Abstract
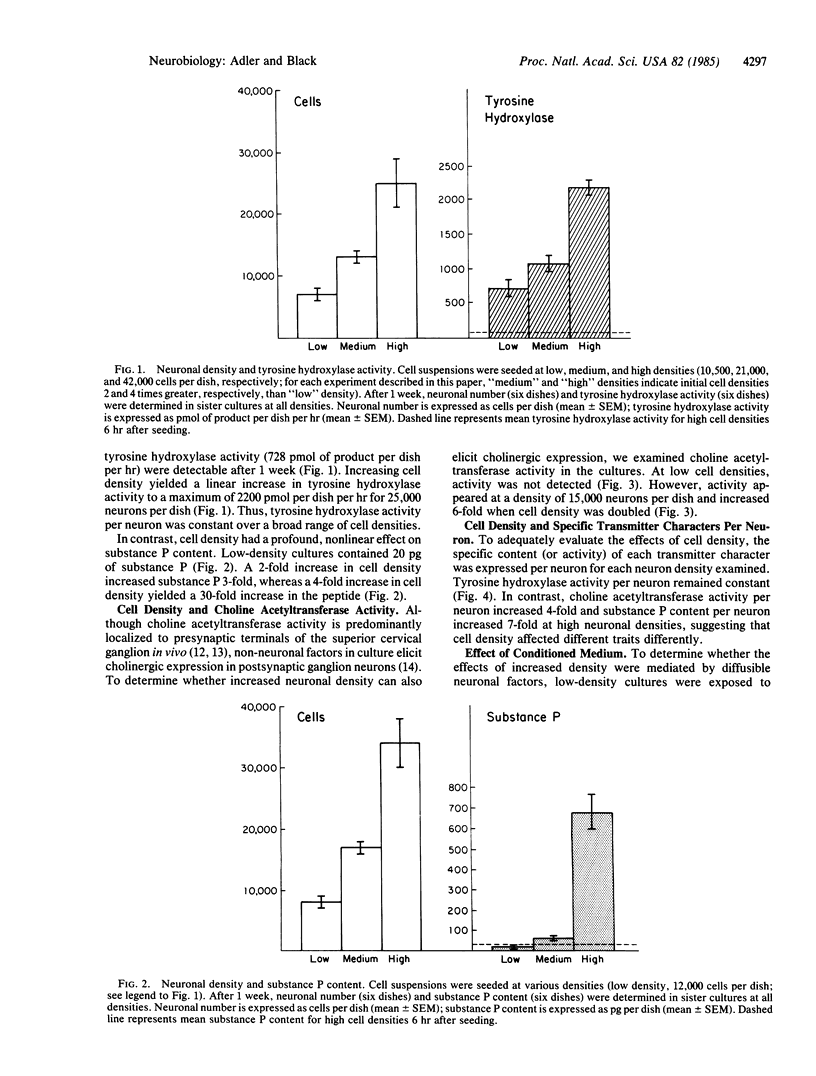
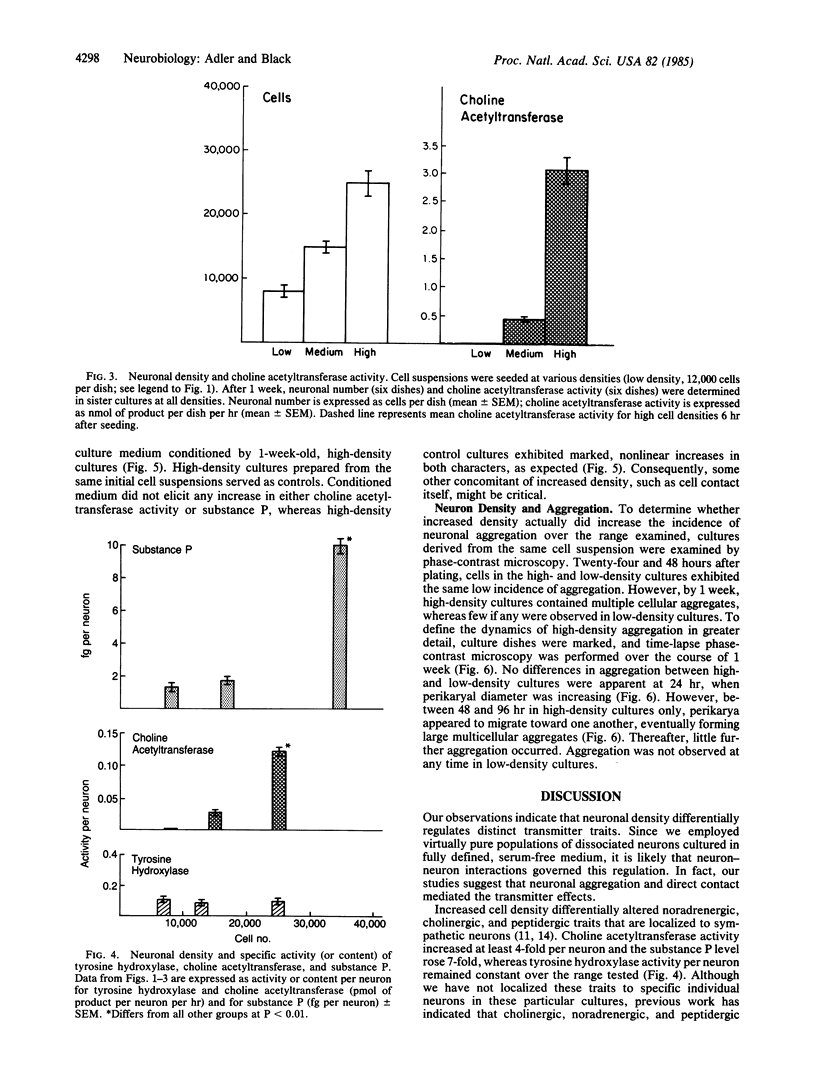
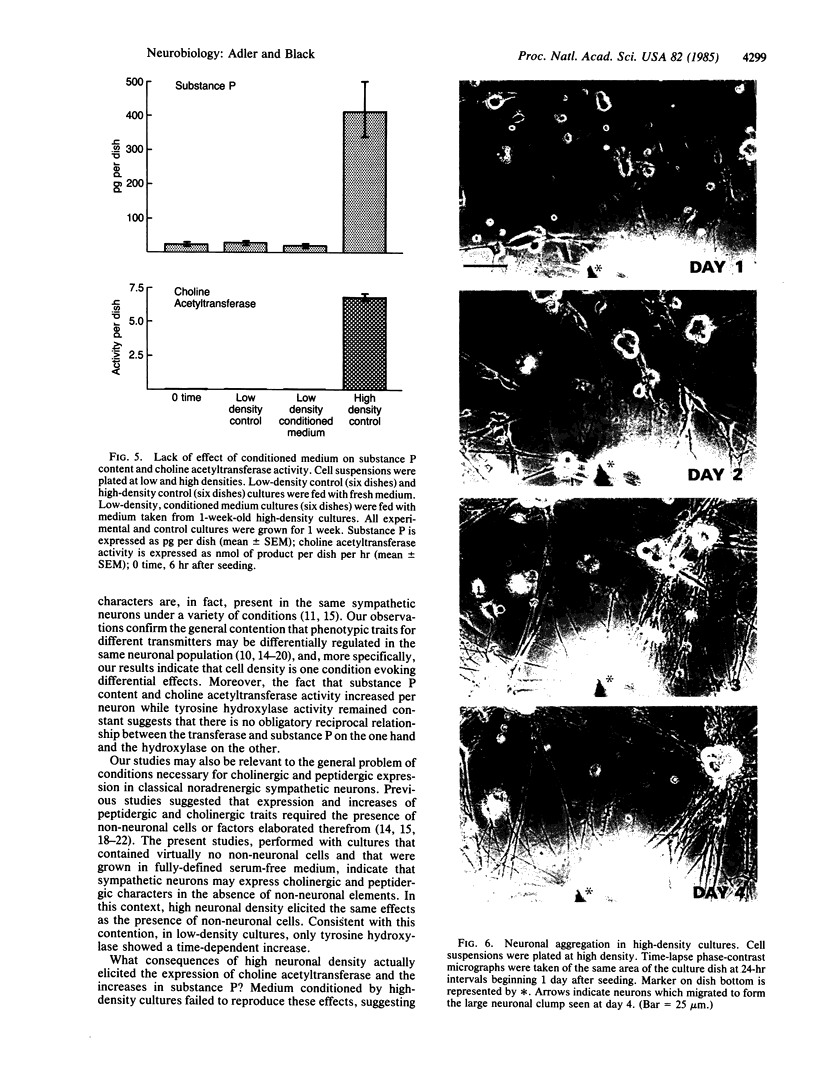
The effects of cell density and aggregation on expression of transmitter traits were examined in dissociated, pure sympathetic neuron cultures, grown in fully defined, serum-free medium. After 1 week at a density of 7-8 X 10(3) neurons per 35-mm dish, moderate levels of tyrosine hydroxylase (tyrosine 3-monooxygenase, EC 1.14.16.2) activity and substance P were detected. When neuron density was increased 4-fold, a 4-fold increase in tyrosine hydroxylase activity was observed; i.e., there was no change in tyrosine hydroxylase activity per neuron. In contrast, substance P increased 30-fold, corresponding to a 7-fold increase in substance P per neuron. Choline O-acetyltransferase (EC 2.3.1.6) activity, not detected at low cell densities, was first detectable at a concentration of 15,000 neurons per dish and increased 6-fold when this cell concentration was doubled. Medium conditioned by high-density cultures failed to reproduce these effects on low-density cultures, suggesting that diffusible factors are not involved in the density-dependent differential regulation. Time-lapse phase-contrast microscopy of high-density cultures showed neuronal migration and progressive aggregation, which did not occur in low-density cultures. Our observations suggest that cell contact may mediate differential expression of transmitter traits.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson A. L., Thoenen H. Cell contact-mediated regulation of tyrosine hydroxylase synthesis in cultured bovine adrenal chromaffin cells. J Cell Biol. 1983 Sep;97(3):925–928. doi: 10.1083/jcb.97.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler J. E., Kessler J. A., Black I. B. Development and regulation of substance P in sensory neurons in vitro. Dev Biol. 1984 Apr;102(2):417–425. doi: 10.1016/0012-1606(84)90206-9. [DOI] [PubMed] [Google Scholar]
- Black I. B., Bloom F. E., Hendry I. A., Iversen L. L. Growth and development of sympathetic ganglion: maturation of transmitter enzymes and synapse formation in the mouse superior cervical ganglion. J Physiol. 1971 May;215(1):24P–25P. [PubMed] [Google Scholar]
- Black I. B., Hendry I. A., Iversen L. L. Trans-synaptic regulation of growth and development of adrenergic neurones in a mouse sympathetic ganglion. Brain Res. 1971 Nov;34(2):229–240. doi: 10.1016/0006-8993(71)90278-2. [DOI] [PubMed] [Google Scholar]
- Bohn M. C., Kessler J. A., Adler J. E., Markey K., Goldstein M., Black I. B. Simultaneous expression of the SP-peptidergic and noradrenergic phenotypes in rat sympathetic neurons. Brain Res. 1984 Apr 30;298(2):378–381. doi: 10.1016/0006-8993(84)91442-2. [DOI] [PubMed] [Google Scholar]
- Bottenstein J. E., Sato G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979 Jan;76(1):514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochard P., Goldstein M., Black I. B. Initial development of the noradrenergic phenotype in autonomic neuroblasts of the rat embryo in vivo. Dev Biol. 1979 Jul;71(1):100–114. doi: 10.1016/0012-1606(79)90085-x. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion molecules. Science. 1983 Feb 4;219(4584):450–457. doi: 10.1126/science.6823544. [DOI] [PubMed] [Google Scholar]
- Fonnum F. A rapid radiochemical method for the determination of choline acetyltransferase. J Neurochem. 1975 Feb;24(2):407–409. doi: 10.1111/j.1471-4159.1975.tb11895.x. [DOI] [PubMed] [Google Scholar]
- Furshpan E. J., MacLeish P. R., O'Lague P. H., Potter D. D. Chemical transmission between rat sympathetic neurons and cardiac myocytes developing in microcultures: evidence for cholinergic, adrenergic, and dual-function neurons. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4225–4229. doi: 10.1073/pnas.73.11.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEBB C. O. Choline acetylase in the developing nervous system of the rabbit and guinea-pig. J Physiol. 1956 Sep 27;133(3):566–570. doi: 10.1113/jphysiol.1956.sp005609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEBB C. O., WAITES G. M. Choline acetylase in antero- and retro-grade degeneration of a cholinergic nerve. J Physiol. 1956 Jun 28;132(3):667–671. doi: 10.1113/jphysiol.1956.sp005556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M., Ross D., Meyers M., Rees R., Bunge R., Wakshull E., Burton H. Synaptic vesicle cytochemistry changes when cultured sympathetic neurones develop cholinergic interactions. Nature. 1976 Jul 22;262(5566):308–310. doi: 10.1038/262308a0. [DOI] [PubMed] [Google Scholar]
- Kessler J. A., Adler J. E., Bell W. O., Black I. B. Substance P and somatostatin metabolism in sympathetic and special sensory ganglia in vitro. Neuroscience. 1983 Jun;9(2):309–318. doi: 10.1016/0306-4522(83)90296-8. [DOI] [PubMed] [Google Scholar]
- Kessler J. A., Adler J. E., Black I. B. Substance P and somatostatin regulate sympathetic noradrenergic function. Science. 1983 Sep 9;221(4615):1059–1061. doi: 10.1126/science.6192502. [DOI] [PubMed] [Google Scholar]
- Kessler J. A., Adler J. E., Bohn M. C., Black I. B. Substance P in principal sympathetic neurons: regulation by impulse activity. Science. 1981 Oct 16;214(4518):335–336. doi: 10.1126/science.6169153. [DOI] [PubMed] [Google Scholar]
- Kessler J. A., Adler J. E., Jonakait G. M., Black I. B. Target organ regulation of substance P in sympathetic neurons in culture. Dev Biol. 1984 May;103(1):71–79. doi: 10.1016/0012-1606(84)90008-3. [DOI] [PubMed] [Google Scholar]
- Landis S. C. Rat sympathetic neurons and cardiac myocytes developing in microcultures: correlation of the fine structure of endings with neurotransmitter function in single neurons. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4220–4224. doi: 10.1073/pnas.73.11.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas C. A., Edgar D., Thoenen H. Regulation of tyrosine hydroxylase and choline acetyltransferase activities by cell density in the PC12 rat pheochromocytoma clonal cell line. Exp Cell Res. 1979 Jun;121(1):79–86. doi: 10.1016/0014-4827(79)90446-4. [DOI] [PubMed] [Google Scholar]
- Moscona A. A., Linser P. Developmental and experimental changes in retinal glia cells: cell interactions and control of phenotype expression and stability. Curr Top Dev Biol. 1983;18:155–188. doi: 10.1016/s0070-2153(08)60582-7. [DOI] [PubMed] [Google Scholar]
- O'Lague P. H., Obata K., Claude P., Furshpan E. J., Potter D. D. Evidence for cholinergic synapses between dissociated rat sympathetic neurons in cell culture. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3602–3606. doi: 10.1073/pnas.71.9.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson P. H., Chun L. L. The induction of acetylcholine synthesis in primary cultures of dissociated rat sympathetic neurons. I. Effects of conditioned medium. Dev Biol. 1977 Apr;56(2):263–280. doi: 10.1016/0012-1606(77)90269-x. [DOI] [PubMed] [Google Scholar]
- Patterson P. H., Chun L. L. The influence of non-neuronal cells on catecholamine and acetylcholine synthesis and accumulation in cultures of dissociated sympathetic neurons. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3607–3610. doi: 10.1073/pnas.71.9.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson P. H. Environmental determination of autonomic neurotransmitter functions. Annu Rev Neurosci. 1978;1:1–17. doi: 10.1146/annurev.ne.01.030178.000245. [DOI] [PubMed] [Google Scholar]
- Rutishauser U., Hoffman S., Edelman G. M. Binding properties of a cell adhesion molecule from neural tissue. Proc Natl Acad Sci U S A. 1982 Jan;79(2):685–689. doi: 10.1073/pnas.79.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]