Abstract
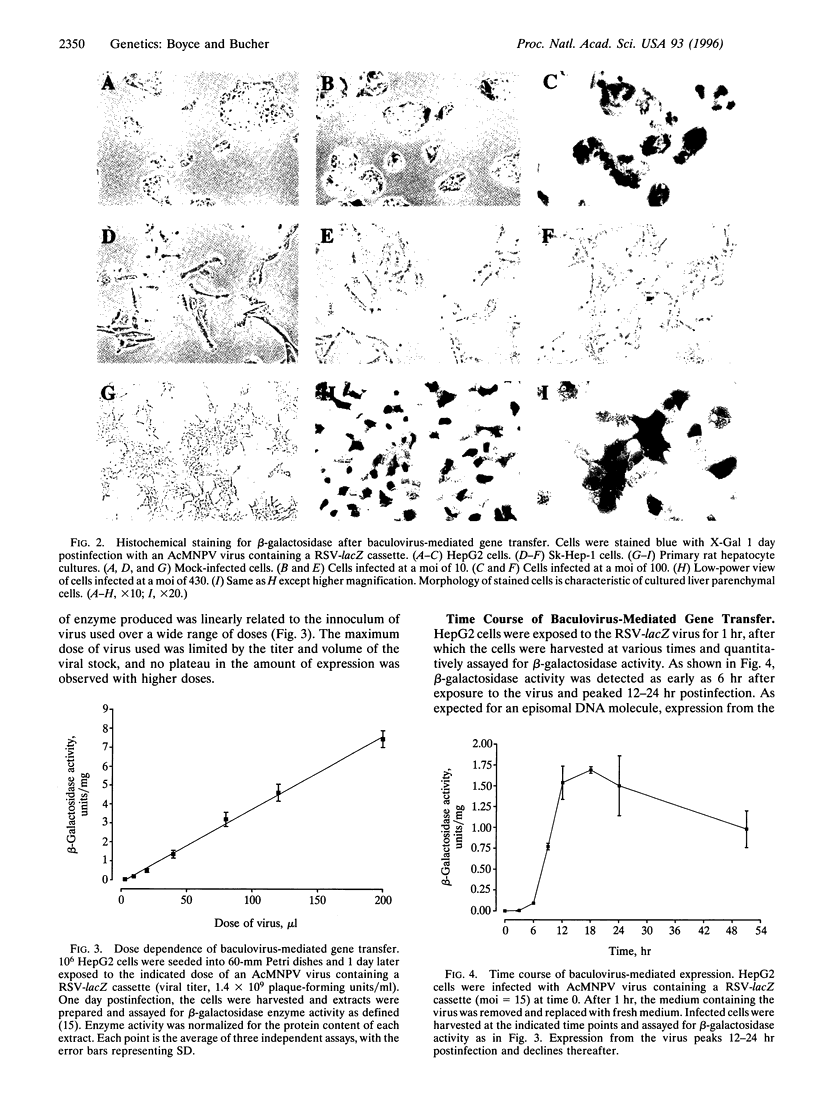
This paper describes the use of the baculovirus Autographa californica multiple nuclear polyhedrosis virus (AcMNPV) as a vector for gene delivery into mammalian cells. A modified AcMNPV virus was prepared that carried the Escherichia coli lacZ reporter gene under control of the Rous sarcoma virus promoter and mammalian RNA processing signals. This modified baculovirus was then used to infect a variety of mammalian cell lines. After infection of the human liver cell lines HepG2, >25% of the cells showed high-level expression of the transduced gene. Over 70% of the cells in primary cultures of rat hepatocytes showed expression of beta-galactosidase after exposure to the virus. Cell lines from other tissues showed less or no expression of lacZ after exposure to the virus. The block to expression in less susceptible cells does not appear to result from the ability to be internalized by the target cell but rather by events subsequent to viral entry. The onset of lacZ expression occurred within 6 hr of infection in HepG2 cells and peaked 12-24 hr postinfection. Because AcMNPV is able to replicate only in insect hosts, is able to carry large (>15 kb) inserts, and is a highly effective gene delivery vehicle for primary cultures of hepatocytes, AcMNPV may be a useful vector for genetic manipulation of liver cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blissard G. W., Wenz J. R. Baculovirus gp64 envelope glycoprotein is sufficient to mediate pH-dependent membrane fusion. J Virol. 1992 Nov;66(11):6829–6835. doi: 10.1128/jvi.66.11.6829-6835.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody S. L., Metzger M., Danel C., Rosenfeld M. A., Crystal R. G. Acute responses of non-human primates to airway delivery of an adenovirus vector containing the human cystic fibrosis transmembrane conductance regulator cDNA. Hum Gene Ther. 1994 Jul;5(7):821–836. doi: 10.1089/hum.1994.5.7-821. [DOI] [PubMed] [Google Scholar]
- Carbonell L. F., Klowden M. J., Miller L. K. Baculovirus-mediated expression of bacterial genes in dipteran and mammalian cells. J Virol. 1985 Oct;56(1):153–160. doi: 10.1128/jvi.56.1.153-160.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell L. F., Miller L. K. Baculovirus interaction with nontarget organisms: a virus-borne reporter gene is not expressed in two mammalian cell lines. Appl Environ Microbiol. 1987 Jul;53(7):1412–1417. doi: 10.1128/aem.53.7.1412-1417.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C., Padmanabhan R., Howard B. H. High efficiency DNA-mediated transformation of primate cells. Science. 1983 Aug 5;221(4610):551–553. doi: 10.1126/science.6306768. [DOI] [PubMed] [Google Scholar]
- Grompe M., Jones S. N., Loulseged H., Caskey C. T. Retroviral-mediated gene transfer of human ornithine transcarbamylase into primary hepatocytes of spf and spf-ash mice. Hum Gene Ther. 1992 Feb;3(1):35–44. doi: 10.1089/hum.1992.3.1-35. [DOI] [PubMed] [Google Scholar]
- Gröner A., Granados R. R., Burand J. P. Interaction of Autographa californica nuclear polyhedrosis virus with two nonpermissive cell lines. Intervirology. 1984;21(4):203–209. doi: 10.1159/000149522. [DOI] [PubMed] [Google Scholar]
- Hartig P. C., Cardon M. C., Kawanishi C. Y. Insect virus: assays for viral replication and persistence in mammalian cells. J Virol Methods. 1991 Feb-Mar;31(2-3):335–344. doi: 10.1016/0166-0934(91)90171-u. [DOI] [PubMed] [Google Scholar]
- Hartig P. C., Cardon M. C. Rapid efficient production of baculovirus expression vectors. J Virol Methods. 1992 Jul;38(1):61–70. doi: 10.1016/0166-0934(92)90169-e. [DOI] [PubMed] [Google Scholar]
- Lin W. C., Culp L. A. Selectable plasmid vectors with alternative and ultrasensitive histochemical marker genes. Biotechniques. 1991 Sep;11(3):344-8, 350-1. [PubMed] [Google Scholar]
- Lu B., Gupta S., Federoff H. Ex vivo hepatic gene transfer in mouse using a defective herpes simplex virus-1 vector. Hepatology. 1995 Mar;21(3):752–759. [PubMed] [Google Scholar]
- Mulligan R. C. The basic science of gene therapy. Science. 1993 May 14;260(5110):926–932. doi: 10.1126/science.8493530. [DOI] [PubMed] [Google Scholar]
- Norton P. A., Coffin J. M. Bacterial beta-galactosidase as a marker of Rous sarcoma virus gene expression and replication. Mol Cell Biol. 1985 Feb;5(2):281–290. doi: 10.1128/mcb.5.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto E., Jones-Trower A., Vanin E. F., Stambaugh K., Mueller S. N., Anderson W. F., McGarrity G. J. Characterization of a replication-competent retrovirus resulting from recombination of packaging and vector sequences. Hum Gene Ther. 1994 May;5(5):567–575. doi: 10.1089/hum.1994.5.5-567. [DOI] [PubMed] [Google Scholar]
- Pennock G. D., Shoemaker C., Miller L. K. Strong and regulated expression of Escherichia coli beta-galactosidase in insect cells with a baculovirus vector. Mol Cell Biol. 1984 Mar;4(3):399–406. doi: 10.1128/mcb.4.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana B., Mischoulon D., Xie Y., Bucher N. L., Farmer S. R. Cell-extracellular matrix interactions can regulate the switch between growth and differentiation in rat hepatocytes: reciprocal expression of C/EBP alpha and immediate-early growth response transcription factors. Mol Cell Biol. 1994 Sep;14(9):5858–5869. doi: 10.1128/mcb.14.9.5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D., Fraser M. J. Production of human beta interferon in insect cells infected with a baculovirus expression vector. Mol Cell Biol. 1983 Dec;3(12):2156–2165. doi: 10.1128/mcb.3.12.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman L. E., Goldsmith P. A. In Vitro Survey of Autographa californica Nuclear Polyhedrosis Virus Interaction with Nontarget Vertebrate Host Cells. Appl Environ Microbiol. 1983 Mar;45(3):1085–1093. doi: 10.1128/aem.45.3.1085-1093.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Nunes F. A., Berencsi K., Gönczöl E., Engelhardt J. F., Wilson J. M. Inactivation of E2a in recombinant adenoviruses improves the prospect for gene therapy in cystic fibrosis. Nat Genet. 1994 Jul;7(3):362–369. doi: 10.1038/ng0794-362. [DOI] [PubMed] [Google Scholar]
- Yee J. K., Miyanohara A., LaPorte P., Bouic K., Burns J. C., Friedmann T. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9564–9568. doi: 10.1073/pnas.91.20.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]