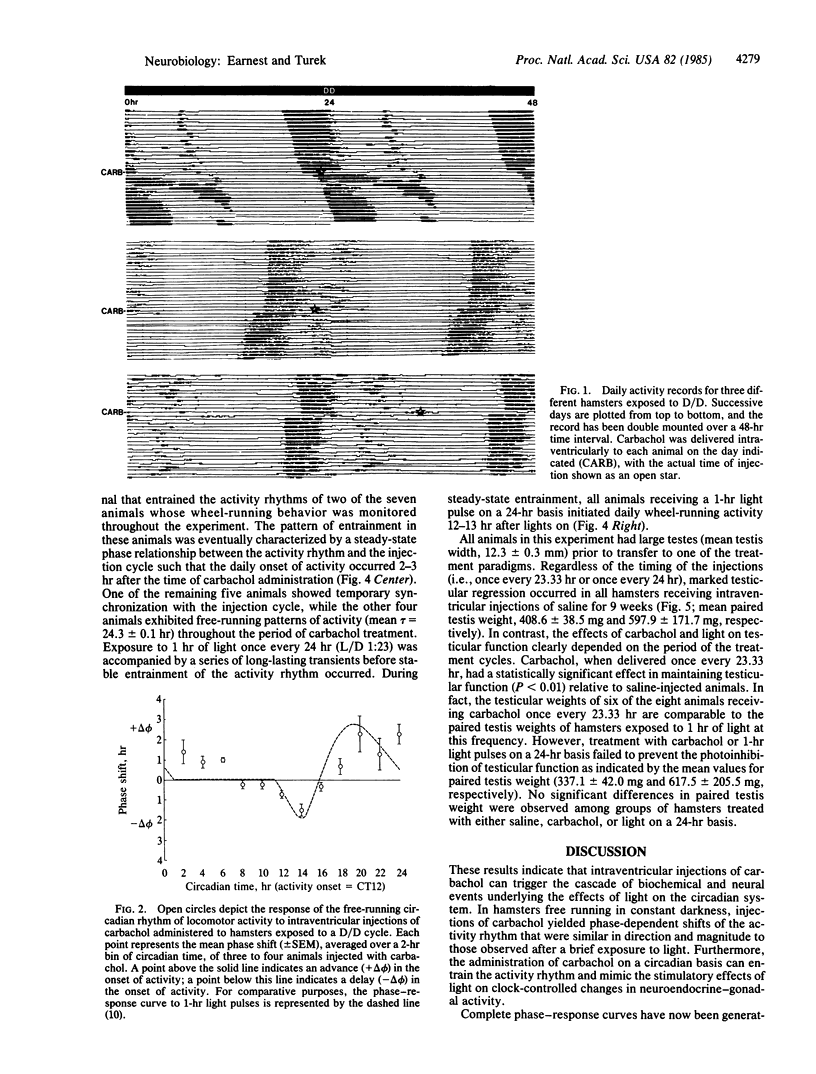
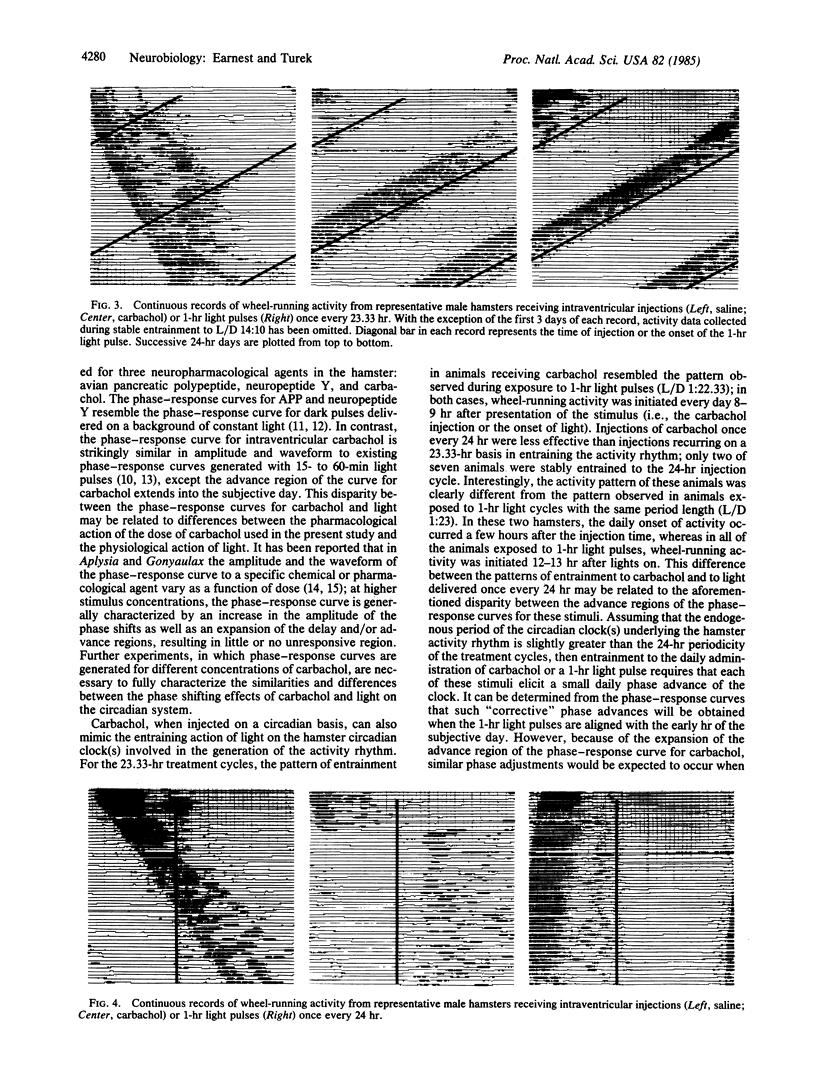
Abstract
A pharmacological approach was used to examine the role of acetylcholine in the photic control of circadian rhythms and seasonal reproductive cycles. The experimental protocol was designed to determine whether the administration of carbachol, a cholinergic agonist, could mimic the effects of brief light pulses on gonadal function and/or the circadian rhythm of wheel-running activity in golden hamsters. Intraventricular injections of carbachol, administered singularly at discrete phase points throughout the circadian cycle, induced phase-dependent shifts in the free-running rhythm of activity similar to those caused by a brief light exposure. Injections of carbachol once every 23.33 hr for 9 weeks entrained the activity rhythm and stimulated the neuroendocrine-gonadal axis in a manner similar to that observed after the presentation of 1-hr light pulses at this frequency. In contrast, the administration of carbachol once every 24 hr did not consistently provide an entraining signal for the activity rhythm and did not stimulate reproductive function. Importantly, the effects of carbachol on the seasonal reproductive response were correlated with the timing of the injections relative to the activity rhythm. These findings suggest that acetylcholine may play an important role in the mechanism by which light regulates circadian rhythms and seasonal reproductive cycles.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers H. E., Ferris C. F., Leeman S. E., Goldman B. D. Avian pancreatic polypeptide phase shifts hamster circadian rhythms when microinjected into the suprachiasmatic region. Science. 1984 Feb 24;223(4638):833–835. doi: 10.1126/science.6546454. [DOI] [PubMed] [Google Scholar]
- Albers H. E., Ferris C. F. Neuropeptide Y: role in light-dark cycle entrainment of hamster circadian rhythms. Neurosci Lett. 1984 Sep 7;50(1-3):163–168. doi: 10.1016/0304-3940(84)90480-4. [DOI] [PubMed] [Google Scholar]
- Brownstein M., Kobayashi R., Palkovits M., Saavedra J. M. Choline acetyltransferase levels in diencephalic nuclei of the rat. J Neurochem. 1975 Jan;24(1):35–38. doi: 10.1111/j.1471-4159.1975.tb07624.x. [DOI] [PubMed] [Google Scholar]
- Corrent G., McAdoo D. J., Eskin A. Serotonin shifts the phase of the circadian rhythm from the Aplysia eye. Science. 1978 Dec 1;202(4371):977–979. doi: 10.1126/science.309655. [DOI] [PubMed] [Google Scholar]
- Earnest D. J., Turek F. W. Effect of one-second light pulses on testicular function and locomotor activity in the golden hamster. Biol Reprod. 1983 Apr;28(3):557–565. doi: 10.1095/biolreprod28.3.557. [DOI] [PubMed] [Google Scholar]
- Earnest D. J., Turek F. W. Role for acetylcholine in mediating effects of light on reproduction. Science. 1983 Jan 7;219(4580):77–79. doi: 10.1126/science.6849121. [DOI] [PubMed] [Google Scholar]
- Elliott J. A. Circadian rhythms and photoperiodic time measurement in mammals. Fed Proc. 1976 Oct;35(12):2339–2346. [PubMed] [Google Scholar]
- Ellis G. B., McKlveen R. E., Turek F. W. Dark pulses affect the circadian rhythm of activity in hamsters kept in constant light. Am J Physiol. 1982 Jan;242(1):R44–R50. doi: 10.1152/ajpregu.1982.242.1.R44. [DOI] [PubMed] [Google Scholar]
- Goldman B. D., Darrow J. M. The pineal gland and mammalian photoperiodism. Neuroendocrinology. 1983 Nov;37(5):386–396. doi: 10.1159/000123579. [DOI] [PubMed] [Google Scholar]
- Moore R. Y. Organization and function of a central nervous system circadian oscillator: the suprachiasmatic hypothalamic nucleus. Fed Proc. 1983 Aug;42(11):2783–2789. [PubMed] [Google Scholar]
- Segal M., Dudai Y., Amsterdam A. Distribution of an alpha-bungarotoxin-binding cholinergic nicotinic receptor in rat brain. Brain Res. 1978 Jun 9;148(1):105–119. doi: 10.1016/0006-8993(78)90381-5. [DOI] [PubMed] [Google Scholar]
- Takahashi J. S., DeCoursey P. J., Bauman L., Menaker M. Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature. 1984 Mar 8;308(5955):186–188. doi: 10.1038/308186a0. [DOI] [PubMed] [Google Scholar]
- Zatz M., Brownstein M. J. Intraventricular carbachol mimics the effects of light on the circadian rhythm in the rat pineal gland. Science. 1979 Jan 26;203(4378):358–361. doi: 10.1126/science.32619. [DOI] [PubMed] [Google Scholar]
- Zatz M., Herkenham M. A. Intraventricular carbachol mimics the phase-shifting effect of light on the circadian rhythm of wheel-running activity. Brain Res. 1981 May 11;212(1):234–238. doi: 10.1016/0006-8993(81)90059-7. [DOI] [PubMed] [Google Scholar]


