Abstract
Background
In a phase III, open-label, comparative, noninferiority study, 638 subjects receiving de novo kidney transplants were randomized to one of three treatment arms: tacrolimus extended-release (Astagraf XL) qd, tacrolimus (Prograf) bid, or cyclosporine (CsA) bid. All subjects received basiliximab induction, mycophenolate mofetil, and corticosteroids. Safety and efficacy follow-up data through 4 years are reported.
Methods
Evaluations included patient and graft survival, study drug discontinuations, laboratory values including renal function and development of new-onset diabetes after transplantation, concomitant medications, and adverse events.
Results
At study termination, 129 Astagraf XL, 113 Prograf, and 79 CsA patients had continued follow-up. Demographic and baseline characteristics were similar in all arms. Four-year Kaplan-Meier estimates of patient survival in the Astagraf XL, Prograf, and CsA groups were 93.2, 91.2, and 91.7%, respectively, while graft survival was 84.7, 82.7, and 83.9%, respectively. At least one serious adverse event was reported in the majority of patients in each group during the study (65.9% Astagraf XL, 69.8% Prograf, and 65.6% CsA). Renal function was not significantly different between Astagraf XL and Prograf. HgbA1c levels were collected every 6 months; the 4-year Kaplan-Meier estimate for incidence of HgbA1c levels ≥6.5% was significantly higher for both tacrolimus formulations compared to CsA; 41.1% (Astagraf XL), 33.6% (Prograf), and 21.3% (CsA).
Conclusions
In this 4-year follow-up report, patients receiving Astagraf XL and Prograf showed comparable efficacy and safety profiles, with a higher incidence of new-onset diabetes after transplantation but superior renal function compared to patients receiving CsA.
Keywords: De novo kidney transplantation, Tacrolimus, Long-term follow-up
Tacrolimus immediate-release (Prograf; Astellas Pharma US, Inc., Northbrook, IL), a twice-daily immunosuppressive agent to prevent graft rejection, has well-defined safety and efficacy profiles (1). However, dosing nonadherence can contribute to graft rejection and late graft loss (2, 3). To improve treatment adherence, a tacrolimus extended-release formulation (Astagraf XL in the US, formerly called MR or MR4, and approved as Advagraf, Graceptor, and Prograf-XL in other countries) was developed for once-daily dosing (1). Comparable steady-state systemic tacrolimus exposure with the two formulations in stable and de novo kidney and liver transplant patients has been demonstrated, allowing monitoring by trough levels as a surrogate for area under the drug concentration-time curve (4, 5). A large, randomized, open-label study was designed to evaluate the safety and efficacy of Astagraf XL compared to Prograf and cyclosporine modified (Neoral; Pharmaceuticals Corp., East Hanover, NJ; CsA) when administered in combination with mycophenolate mofetil (MMF), corticosteroids, and basiliximab induction in de novo kidney transplant recipients (1). One-year results were published in 2007 (1). Two-year data showed no unexpected safety or efficacy signals with Astagraf XL in renal transplant recipients (6). Since then, extended-release tacrolimus has been approved in 74 countries for prevention of rejection after kidney transplantation. Herein, we report 4-year follow-up results of the original study.
RESULTS
This was a 1-year efficacy and safety study (beginning in June 2003) followed by a clinical continuation phase.
Patient Disposition
In the Astagraf XL, Prograf, and CsA groups, 226, 219, and 223 patients, respectively, were randomized; of those patients, 214, 212, and 212, respectively, received at least one dose of study drug. Most patients entered the clinical continuation phase (182 Astagraf XL, 179 Prograf, and 151 CsA). When terminated by the sponsor in March 2009, 129, 113, and 79 patients in the Astagraf XL, Prograf, and CsA groups, respectively, remained on study (Fig. 1). Median follow-up of those remaining on study was 4.7 years (range: 4.0–5.3 years).
FIGURE 1.
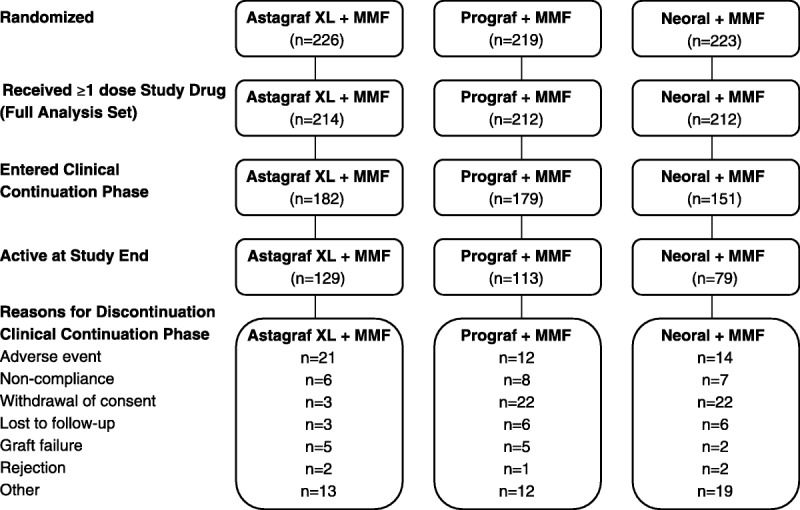
Patient Disposition. All randomized patients: screened and eligible for inclusion in the study. Full Analysis Set: All randomized patients who received at least 1 dose of study medication.
A higher proportion of CsA (22.0%) patients changed primary immunosuppressive treatment than Astagraf XL (11.5%) and Prograf (3.2%) patients. One of 26 patients discontinued Astagraf XL because of severe rejection; the majority discontinued because of adverse events (AEs). Crossover to another study treatment regimen to address AEs or severe refractory rejection resulting in study drug discontinuation was permitted; however, crossover to the Astagraf XL arm was not permitted.
Demographics
Patients eligible for inclusion in the Full Analysis Set had similar demographics and other baseline characteristics as in the 1-year report (1). The study population was primarily male (>60%), white (>70%), and under 65 years of age (>88%). The mean age (SD) was 48 (13) years in the Astagraf XL and CsA groups and 49 (13) years in the Prograf group. In the Astagraf XL, Prograf, and CsA groups, organs from living donors were used for 48.1, 50.0, and 52.4% of patients, respectively.
Immunosuppressive Drugs
Mean trough concentrations of Astagraf XL and Prograf were similar up to 5 years’ follow-up, with mean concentrations starting at 1 year ranging from 6.5 to 7.5 ng/mL in Astagraf XL and 6.1 to 7.8 ng/mL in Prograf. Mean CsA values ranged from 115.0 to 177.4 ng/mL.
MMF mean total daily dose during the extension was 1,572, 1,551, and 1,774 mg in the Astagraf XL, Prograf, and CsA groups, respectively. Mean corticosteroid dosing was within the same range in all treatment groups (5 to 10 mg prednisone per day) with the exception of the 48-month visit in the CsA group where the mean dose increased to 11 mg per day.
Efficacy Results
All regimens provided similar efficacy over the clinical continuation phase and had high patient and graft survival rates by 4-year Kaplan-Meier estimates. In the Astagraf XL, Prograf, and CsA groups, patient survival was 93.8% (95% CI: 90.5%, 97.2%), 93.2% (95% CI: 89.8%, 96.7%), and 92.5% (95% CI: 88.6%, 96.3%), respectively (Fig. 2A), while graft survival was 88.1% (95% CI: 83.7%, 92.6%), 85.4% (95% CI: 80.5%, 90.4%), and 85.3% (95% CI: 80.3%, 90.4%), respectively (Fig. 2B) at 4 years’ follow-up.
FIGURE 2.
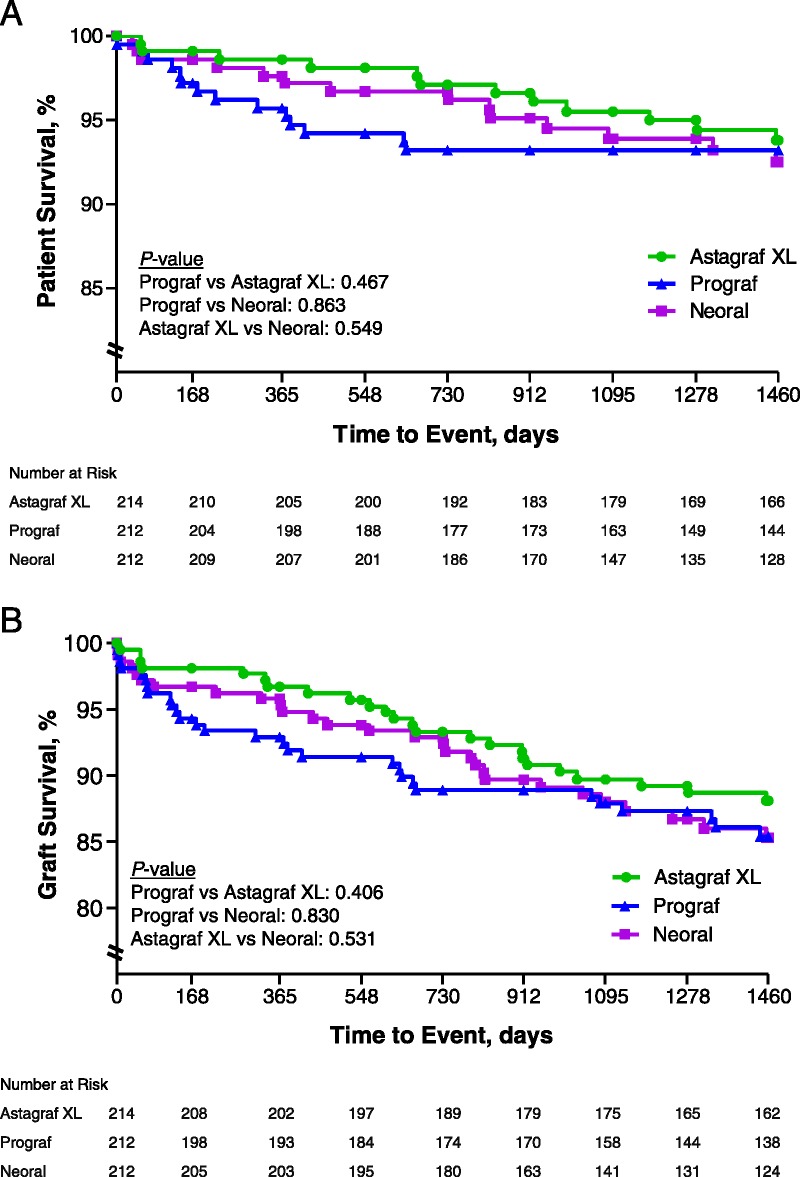
Patient (A) and graft (B) survival over 4 years in the three treatment arms. Percent of patient (A) and graft (B) survival over 4 years in three treatment groups, measured in days. Number at risk indicates the number of active patients at each time interval.
Subgroup analyses of death and graft loss showed no obvious sex or age effect.
Graft loss had a numerically higher incidence in black patients than in white patients across treatment groups. Graft loss for Astagraf XL was 11.9% (19/160) in white and 19.5% (8/41) in black patients, for Prograf was 10.5% (16/152) in white and 31.4% (16/51) in black patients, and for CsA was 12.3% (20/163) in white and 22.2% (8/36) in black patients. For patients ≥65 years of age, graft loss rates were 16.7% (4/24), 13.0% (3/23), and 30.0% (6/20) for Astagraf XL, Prograf, and CsA groups, respectively, compared to 12.6% (24/190), 15.3% (29/189), and 12.5% (24/192) for patients aged <65 years. For females, graft loss rates were 9.2% (7/76), 10.5% (8/76), and 15.9% (13/82) for Astagraf XL, Prograf, and CsA groups, respectively, compared to 15.2% (21/138), 17.6% (24/136), and 13.1% (17/130) in male patients.
As measured by the Cockcroft-Gault formula for glomerular filtration rate (GFR), the adjusted mean difference in renal function of Astagraf XL-Prograf was 0.8 (95% CI: −1.0, 2.5) mL/min, Astagraf XL-CsA was 2.3 (95% CI: 0.5, 4.1) mL/min, and CsA-Prograf was −1.5 (95% CI: −3.3, 0.2) mL/min. The difference was not statistically different when comparing Astagraf XL and Prograf (P=0.3930) nor Prograf and CsA (P=0.0889) but was statistically different when comparing Astagraf XL and CsA (P=0.0118), indicating higher mean renal function in the Astagraf XL group (Fig. 3). When measured using modification of diet in renal disease (MDRD), the adjusted mean differences were 0.6 (95% CI: −1.3, 2.6) mL/min for Astagraf XL compared to Prograf, 2.7 (95% CI: 0.7, 4.7) mL/min for Astagraf XL compared to CsA, and −2.1 (95% CI: −4.1, -0.1) mL/min for CsA compared to Prograf. Statistical conclusions were similar comparing Astagraf XL to other regimens (Astagraf XL vs. CsA, P=0.0083; Astagraf XL vs. Prograf, P=0.5400) but comparing Prograf and CsA yielded a statistically significant difference (P=0.0398) in overall function of the kidneys over 48 months. There was no treatment-visit interaction in either analysis (P≥0.8779).
FIGURE 3.
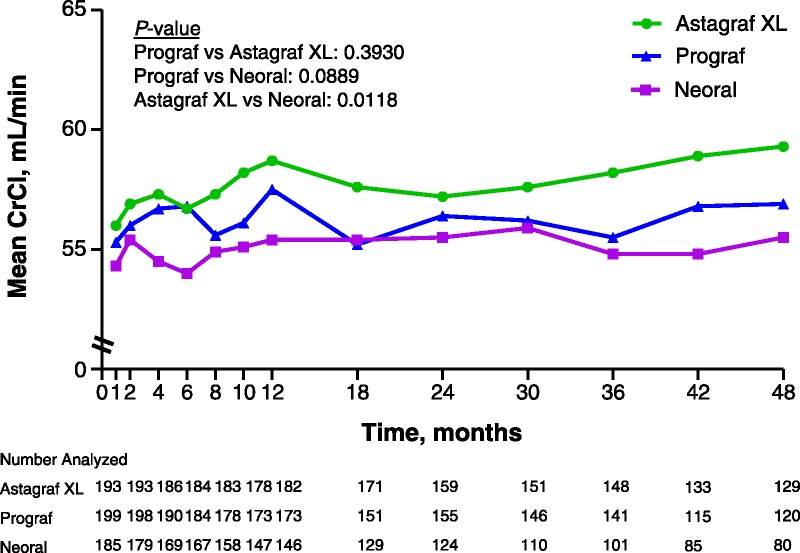
Renal function measured by Cockcroft-Gault equation over 4 years in the three treatment arms. Renal function measured by CrCl in milliliters per minute over 4 years in the three treatment arms. Number analyzed indicates the number of active patients who had a laboratory assessment.
Safety
Forty-five deaths including one patient randomized to the CsA group who never received study drug occurred. Twenty-three deaths while on randomized therapy or within 10 days of discontinuing and 21 deaths more than 10 days after last dose of randomized study drug occurred. Eighteen deaths (six Astagraf XL, eight Prograf, and four CsA) were related to infectious processes and 14 deaths were cardiac-related (five Astagraf XL, three Prograf, and six CsA). Four deaths occurred after 48 months (one Astagraf XL, two Prograf, and one CsA).
Fifty graft losses occurred during the study, including 16 (five Astagraf XL, four Prograf, and seven CsA) resulting from chronic allograft nephropathy and eight (four Astagraf XL and four Prograf) resulting from acute rejection. Other causes included vascular thrombosis (one Astagraf XL, two Prograf, and two CsA) and polyomavirus nephropathy (one Astagraf XL, one Prograf, and one CsA).
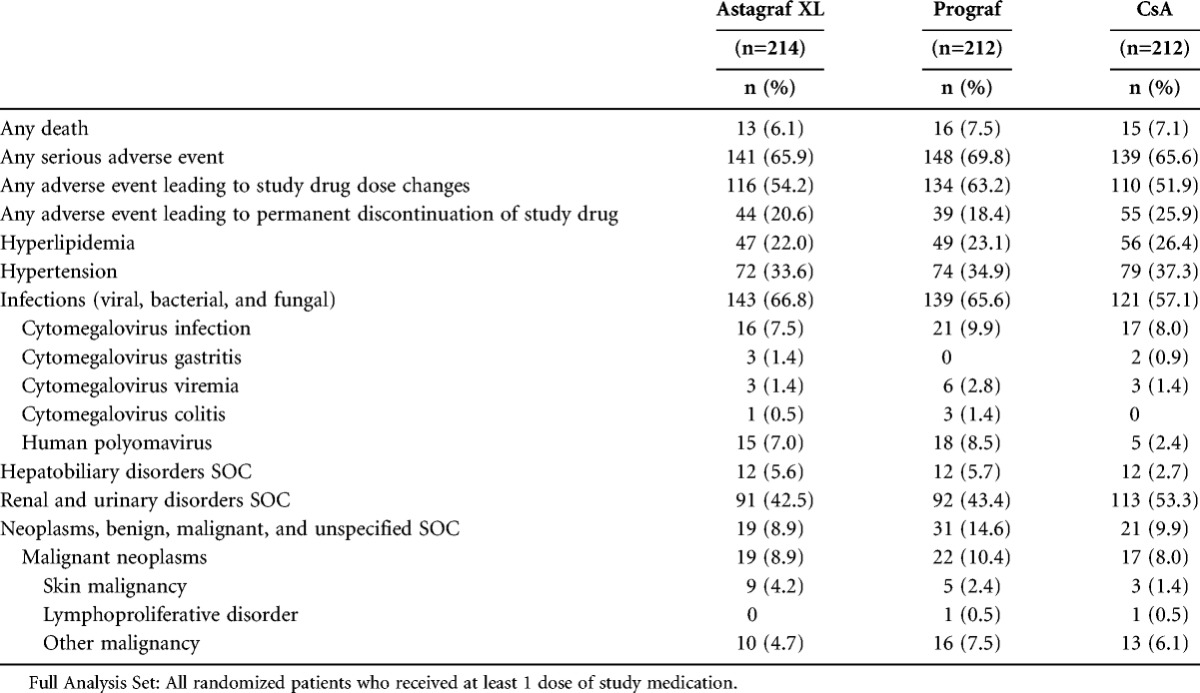
At least one serious adverse event (SAE) was reported in most patients in each group (65.9% of Astagraf XL, 69.8% of Prograf, and 65.6% of CsA patients). The three most commonly reported SAEs in all groups were increased serum creatinine, cytomegalovirus infection, and urinary tract infection (reported in approximately 5–8% of patients across groups). Polyomavirus infection occurred in 7.0, 8.5, and 2.4% of patients in the Astagraf XL, Prograf, and CsA groups, respectively. Events in all except 39, 38, and 40 patients in the Astagraf XL, Prograf, and CsA groups, respectively, were reported as recovered (with or without residual effects).
An overview of the number and percentage of patients with treatment-emergent AEs is provided (Table 1). The incidence of infection of any kind was higher in Astagraf XL (66.8%) and Prograf (65.6%) groups versus the CsA (57.1%) group. AEs coded to the system organ class Neoplasms Benign, Malignant, and Unspecified were reported in 14.6% of patients in Prograf compared to 8.9% of patients in Astagraf XL and 9.9% of patients in CsA. White blood cell counts <2.0×109/L were observed in 5.3, 6.2, and 4.8% of patients in the Astagraf XL, Prograf, and CsA groups, respectively. There was no observed increase in the incidence of infection, renal disorders, hepatic dysfunction, neoplasms, hyperlipidemia, or hypertension over time in any group.
TABLE 1.
Overview of treatment-emergent adverse events
Overall, the incidence of discontinuation resulting from AE was 55/212 (25.9%) in the CsA, 44/214 (20.6%) in the Astagraf XL, and 39/212 (18.4%) in the Prograf group. No AEs leading to Astagraf XL or Prograf discontinuation were reported in more than three patients per group. Human polyomavirus resulted in study drug discontinuation in three patients in the Astagraf XL and Prograf groups, and no patients in the CsA group. In comparison, the most common reason for study drug discontinuation in the CsA group was gingival hyperplasia (reported in seven patients) and was not reported in the Astagraf XL or Prograf groups.
At least one change in study drug dose was made because of AE(s) in 54.2% of the Astagraf XL, 63.2% of the Prograf, and 51.9% of the CsA group (P=0.0444; Fisher exact test [two-tailed]). Higher rates of patients experienced tremor leading to change in study drug in the Astagraf XL (12.6%) and Prograf (10.8%) groups than in the CsA group (4.2%). Other differences were shown in rates leading to changes in study drug dose (neuropathy [1.9% Astagraf XL, 0 Prograf, and 0 CsA], human polyomavirus infection [4.7% Astagraf XL, 3.8% Prograf, and 0 CsA], cytomegalovirus infection [0 Astagraf XL, 2.8% Prograf, and 1.4% CsA], diarrhea [2.3% Astagraf XL, 5.2% Prograf, and 0 CsA], gingival hyperplasia [0 Astagraf XL, 0 Prograf, and 3.8% CsA], and hirsutism [0 Astagraf XL, 0 Prograf, and 2.4% CsA]).
Evidence of treatment-emergent glucose intolerance in the continuation phase was more common in the Astagraf XL and Prograf groups versus the CsA group. Patients who received either tacrolimus treatment had a higher incidence of glucose intolerance (fasting plasma glucose ≥126 mg/dL, HgbA1c ≥6%, insulin use, oral hypoglycemic agent use, or other antidiabetic use) than those in the CsA treatment group from month 18 through month 60; no meaningful differences were observed for any subgroup. Based on current American Diabetic Association recommendations, HgbA1c ≥6.5% was subsequently analyzed (7). Rates of HgbA1c ≥6.5% over time were statistically significantly lower in the CsA group compared to the tacrolimus-based groups but not between Astagraf XL and Prograf (log rank test: Prograf vs. CsA P=0.01; Astagraf XL vs. CsA P=0.0006; Prograf vs. Astagraf XL P=0.38) (Fig. 4). The 4-year Kaplan-Meier rate estimate of HgbA1c levels ≥6.5% was 41.1% (95% CI: 32.8%, 49.4%) in Astagraf XL, 33.6% (95% CI: 25.3%, 41.9%) in Prograf, and 21.3% (95% CI: 13.3%, 29.2%) in CsA.
FIGURE 4.
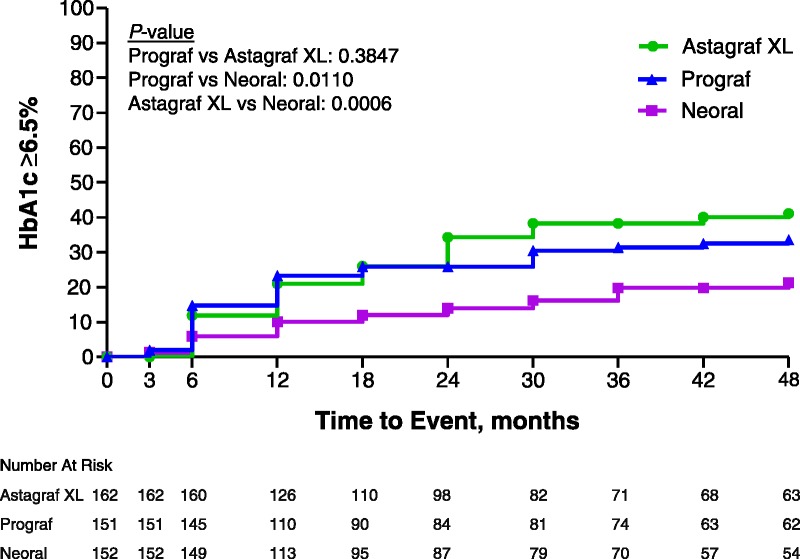
Cumulative incidence of HgbA1c levels ≥6.5% over 4 years in the three treatment arms. Percent of patients with HgbA1c level ≥6.5% over 4 years in three treatment arms, measured in months. Number at risk indicates the number of active patients at each time interval.
DISCUSSION
Data from the clinical continuation corroborate and extend 1-year findings. Evidence from this trial showed similar efficacy between regimens over 48 months’ follow-up. Patient and graft survival rates were high (>90 and >80%, respectively) with no statistically significant pairwise comparison differences between groups; however, this study included a relatively low-risk population. Adherence was not evaluated; dropout resulting from noncompliance was similar between treatment arms. The higher rate of graft loss in black subjects at 4 years in all three treatment arms is compatible with 5-year data from the Scientific Registry of Transplant Recipients (8).
Long-term efficacy observed is similar to recent studies. In the European multicenter study in kidney transplant recipients, 1-year patient and graft survival in the Advagraf arm were 96.9 and 91.5%, respectively (4), and 98.6 and 96.7% in the current study. In two large kidney transplant trials, 3-year results have been reported. In the SYMPHONY study, patient and graft survival in the low-dose tacrolimus arm were 95 and 90%, respectively (9). In the belatacept trial, patient survival with functioning graft in two calcineurin-free belatacept arms and the CsA arm were 92, 92, and 89%, while graft survival rates were approximately 87, 88, and 84%, respectively (10). In the current study, 3-year patient survival rates in the Astagraf XL, Prograf, and CsA arms was 95.5, 93.2, and 93.9%, and graft survival rates were 89.7, 87.9, and 88.0%, respectively.
Renal function was significantly better over 4 years for Astagraf XL than CsA when calculated by both the Cockroft-Gault and MDRD formulas; Prograf was significantly better than CsA only using the MDRD formula. The lack of interaction between time and treatment (test for interaction: P≥0.8779) indicated that there was no evidence of differing rates of improvement in renal function over time between the treatment groups (i.e., the slope over time was similar between treatment groups); therefore, average differences in renal function were consistent after the kidney stabilized and were maintained through 4 years post-transplant. Having adjusted for month 1 renal function and time, this analysis supports the conclusion that Astagraf XL showed a benefit in renal function which was maintained through the horizon of collected data. However, the sponsor supplied Astagraf XL for the duration of the study and Prograf and CsA for only the first year. Other studies have shown better renal function with tacrolimus than CsA (11, 12).
The incidence of new onset diabetes after transplant (NODAT) was significantly higher in tacrolimus arms, and the 1-year difference persisted throughout follow-up. Between the tacrolimus arms, no meaningful difference in the incidence of diabetes markers existed. The higher incidence of NODAT with tacrolimus than CsA has been widely documented in literature (11, 13–15). The long-term relevance of NODAT with Astagraf XL and extrapolations to other populations should be interpreted with caution and evaluated further. Comparative rates of NODAT with tacrolimus formulations have been reported in two other large clinical trials. In patients without a history of or baseline diabetes mellitus, the OSAKA trial reported NODAT in 16.1% of 274 patients treated with Prograf and 13.2% of 265 patients receiving the same dose of Astagraf XL (16). In the European study (4), the rate was 20.5% in both treatment arms, similar to the 1-year rates of 21.0 and 23.3% in Astagraf XL and Prograf, respectively, in this study. In addition, a systematic review of once-daily versus twice-daily tacrolimus revealed no statistically significant difference in NODAT incidence between formulations (17).
Data for cytomegalovirus (CMV) and human polyomavirus infections were only collected by AE reporting. Incidence of CMV infections was similar between treatment arms. The absence of systematic reporting for CMV disease likely contributed to the low rate of CMV infections.
Human polyomavirus infections and changes in study drug dose were numerically more frequently reported in patients on tacrolimus than CsA. The same caveat as for the CMV infections applies because systematic reporting was not done. If a difference in the incidence of human polyomavirus infections existed, it could be related to concomitant dosing with MMF and the pharmacokinetic interaction between CsA and mycophenolate acid, which was not as widely known, and to MMF given at the same dose to patients on tacrolimus and CsA (18).
Statistical comparisons of treatment groups within the continuation phase should be interpreted with caution. As shown in the patient disposition (Fig. 1), fewer patients entered the “clinical continuation” from CsA (151) compared to Astagraf XL (182). Additionally, the Prograf and CsA arms included 22 patients classified as “withdrawal of consent” during the continuation phase whereas three patients in the Astagraf XL group had this classification for reasons for discontinuation (Fig. 1). There was a difference by treatment arm in the amount of missing data and therefore censoring in the continuation phase of the study, which may be informative rather than random. It is also likely that these findings relate to the commercial availability of Prograf and CsA and not Astagraf XL and because the sponsor supplied Astagraf XL for the duration of the study and only for the first year in subjects randomized to Prograf and CsA. Given this difference in missing data, censoring by treatment arm, and the potential for informative censoring, comparisons between groups may be biased or confounded.
The long-term safety profile of each treatment regimen in this study was consistent with extended use of immunosuppressive regimens in transplant recipients. These findings provide long-term follow-up and confirmation of 1-year findings in de novo renal transplant recipients for administration of Astagraf XL in combination with MMF and steroids.
MATERIALS AND METHODS
Silva et al. (1) describes detailed methodology for the first year of this study; a summary is provided below. This was a randomized, open-label, comparative study in de novo kidney transplant recipients ≥12 years of age. Eligible patients were randomized in a 1:1:1 ratio to one of three parallel treatment groups:
Astagraf XL
Prograf
CsA
Dose adjustments of primary immunosuppressants were allowed on the basis of clinical evidence of efficacy, occurrence of AEs, and whole blood trough concentrations. All patients were to receive basiliximab induction therapy (20 mg iv on day 0 and a second dose between day 3 and 5) and corticosteroid treatment.
All patients were to receive MMF and corticosteroids concomitantly with study drug. The MMF dose was 1 g twice daily (bid) (1.5 g bid was permitted in black patients) throughout the study. Dose-equivalent bid, three times daily, or four times daily dosing was permitted if tolerability was a concern.
Patients could cross over to another regimen within the study to address AEs or severe refractory rejection that led to discontinuation of study drug; however, crossover to the Astagraf XL arm was not permitted. Patients who crossed over to another regimen or discontinued primary study drug (but did not withdraw consent) were to be followed throughout.
Patients were evaluated frequently during the first year and were eligible to continue the clinical continuation upon completion. The first visit of the clinical continuation phase occurred at month 18 with subsequent visits every 6 months for safety and efficacy follow-up. However, biopsy-proven acute rejection data were only collected as AE reporting past year 1.
All patients who received at least one dose of study drug were analyzed. Treatment-emergent AEs (reported as starting from first dose of study drug through the end of study) were summarized. Patient and graft survival were presented through 4 years posttransplant using Kaplan-Meier analysis with censoring on the day of last follow-up. Kaplan-Meier analysis was also used to analyze events of HgbA1c ≥6.5% through 4 years with last follow-up visit used as censoring time for those without an observed event. Renal function over 4 years was assessed using a repeated measures mixed-model analysis of covariance with treatment and scheduled visit as factors while adjusting for month 1 renal function. In this analysis, the month 1 renal function was used as a surrogate for baseline covariate to allow this parameter to stabilize following kidney transplantation; therefore, claims for posttransplant differences between treatment arms have accounted for any differences present at month 1. The effect of time was also adjusted for by the model.
ACKNOWLEDGMENTS
This study was supported by Astellas Pharma US, Inc., Northbrook, IL. Statistical support was provided by Astellas Scientific and Medical Affairs, Inc. (Billy Franks). The manuscript was drafted by the medical writing department of Astellas Pharma Global Development (Kirsten Helmcke), which also provided editorial assistance to the authors. The authors made a substantial contribution to the study’s conception and design, acquisition of data, and analysis and interpretation of data; participated in the drafting and review of the manuscript; and approved the final version.
Footnotes
The authors declare no funding for this study. H.-U.M.-K., R.C, J.H, W.E.F., and M.R.F. are full-time employees at Astellas Pharma, sponsor of this study.
The other authors declare no conflicts of interest.
H.T.S. Jr, H.C.Y., H.-U.M.-K., R.C., J.H., W.E.F., and M.R.F. participated in designing the research, analyzing the data, and reviewing the article. R.C. was responsible for statistical analyses. M.R.F. participated in the writing of the article.
Received 14 August 2013. Revision requested 29 August 2013.
Accepted 27 September 2013.
REFERENCES
- 1. Silva HT, Yang HC, Abouljoud M, et al. One-year results with extended-release tacrolimus/MMF, tacrolimus/MMF and cyclosporine/MMF in de novo kidney transplant recipients. Am J Transplant 2007; 7: 595. [DOI] [PubMed] [Google Scholar]
- 2. De Geest S, Borgermans L, Gemoets H, et al. Incidence, determinants, and consequences of subclinical noncompliance with immunosuppressive therapy in renal transplant recipients. Transplantation 1995; 59: 340. [PubMed] [Google Scholar]
- 3. Weng FL, Israni AK, Joffe MM, et al. Race and electronically measured adherence to immunosuppressive medication after deceased donor renal transplantation. J Am Soc Nephrol 2005; 16: 1839. [DOI] [PubMed] [Google Scholar]
- 4. Krämer BK, Charpentier B, Bäckman L, et al. Tacrolimus once daily (ADVAGRAF) versus twice daily (PROGRAF) in de novo renal transplantation: a randomized phase III study. Am J Transplant 2010; 10: 2632. [DOI] [PubMed] [Google Scholar]
- 5. Trunecka P, Boillot O, Seehofer D, et al. Once-daily prolonged-release tacrolimus (ADVAGRAF) versus twice-daily tracrolimus (PROGRAF) in liver transplantation. Am J Transplant 2010; 10: 2313. [DOI] [PubMed] [Google Scholar]
- 6. Yang H. A phase III, randomized, open-label, comparative, multicenter study to assess the safety and efficacy of Prograf® (tacrolimus)/MMF, extended release (XL) tacrolimus/MMF and Neoral® (cyclosporine)/MMF in de novo kidney transplant recipients: 2 year results (Abstr #144). Am J Transplant 2007; 7 (suppl 2): 183 [Google Scholar]
- 7. Nathan DM, Balkau B, Bonora E, et al. Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009; 32: 1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) OPTN/SRTR 2010 Annual Data Report. Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; 2011 [Google Scholar]
- 9. Ekberg H, Bernasconi C, Tedesco-Silva H, et al. Calcineurin inhibitor minimization in the Symphony study: observational results 3 years after transplantation. Am J Transplant 2009; 9: 1876. [DOI] [PubMed] [Google Scholar]
- 10. Vincenti F, Larsen CP, Alberu J, et al. Three-year outcomes from BENEFIT, a randomized, active-controlled, parallel-group study in adult kidney transplant recipients. Am J Transplant 2012; 12: 210. [DOI] [PubMed] [Google Scholar]
- 11. Ekberg H, Tedesco-Silva H, Demirbas A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 2007; 357: 2562. [DOI] [PubMed] [Google Scholar]
- 12. Kaplan B, Schold JD, Meier-Kriesche HU. Long-term graft survival with neoral and tacrolimus: a paired kidney analysis. J Am Soc Nephrol 2003; 14: 2980. [DOI] [PubMed] [Google Scholar]
- 13. Kasiske BL, Snyder JJ, Gilbertson D, et al. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant 2003; 3: 178. [DOI] [PubMed] [Google Scholar]
- 14. Vincenti F, Friman S, Scheuermann E, et al. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant 2007; 7: 1506. [DOI] [PubMed] [Google Scholar]
- 15. First MR, Dhadda S, Croy R, et al. New onset diabetes after transplantation (NODAT): an evaluation of definitions in clinical trials. Transplantation 2013; 96: 58. [DOI] [PubMed] [Google Scholar]
- 16. Albano L, Banas B, Klempnauer JL, et al. OSAKA trial: a randomized, controlled trial comparing tacrolimus QD and BD in kidney transplantation. Transplantation 2013; doi: 10.1097/TP.0b013e3182a203bd) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ho ETL, Wong G, Craig JC, et al. Once-daily extended-release versus twice-daily standard-release tacrolimus in kidney transplant recipients. Transplantation 2013; 95: 1120. [DOI] [PubMed] [Google Scholar]
- 18. van Gelder T, Klupp J, Barten MT, et al. Comparison of the effects of tacrolimus and cyclosporine on the pharmacokinetics of mycophenolic acid. Ther Drug Monit 2001; 23: 119. [DOI] [PubMed] [Google Scholar]


