Abstract
While therapeutic drugs are routinely self-administered by patients, there is little precedent for self-vaccination. Convenient self-vaccination may expand vaccination coverage and reduce administration costs. Microneedle patches are in development for many vaccines, but no reports exist on usability or acceptability. We hypothesized that naïve patients could apply patches and that self-administered patches would improve stated intent to receive an influenza vaccine. We conducted a randomized, repeated measures study with 91 venue-recruited adults. To simulate vaccination, subjects received placebo microneedle patches given three times by self-administration and once by the investigator, as well as an intramuscular injection of saline. Seventy participants inserted patches with thumb pressure alone and the remainder used snap-based devices that closed shut at a certain force. Usability was assessed by skin staining and acceptability was measured with an adaptive-choice analysis. The best usability was seen with the snap device, with users inserting a median value of 93–96% of microneedles over three repetitions. When a self-administered microneedle patch was offered, intent to vaccinate increased from 44% to 65% (CI: 55–74%). The majority of those intending vaccination would prefer to self-vaccinate: 64% (CI: 51–75%). There were no serious adverse events associated with use of microneedle patches. The findings from this initial study indicate that microneedle patches for self-vaccination against influenza are usable and may lead to improved vaccination coverage.
Keywords: microneedle, human study, usability, acceptability, influenza
Introduction
Seasonal influenza causes three to five million cases of severe illness and 250,000–500,000 deaths annually [1]. Two key challenges for influenza vaccination are low voluntary coverage rates in adults and the expense of yearly vaccine administration.
The United States (US) achieves only 42% influenza vaccination coverage [2]. The average coverage of countries in the European Economic Area is 47% for elderly adults and 12% for the entire population [3]. These low levels are caused in part by fear of needles and inconvenience for patients. Needle phobia causes at least 7–8% of vaccination non-compliance [4], and inconvenience ranks as high as second as a reason for skipping influenza vaccination [5–7].
Although increased vaccination coverage reduces morbidity and mortality, it further increases vaccination costs. Two leading factors in the cost of influenza vaccination are administration costs and patient time cost, which can outweigh the cost of the vaccine itself by a ratio of 3.3 to 1 [8]. Administration by minimally trained workers or by patients themselves may expand the reach of vaccines and reduce vaccination costs [9].
To improve coverage and reduce costs, we propose using microneedle patches for high-throughput vaccination by healthcare personnel or self-vaccination by patients themselves. Microneedle patches contain arrays of needles measuring hundreds of microns in length that target vaccine delivery to the skin [10,11].
Microneedle patches have been studied for self-administration for cosmetic applications and for delivery of parathyroid hormone [12], but with no direct published data on usability. Several other delivery methods have been considered for self-vaccination: intranasal, sublingual, oral, inhaled, edible and transcutaneous vaccines [13–15]. To date, only one vaccine is approved for self-administration in patients’ homes, oral typhoid, with an estimated 3 million vaccine series administered per year worldwide [16,17]. Microneedle patches are especially attractive for self-vaccination because they are compatible with live, inactivated and subunit vaccines [18,19], administer a consistent dose [20,21], offer thermostability [22,23] and can be manufactured inexpensively [11]. Moreover, microneedle-based influenza vaccines are expected to be well accepted by practitioners and the general public [24–26] and have the potential to be more immunogenic [27–29].
Despite interest in self-administration of influenza vaccines, there is no published data on self-administration of microneedle patches or on the effect of microneedle patches and self-vaccination on vaccination coverage. We therefore conducted a study on the usability and acceptability of microneedle patches for influenza vaccination to test two central hypotheses: (i) participants can correctly apply microneedle patches with minimal training and, (ii) intent to vaccinate increases if a self-administered microneedle patch is offered to participants. Two groups of participants tested different insertion methods: applying force with the thumb alone or with a low-cost snap-based device.
Materials and Methods
Fabrication of Microneedle Patches and Snap Based Devices
Etched, stainless steel microneedles were mounted on adhesive foam backing (TM9942, MacTac, Stow, OH) and packaged with polyacetal. Each patch contained 50 hexagonally-packed microneedles, 750 μm long, with a row spacing and column spacing of 1.6 and 1.0 mm, respectively. Parts were assembled with double sided adhesive (1522, 3M, Minneapolis, MN) and sent for ethylene oxide sterilization. Nearly identical microneedle designs have been shown to insert effectively into skin [30,31]. We fabricated a snap-based device to facilitate insertion using polypropylene screw caps (91620A200, McMaster-Carr, Atlanta, GA). We used a resistive strain gauge load cell (RSP1-010M-A, Loadstar Sensors, Fremont, CA) to evaluate these devices compared to the force an experienced, blinded investigator uses to insert microneedle patches.
Study Approval and Participant Recruiting
This study was approved by the Georgia Institute of Technology Institutional Review Board, and informed consent was obtained from all participants. We used a venue sampling method [32] to obtain a high response rate. Eligible participants were healthy, non-pregnant adults with no diseased skin, no pain perception problems, and no known allergies to compounds used in the study. Seventy participants recruited from Atlanta, GA between September 2011 and May 2012 inserted patches without snap-based devices. Twenty-one participants recruited between October and December 2012 used snap-based devices. Sampling error could affect usability and acceptability results. Because males and participants with a household income less than $20,000 were initially overrepresented, we modified our venue list during the study to emphasize venues that would attract other subjects. All participants were naïve concerning microneedle use.
Microneedle Administrations
Participants experienced three un-blinded procedures: self-administration of three microneedle patches, investigator-administration of a microneedle patch, and investigator administration of 0.5 mL saline by IM injection (22–23gauge needle, 1–1.5 inches long). Patches were administered to the volar forearm, and participants were instructed to clinch their fists. For insertion with thumb pressure alone, the procedures were randomized, but self-administration always preceded investigator administration for insertion with the snap-based devices. Instructions provided to participants are provided in Supplemental Information. The investigator intervened if the participant placed a patch on incorrectly (e.g. upside down) or if participants had a failed administration attempt. In these cases, participants were told to push harder. Patches were removed immediately after administration, and participants were told that patch vaccination would require a wear time of 1 min. Pain ratings were collected using a visual analog scale (VAS). All participant input was collected using a computer.
Skin Staining to Measure Usability
Dyes were applied to assess usability. Power analysis was based on the hypothesis that at least 85% of people could correctly administer a patch. Expecting 67 of 70 participants to succeed, we had 83% power for this hypothesis. The group using snap-based devices was a pilot study. Gentian violet or fluorescein dye was applied to different skin tones at investigator’s discretion. Gentian violet 1% (Humco, Texarkana, TX) was pooled for 1 min, dabbed with gauze, and cleaned with alcohol after 5 min. Fluorescein 10% (Akorn, Lake Forest, IL) was diluted to 2% in saline, applied lightly with a cotton swab, dabbed with gauze, and cleaned with alcohol after 5 s, to provide staining on dark-toned skin. Fluorescein stains were imaged under blue LED light with blue glass as an excitation filter and a Tiffen 5558filter for emission (Hauppauge, NY). Usability was quantified by counting insertion sites, uniformly and linearly narrowing the color balance on some images. Uncertain points, such as those covered by blood, were excluded from counts. The number of stained sites visible after microneedle insertion has previously been correlated with trans-epidermal water loss, a measure of skin puncture [33].
Acceptability Questionnaire
An adaptive choice survey solicited participants’ preference for vaccination options after device administration. Participants chose between an IM injection, no vaccination at all, or a microneedle patch option. The IM injection had a fixed price (the lower of $25 or the participant’s current out-of-pocket cost for influenza vaccination). The patch price changed according to a binary search algorithm. Each binary search had four steps starting at a random price, bounded between the IM price ± $20. We asked about three vaccine patch options: self-administration at home, self-administration with a healthcare worker nearby, and healthcare-worker-administration. We repeated these measurements for a hypothetical “high-protection” patch offering a 50% smaller chance in getting influenza after vaccination.
Behavioral Questionnaire
To answer what factors drove acceptance of microneedle patches, we included a questionnaire with constructs borrowed from the Theory of Reasoned Action [34]. Analysis in SPSS v20 using the Varimax rotation method separated the questionnaire items into a four-factor solution. Internal consistencies achieved on the four scales demonstrated a high level of reliability.
Each participant had a normalized score for each factor. We regressed these scores to a binary outcome measure of whether a participant would choose a microneedle patch offered at the same price as an IM injection, based on the acceptability data (Minitab v15, binary logistic regression). See details in Supplemental Information.
Results
Usability
We determined if participants could apply microneedle patches with minimal training. Subjects self-administered placebo microneedle patches three times, had a placebo microneedle patch administered by study personnel and received an IM injection of saline in randomized order. Participants were well distributed in terms physical and socioeconomic factors (Table 1).
Table 1.
| Trait | Value | Count | Percentage of Sample (n=91) |
|---|---|---|---|
| Gender | Male | 55 | 60% |
| Female | 36 | 40% | |
| Race/Ethnicity | White | 38 | 42% |
| African American / Black | 43 | 47% | |
| Other | 10 | 11% | |
| Education | High school or less | 34 | 37% |
| Associates degree | 17 | 19% | |
| College degree or more | 40 | 44% | |
| Age | 18–19 | 4 | 4% |
| 20–29 | 35 | 38% | |
| 30–39 | 16 | 18% | |
| 40–49 | 19 | 21% | |
| 50+ | 17 | 19% | |
| Income | Less than $20,000 | 25 | 28% |
| $20,000 – $40,000 | 20 | 22% | |
| More than $40,000 | 35 | 38% | |
| No income or unknown | 11 | 12% |
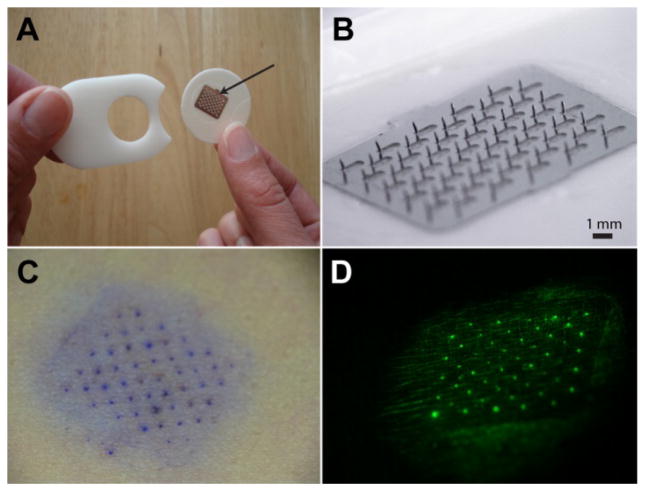
Fig. 1A and 1B show the prototype microneedle patch used in this study. The patch was 3 cm in diameter with an array of 100 microneedles each measuring 750 μm in length. Fig. 1C and 1D display examples of gentian violet and fluorescein skin staining.
Figure 1.
A placebo microneedle patch and examples of skin stains applied to evaluate usability. (A) A 12x10 mm microneedle array (arrow) on a 30 mm-diameter foam adhesive patch with a polyacetal liner that protects the microneedles in packaging. (B) The microneedle array under magnification, showing 100 microneedles each measuring 750 μm long. (C) Gentian violet skin stain and (D) fluorescein skin stain labeling sites of microneedle penetration into the skin of human participants.
Fig. 2A illustrates the snap-based device used by twenty of the participants. This device made a snapping sound when a force of approximately 37 N was applied. As shown in Fig 2B, this force is similar to that applied by an experienced investigator.
Figure 2.
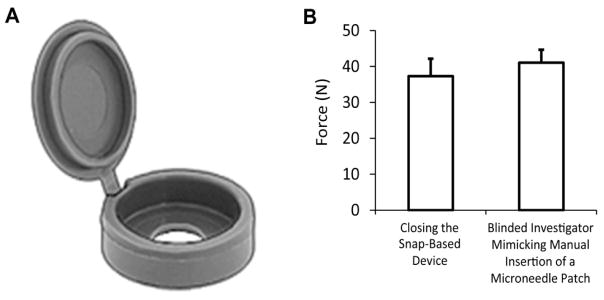
Design of the snap-based device providing force feedback during microneedle patch administration. (A) Three-dimensional rendering of the device measuring 1.4 cm in diameter and 0.4 cm in height in the closed position. The top portion hinges and closes onto the bottom portion with a snapping noise. Devices were placed on the back of microneedle patches to assist with insertion by providing audible feedback to the user when sufficient force was applied. (B) The force required to close the device (n=10) compared to blinded thumb presses by an experienced investigator estimating the force required for microneedle insertion into skin (n=10). Error bars show standard deviation.
Fig. 3 charts the usability data for each self-administration attempt, the best attempt out of three, and the usability of microneedles administered by study personnel. Usability data for three participants were unavailable due to uninterpretable fluorescein stains.
Figure 3.
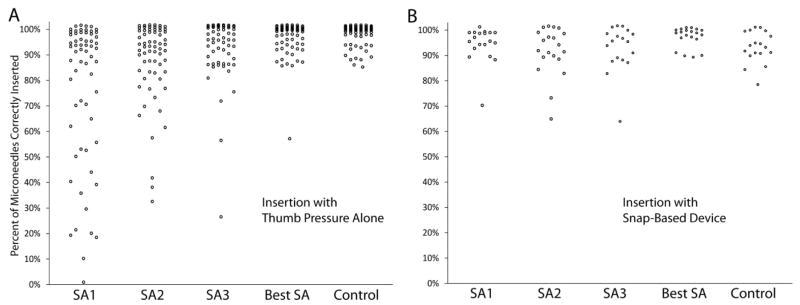
Usability of microneedle patches. (A) Usability for insertion with thumb pressure alone (n=69). (B) Usability with the snap-based device (n=20). The y axis shows the percent of microneedles in a patch that punctured skin, as determined by a skin staining assay. Attempts SA1 through SA3 are participant self-administrations. Best SA is the highest percent administered from the three attempts by a participant. The control is investigator administration. A random jitter was applied to separate overlapping points (±1% on the y-axis).
Without the snap-based device, the median number of insertion sites observed on the first attempt was 90%. The variability between participants was high with an interquartile range (IQR) of 44%. On the second and third attempt, the median number of insertion sites observed increased to 94% and the variability decreased (IQR: 13–15%). The improvement in administration success was statistically significant (p = 0.003, n = 57, Friedman’s rank test), indicating a learning curve. This suggested the need for a device to assist with microneedle insertion.
With the snap-based device, the median number of insertion sites observed on the first attempt was 96%, and the variability between subjects was lower than before (IQR: 5%). The improvement in the number of insertion sites observed on the first attempt was statistically significant (p = 0.006, Mann-Whitney U). The second and third attempts performed similarly well (median percent inserted: 93–95%, IQR: 9–10%). This shows that a snap-based device that provides feedback to the user regarding insertion force improved microneedle insertion success.
All insertions were well tolerated with only very mild, transient erythema. One unrelated adverse event occurred, a viral pneumonia case four days after the study. Two participants withdrew due to lightheadedness after procedures.
Acceptability
We used a discrete choice analysis to assess whether more participants would express intent to be vaccinated against influenza if offered a microneedle patch. At the baseline, 44% (CI: 34–54%) of participants expressed intent to be vaccinated during the coming year given currently available vaccination methods (i.e., “normally vaccinated” participants). This number of normally vaccinated participants is higher than the 33% influenza vaccination coverage reported in the US in 2011–12 for adults age 18–64 [2].
Microneedle-patch vaccination by a healthcare worker
When participants were offered the choice of vaccination using a microneedle patch administered by a healthcare professional in addition to conventional IM injection, intent to vaccinate increased to 61% (CI: 50–70%). This represents a 17 percentage point increase in expected vaccination coverage overall just due to offering a microneedle patch.
Considering just the normally vaccinated participants, 51% expressed a preference for the microneedle patch; the remainder preferred IM injection. Of greater significance, among the normally unvaccinated participants, 30% (CI: 19–44%) expressed willingness to get vaccinated if offered the microneedle patch, and all of these participants preferred the microneedle patch over IM injection (Fig. 4A). This suggests that a large fraction of those who normally do not get vaccinated could be convinced to be vaccinated if offered a microneedle patch.
Figure 4.
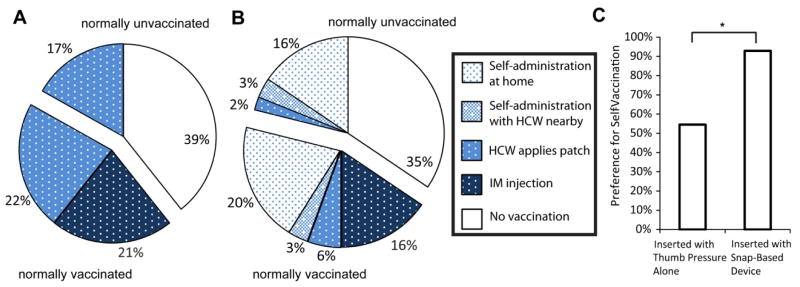
Acceptability of microneedle patches and self-vaccination assuming patches have similar effectiveness and cost to injections. Participant preference for four vaccination options is shown: i) IM injection, ii) healthcare worker (HCW) applies a patch, iii) self-administration of a patch with a healthcare worker nearby and iv) self-administration of a patch at home. The unfilled portion shows participants who would remain unvaccinated. (A) Microneedle patches without self-vaccination options (i.e., only options (i) and (ii)). (B) Microneedle patches with self-vaccination options (i.e., options (i) through (iv)). (C) Comparison of self-vaccination acceptability for participants who inserted with thumb pressure alone or with the snap-based device. Self-vaccination preference was significantly higher for those using the snap-based device (p = 0.004).
Microneedle-patch self-vaccination
We next offered the option to self-vaccinate using a microneedle patch, either at home or in the presence of a healthcare worker. Given these self-administration options, intent to vaccinate increased to 65% from the baseline 44%, corresponding to a 21 percentage point increase in intended vaccination coverage. Among normally unvaccinated participants, 38% (CI: 26–52%) expressed willingness to get vaccinated (Fig. 4B). Use of the snap-based device did not significantly affect intent to vaccinate.
Among those expressing intent to be vaccinated by any method, 55% preferred to self-administer the microneedle patch at home, 9% preferred to self-administer the microneedle patch in the presence of a healthcare worker, 12% preferred to have a healthcare worker administer the microneedle patch, and 24% preferred IM injection. This means that 76% preferred microneedle-patch vaccination over IM vaccination and that 64% preferred self-vaccination over vaccination by a healthcare worker. The acceptance of self-vaccination was significantly higher than in the group using the snap-based device (p = 0.004, Chi-square, Fig. 4C). Concerning the effect of price and efficacy on microneedle patch acceptability, we found as expected that acceptability of microneedles decreased if users were asked to pay more and increased if participants were told the microneedle patch would be more effective at preventing influenza. Further details are presented in Supplemental Information.
Although most participants chose self-administration if made available, there was only a small increase in intent to vaccinate compared to the case where microneedle patches were offered, but self-administration was unavailable. Although the large majority of participants preferred microneedle patches and preferred to use them for self-vaccination, it was the microneedle patch itself that was the primary deciding factor that increased intent to vaccinate.
Pain
We compared the pain of microneedle administration and IM injection for participants who achieved at least 85% of needles inserted on their first attempt. The median pain scores, out of 100, were 1.5 for self-administration, 1.5 for investigator administration, and 15 for IM injection (IQR: 5, 8, and 30, respectively). There was no significant difference in pain for insertion with the snap-based device (median: 2.5, IQR: 5) and insertion without the device (median: 1, IQR: 7; p>0.5, Mann-Whitney U). For both groups, statistical analysis showed microneedle patch administrations were significantly less painful than IM injection (Repeated Measures ANOVA: p<0.002). In both cases, there was no significant difference between self-administration and investigator administration.
Factors Affecting Microneedle Patch Uptake
We measured psychosocial indicators of microneedle acceptability with constructs from the Theory of Reasoned Action [34]. For 21 items in the questionnaire, an exploratory principal components factor analysis yielded four primary factors covering 65% of the total variance among subjects (see Table S1 in supplemental information). The four factors were: attitude towards microneedles, normative approval, behavioral beliefs, and outcome evaluations. Normative approval measured participants’ perceived approval the microneedle patch by doctors, family, and friends. Behavioral beliefs measured the perceived convenience and reliability of microneedle patches. Outcome evaluations related to physical side effects of microneedles and injections.
The significant predictors of uptake were behavioral beliefs about usability and reliability (p = 0.001), normative approval (p = 0.02), and positive attitude towards microneedles (p = 0.046). This indicates that acceptability of microneedles correlated with patient beliefs (i) that microneedles are convenient and reliable to use and (ii) that doctors, family and friends approve of microneedle vaccination. Outcome evaluations (p = 0.74) positively correlated with uptake, but the correlation was not statistically significant, suggesting that the pain reduction associated with microneedles was less important to acceptability. Every participant agreed or strongly agreed that microneedle patches were easy to administer, and no participant disagreed that microneedle patches could administer flu vaccine reliably. This contrasts with a recent focus group study [35] in which 84% of participants thought it might be difficult to verify correct microneedle administration. In addition, our Likert scores for preference of microneedle patches over traditional injections were slightly higher (3.3 ± 0.8 compared to 2.7 ± 1.1, mean ± standard deviation, estimated from data in Table IV in [35]). These differences may be due to sampling from different populations. In addition, participants in this study experienced microneedle administration prior to completing their questionnaire.
Discussion
This study sought to assess if self-administered microneedle patches are usable and acceptable for influenza vaccination. Concerning usability, almost all participants, in a relatively large, diverse population, were able to self-administer microneedle patches with minimal assistance. With administration by thumb pressure alone, some participants needed multiple attempts and instruction to push harder in order to successfully self-administer microneedles. With a simple, low-cost snap-based device, usability improved significantly. Usability of the microneedle with this device appeared to be similar to usability rates seen for the only self-administered vaccine currently approved in the US: oral typhoid vaccine [16,36]. These results suggest that microneedle patch administration with a simple device providing insertion-force feedback is feasible and could be a reliable self-vaccination method. Having shown feasibility, the next step is to reproduce the usability results with refined instructions in a more realistic patient setting. In addition, future studies may benefit from investigating alternative administration sites, depth of microneedle administration, or extent of drug delivery.
The quantitative acceptability analysis suggests that offering a self-administered microneedle patch could improve vaccination coverage from 44% to 65%, which could have a significant impact on reducing influenza hospitalizations, deaths, and lost productivity if the findings of this moderately sized study are predictive of the US population as a whole. The microneedle patch alone was sufficient to convert 30% of normally unvaccinated participants to willing vaccinees, while the added convenience of self-administration further increased intent to vaccinate to 38%. Most participants preferred self-administration and the perceived convenience of patches was the most significant factor predicting microneedle uptake. We conclude that although participants preferred self-administration more, particularly in the group using the snap-based device, microneedle patches alone may be sufficient for improving hypothetical coverage in this population.
A separate study compared two groups receiving either self-administration and healthcare-provider-administration of influenza vaccine using a hollow microneedle device [37]. Given the option of self-administration or healthcare-provider-administration, 42% preferred the method they experienced, 42% were ambivalent, and 16% preferred the other method. Among participations who experienced self-vaccination, the preference for self-administration was comparable to what we observed. The reduced acceptance among those who had not experienced self-vaccination may again explain the discrepancies observed in survey responses for our sample that experienced microneedle self-administration and the sample in the focus group study that did not [35].
Beyond affecting the acceptability of influenza vaccines, another rationale for pursuing self-administered vaccines is to reduce the cost of vaccination. Influenza vaccine administration outweighs the cost of the vaccine itself in the United States, as evidenced by the Centers for Medicare and Medicaid services which reimburses $12.40 for the vaccine [38], but $26.20 for vaccine administration (average across all localities [39]). The long-term cost of a microneedle patch for influenza vaccination is expected to be close to the cost of a dose for intramuscular vaccination, but there will likely be significant initial costs associated with designing and implementing the new manufacturing process. Concerning administration, we expect that the cost of self-vaccination would be close to the cost of stocking, selling, and documenting an influenza vaccination in a pharmacy, approximately $5.50 (range $4.60-$11.70) in 2012 USD [8]. Therefore, there are significant potential savings in self-vaccination for healthcare payers if changes in dose cost, reimbursement, and distribution are not too costly. An ideal setting for introducing self-administered vaccines may be a region that provides free influenza vaccines from a single payer, such as Ontario, Canada [40]. More research is needed to develop an economic analysis of self-vaccination and consider how to accommodate self-administration in the different healthcare payers systems used worldwide.
The usability and acceptability results presented here have broad implications beyond seasonal influenza. Simple administration with microneedle patches could enable vaccination by minimally trained workers or by patients themselves in other settings. For example, our laboratory is developing microneedle patches for polio and measles vaccination [41] to obviate the need for trained healthcare workers in the effort to eliminate or eradicate these diseases.
For future work, we need to improve microneedle patch administration to approach 100% reliable insertion on the first attempt, reproduce acceptability results with larger and broader populations, conduct clinical trials on the immunogenicity and safety of self-vaccination, and scale up manufacturing. Because a self-administered vaccine patch would represent a paradigm shift in healthcare, we are also assessing acceptance among healthcare providers and other stakeholders. Regulatory approval will require addressing safety concerns like anaphylaxis and syncope and legal topics such as waste disposal, mailing of biologicals, over-the-counter policy, compensation for vaccine injuries, and the validity of self-vaccination for school children and healthcare workers. We will also need to find proper ways to document self-administered vaccines and reimburse patients.
The primary limitations of this study are small sample size, volunteer bias, use of stated preference for willingness-to-pay, potential bias due to experience with self-administration, and traditional biases associated with questionnaires. Additionally, skin staining indicated puncture of the skin surface rather than confirmed drug delivery or depth of insertion, and this study did not use other measures of skin barrier disruption such as trans-epidermal water loss that have been used previously [30,31]. Potential improvements include using larger samples of subjects, using influenza vaccine instead of skin stains to validate usability, and using marketing research techniques to eliminate volunteer and questionnaire biases.
Overall, these first-in-humans results on microneedle patch usability and acceptability suggest patients can use microneedle patches, microneedle patches may improve vaccination coverage, and self-vaccination is well accepted by patients.
Supplementary Material
Highlights.
First-in-humans study of microneedle patch usability and acceptability
Users can correctly apply microneedle patches
A snap-based device providing force feedback to users improved usability
Microneedle patches increased intent to vaccinate from 44% to 65%
64% of participants intending vaccination would prefer to self-vaccinate
Acknowledgments
Donna Bondy provided administrative assistance. Yoo Chun Kim, Winston Pewin, Brian Bondy and Betul Benzer helped fabricate patches. Sharon Norman aided survey development. Samantha Andrews, Chris Edens, and Matt Mistilis helped develop staining methods. Brooke Hixson, Marc Padilla, and Patrick Kelly helped with recruiting. Devin McAllister, Sebastien Henry, Darin Zehrung, and the vaccine technology team at PATH provided advice during study design. Mark Prausnitz is an inventor of patents that have been licensed to companies developing microneedle-based products, is a paid advisor to companies developing microneedle-based products, and is a founder/shareholder of companies developing microneedle-based products.
Footnotes
Author Contributions
JJN designed devices, programmed the questionnaire, coordinated the study, and wrote the manuscript. JMA and MAM managed participant recruitment and assisted with study procedures. PMF and MIM assisted with study design and data analysis. MRP directed the study as the principal investigator.
This potential conflict of interest has been disclosed and is managed by Georgia Tech and Emory University.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
James J. Norman, Email: jnorman3@gatech.edu.
Jaya M. Arya, Email: jarya3@gatech.edu.
Maxine A. McClain, Email: mx.mcclain@gmail.com.
Paula M. Frew, Email: pfrew@emory.edu.
Martin I. Meltzer, Email: qzm4@cdc.gov.
Mark R. Prausnitz, Email: prausnitz@gatech.edu.
References
- 1.World Health Organization. [Accessed Dec 1, 2013];Seasonal Influenza Fact Sheet. 2009 http://www.who.int/mediacentre/factsheets/fs211/en/
- 2.McIntyre AF, Gonzalez-Feliciano AG, Santibanez TA, Bryan LN, Greby SM, Biggers BB, et al. [Accessed Dec 1, 2013];Flu Vaccination Coverage, United States, 2011–12 Influenza Season. 2012 http://www.cdc.gov/flu/fluvaxview/coverage_1112estimates.htm.
- 3.O’Flanagan D, Cotter S, Mereckiene J. [Accessed Dec 1, 2013];Seasonal influenza vaccination in EU/EEA, influenza season 2011–12. 2013 http://venice.cineca.org/VENICE_Seasonal_Influenza_2011-12_1.0v.pdf.
- 4.Taddio A, Ipp M, Thivakaran S, Jamal A, Parikh C, Smart S, et al. Survey of the prevalence of immunization non-compliance due to needle fears in children and adults. Vaccine. 2012;30:4807–12. doi: 10.1016/j.vaccine.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Harris KM, Maurer J, Uscher-Pines L, Kellerman AL, Lurie N. Seasonal Flu Vaccination: Why Don’t More Americans Get It? [Accessed Dec 1, 2013];RAND Corp Res Briefs. 2011 http://www.rand.org/content/dam/rand/pubs/research_briefs/2011/RAND_RB9572.pdf.
- 6.Johnson DR, Nichol KL, Lipczynski K. Barriers to adult immunization. Am J Med. 2008;121:S28–35. doi: 10.1016/j.amjmed.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Uscher-Pines L, Maurer J, Kellerman A, Harris KM. Healthy young and middle age adults: what will it take to vaccinate them for influenza? Vaccine. 2010;28:7420–2. doi: 10.1016/j.vaccine.2010.08.095. [DOI] [PubMed] [Google Scholar]
- 8.Prosser LA, O’Brien MA, Molinari NA, Hohman KH, Nichol KL, Messonnier ML, et al. Non-traditional settings for influenza vaccination of adults: costs and cost effectiveness. Pharmacoeconomics. 2008;26:163–78. doi: 10.2165/00019053-200826020-00006. [DOI] [PubMed] [Google Scholar]
- 9.Glenn GM, Kenney RT. Mass vaccination: solutions in the skin. Curr Top Microbiol Immunol. 2006;304:247–68. doi: 10.1007/3-540-36583-4_14. [DOI] [PubMed] [Google Scholar]
- 10.Donnelly RF, Singh TR, Morrow DI, Woolfson AD. Microneedle-mediated transdermal and intradermal drug delivery. Chichester, West Sussex: Wiley-Blackwell; 2012. [Google Scholar]
- 11.Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547–68. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cosman F, Lane NE, Bolognese MA, Zanchetta JR, Garcia-Hernandez PA, Sees K, et al. Effect of transdermal teriparatide administration on bone mineral density in postmenopausal women. J Clin Endocrinol Metab. 2010;95:151–8. doi: 10.1210/jc.2009-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ambrose CS, Wu X. The safety and effectiveness of self-administration of intranasal live attenuated influenza vaccine in adults. Vaccine. 2012;31:857–60. doi: 10.1016/j.vaccine.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 14.Mason HS, Haq TA, Clements JD, Arntzen CJ. Edible vaccine protects mice against Escherichia coli heat-labile enterotoxin (LT): potatoes expressing a synthetic LT-B gene. Vaccine. 1998;16:1336–43. doi: 10.1016/s0264-410x(98)80020-0. [DOI] [PubMed] [Google Scholar]
- 15.Glenn GM, Flyer DC, Ellingsworth LR, Frech SA, Frerichs DM, Seid RC, et al. Transcutaneous immunization with heat-labile enterotoxin: development of a needle-free vaccine patch. Expert Rev Vaccines. 2007;6:809–19. doi: 10.1586/14760584.6.5.809. [DOI] [PubMed] [Google Scholar]
- 16.Cryz SJ., Jr Patient compliance in the use of Vivotif Berna(R) vaccine, typhoid vaccine, live oral Ty21a. J Travel Med. 1998;5:14–7. doi: 10.1111/j.1708-8305.1998.tb00449.x. [DOI] [PubMed] [Google Scholar]
- 17.Fondation Merieux. [Accessed Dec 1, 2013];Report of the Meeting on Typhoid Fever, a Neglected Disease: Towards a Vaccine Introduction Policy. 2007 http://www.who.int/immunization/sage/typhoid_meeting_july.pdf.
- 18.Kommareddy S, Baudner BC, Oh S, Kwon SY, Singh M, O’Hagan DT. Dissolvable microneedle patches for the delivery of cell-culture-derived influenza vaccine antigens. J Pharm Sci. 2012;101:1021–7. doi: 10.1002/jps.23019. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan SP, Koutsonanos DG, Del Pilar Martin M, Lee JW, Zarnitsyn V, Choi S-O, et al. Dissolving polymer microneedle patches for influenza vaccination. Nat Med. 2010;16:915–20. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6:148–58. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Brown K, Siebenaler K, Determan A, Dohmeier D, Hansen K. Development of lidocaine-coated microneedle product for rapid, safe, and prolonged local analgesic action. Pharm Res. 2012;29:170–7. doi: 10.1007/s11095-011-0524-4. [DOI] [PubMed] [Google Scholar]
- 22.Quan F-S, Bondy BJ, Choi H-J, Prausnitz MR, Kang S-M, Yoo D-G, et al. Stability of influenza vaccine coated onto microneedles. Biomaterials. 2012;33:3756–69. doi: 10.1016/j.biomaterials.2012.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X, Fernando GJ, Crichton ML, Flaim C, Yukiko SR, Fairmaid EJ, et al. Improving the reach of vaccines to low-resource regions, with a needle-free vaccine delivery device and long-term thermostabilization. J Control Release. 2011;152:349–55. doi: 10.1016/j.jconrel.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 24.Arnou R, Frank M, Hagel T, Prebet A. Willingness to vaccinate or get vaccinated with an intradermal seasonal influenza vaccine: a survey of general practitioners and the general public in France and Germany. Adv Ther. 2011;28:555–65. doi: 10.1007/s12325-011-0035-z. [DOI] [PubMed] [Google Scholar]
- 25.Eizenberg P, Booy R, Naser N, Mason G, Stamboulian D, Weber F. Acceptance of Intanza(R) 9 mug intradermal influenza vaccine in routine clinical practice in Australia and Argentina. Adv Ther. 2011;28:640–9. doi: 10.1007/s12325-011-0042-0. [DOI] [PubMed] [Google Scholar]
- 26.Prymula R, Usluer G, Altinel S, Sichova R, Weber F. Acceptance and opinions of Intanza/IDflu intradermal influenza vaccine in the Czech Republic and Turkey. Adv Ther. 2012;29:41–52. doi: 10.1007/s12325-011-0090-5. [DOI] [PubMed] [Google Scholar]
- 27.Arnou R, Eavis P, Pardo J-RDJ, Ambrozaitis A, Kazek M-P, Weber F. Immunogenicity, large scale safety and lot consistency of an intradermal influenza vaccine in adults aged 18–60 years: Randomized, controlled, phase III trial. Hum Vaccin. 2010;6:346–54. doi: 10.4161/hv.6.4.10961. [DOI] [PubMed] [Google Scholar]
- 28.Arnou R, Icardi G, De Decker M, Ambrozaitis A, Kazek M-P, Weber F, et al. Intradermal influenza vaccine for older adults: a randomized controlled multicenter phase III study. Vaccine. 2009;27:7304–12. doi: 10.1016/j.vaccine.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 29.Van Damme P, Oosterhuis-Kafeja F, Van der Wielen M, Almagor Y, Sharon O, Levin Y. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine. 2009;27:454–9. doi: 10.1016/j.vaccine.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 30.Gupta J, Gill HS, Andrews SN, Prausnitz MR. Kinetics of skin resealing after insertion of microneedles in human subjects. J Control Release. 2011;154:148–55. doi: 10.1016/j.jconrel.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brogden NK, Ghosh P, Hardi L, Crofford LJ, Stinchcomb AL. Development of in vivo impedance spectroscopy techniques for measurement of micropore formation following microneedle insertion. J Pharm Sci. 2013;102:1948–56. doi: 10.1002/jps.23544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore K, Painter JE, Kulb C, Frew PM, Hixson B, del Rio C, et al. Factors mediating seasonal and influenza A (H1N1) vaccine acceptance among ethnically diverse populations in the urban south. Vaccine. 2012;30:4200–8. doi: 10.1016/j.vaccine.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haq MI, Smith E, John DN, Kalavala M, Edwards C, Anstey A, et al. Clinical administration of microneedles: skin puncture, pain and sensation. Biomed Microdevices. 2009;11:35–47. doi: 10.1007/s10544-008-9208-1. [DOI] [PubMed] [Google Scholar]
- 34.Ajzen I, Fishbein M. Understanding attitudes and predicting social behavior. Englewood Cliffs, N.J: Prentice-Hall; 1980. [Google Scholar]
- 35.Birchall JC, Clemo R, Anstey A, John DN. Microneedles in clinical practice--an exploratory study into the opinions of healthcare professionals and the public. Pharm Res. 2011;28:95–106. doi: 10.1007/s11095-010-0101-2. [DOI] [PubMed] [Google Scholar]
- 36.Mekmullica J, Pancharoen C. Acceptability of oral typhoid vaccine in Thai children. Southeast Asian J Trop Med Public Heal. 2003;34:334–6. [PubMed] [Google Scholar]
- 37.Coleman BL, McGeer AJ, Halperin SA, Langley JM, Shamout Y, Taddio A, et al. A randomized control trial comparing immunogenicity, safety, and preference for self- versus nurse-administered intradermal influenza vaccine. Vaccine. 2012;30:6287–93. doi: 10.1016/j.vaccine.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Centers for Medicare & Medicaid Services. [Accessed Dec 1, 2013];Seasonal Influenza Vaccines Pricing. 2013 http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/VaccinesPricing.html.
- 39.Centers for Medicare & Medicaid Services. [Accessed Dec 1, 2013];Physician Fee Schedule Search - CPT 90471. 2013 http://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx.
- 40.Sander B, Kwong JC, Bauch CT, Maetzel A, McGeer A, Raboud JM, et al. Economic appraisal of Ontario’s Universal Influenza Immunization Program: a cost-utility analysis. PLoS Med. 2010;7:e1000256. doi: 10.1371/journal.pmed.1000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edens C, Collins ML, Ayers J, Rota PA, Prausnitz MR. Measles vaccination using a microneedle patch. Vaccine. 2013;31:3403–9. doi: 10.1016/j.vaccine.2012.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.