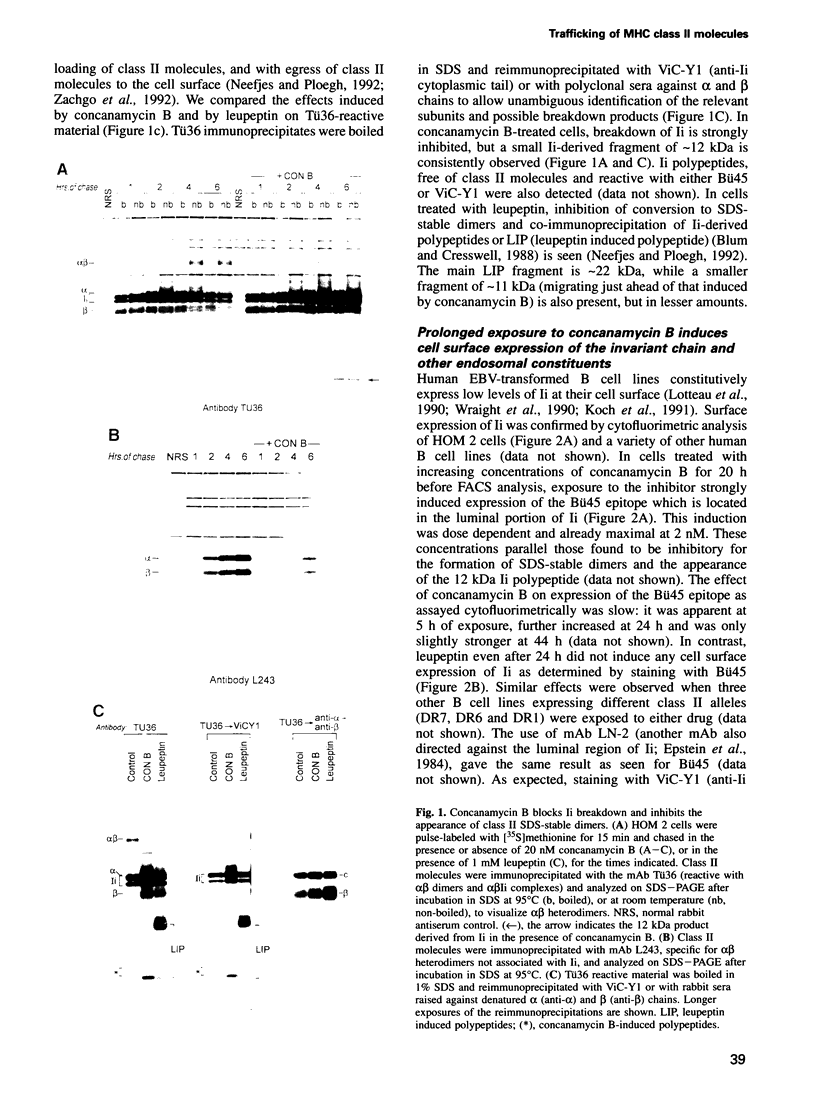
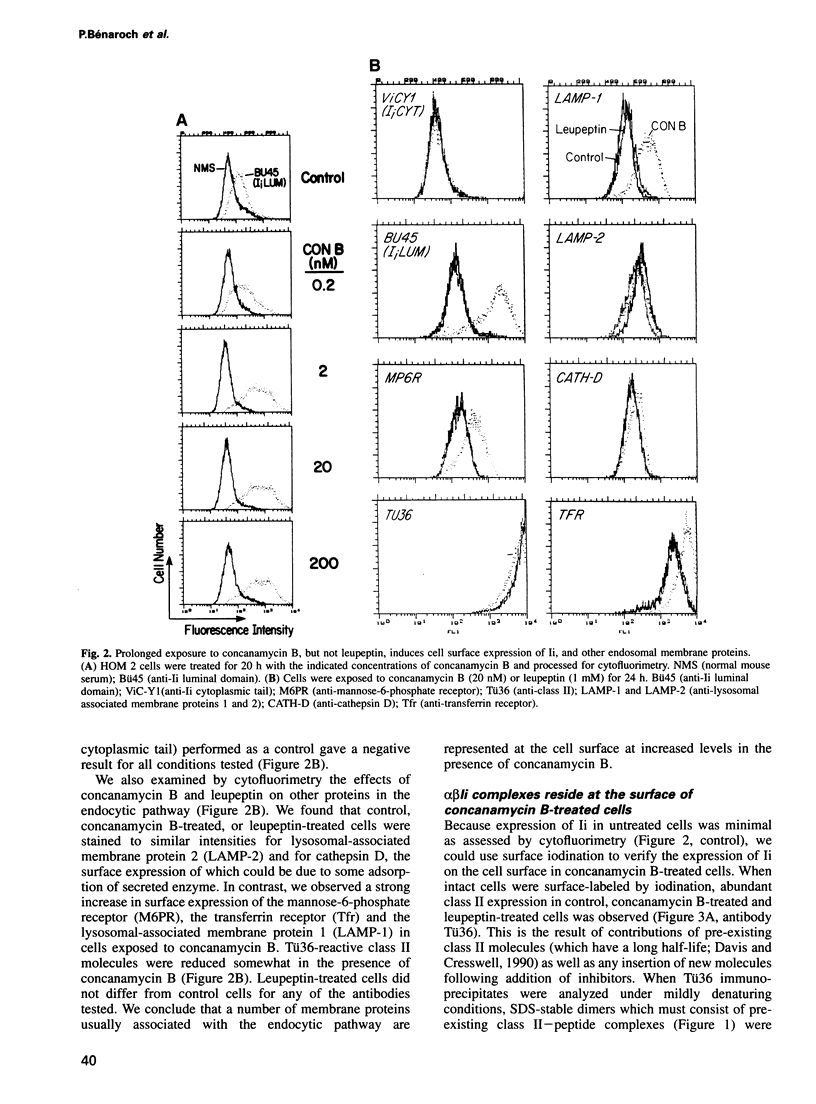
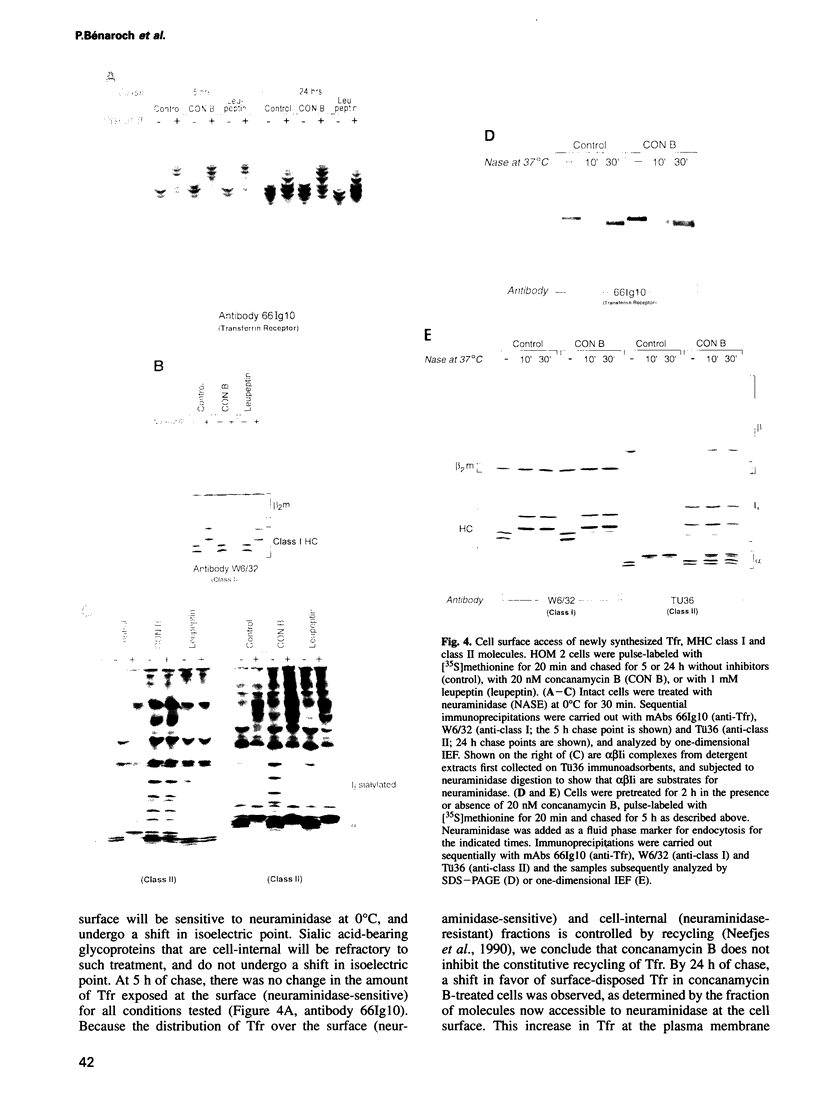
Abstract
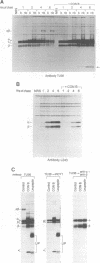
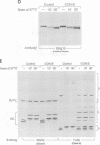
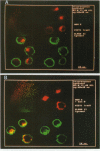
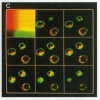
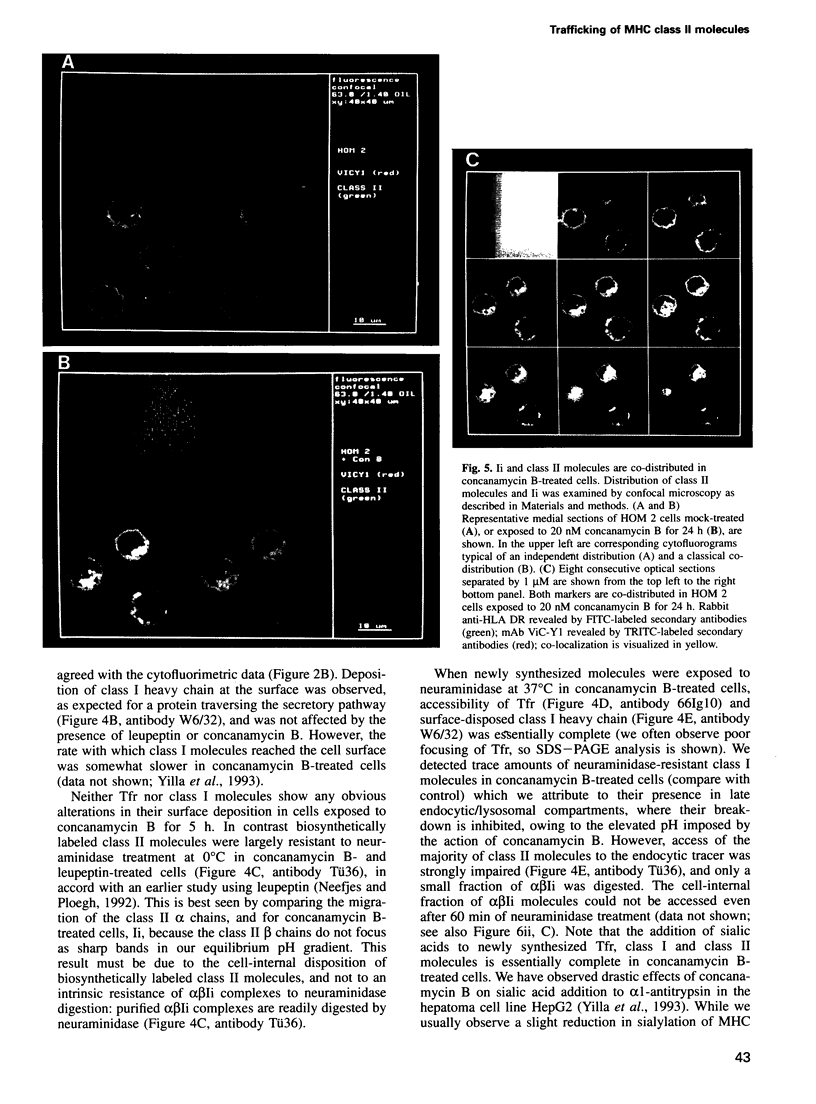
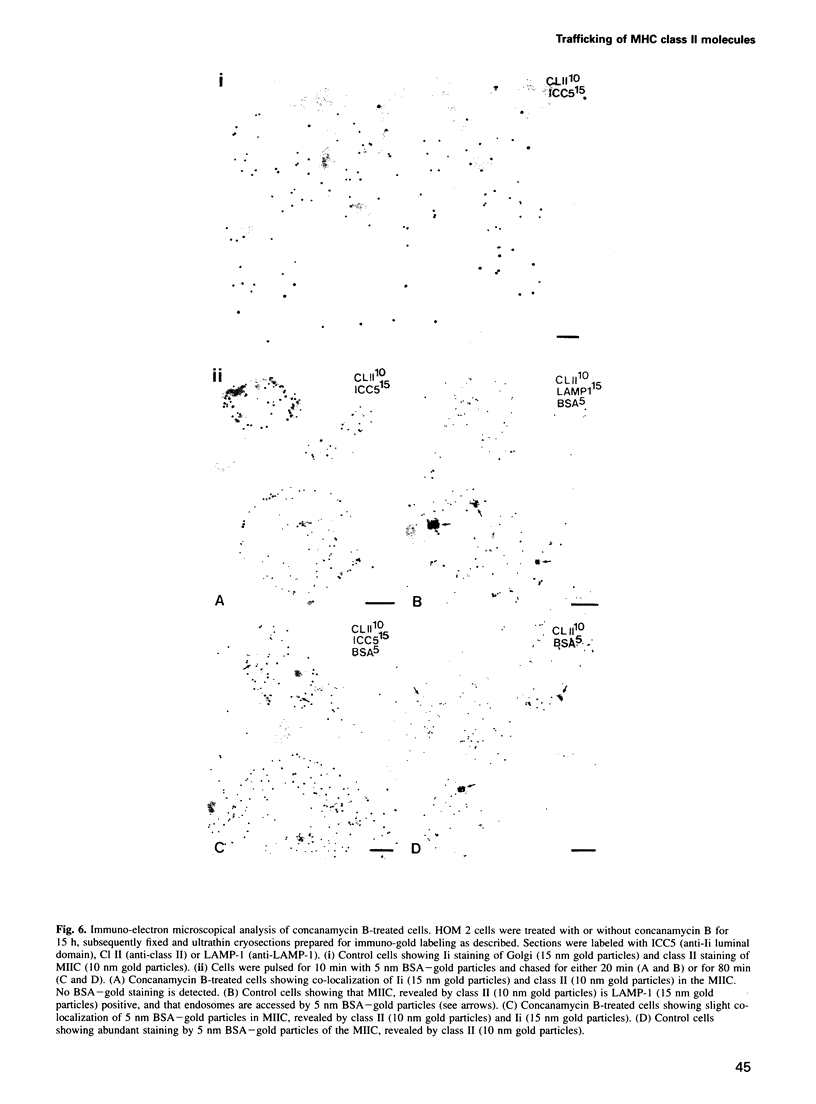
We have examined trafficking of major histocompatibility complex (MHC) class II molecules in human B cells exposed to concanamycin B, a highly specific inhibitor of the vacuolar H(+)-ATPases required for acidification of the vacuolar system and for early to late endosomal transport. Neutralization of vacuolar compartments prevents breakdown of the invariant chain (Ii) and blocks conversion of MHC class II molecules to peptide-loaded, SDS-stable alpha beta dimers. Ii remains associated with alpha beta and this complex accumulates internally, as ascertained biochemically and by morphological methods. In concanamycin B-treated cells, a slow increase (> 20-fold) in surface expression of Ii, mostly complexed with alpha beta, is detected. This surface-disposed fraction of alpha beta Ii is nevertheless a minor population that reaches the cell surface directly, or is routed via early endosomes as intermediary stations. In inhibitor-treated cells, the bulk of newly synthesized alpha beta Ii is no longer accessible to fluid phase endocytic markers. It is concluded that the majority of alpha beta Ii is targeted directly from the trans-Golgi network to the compartment for peptide loading, bypassing the cell surface and early endosomes en route to the endocytic pathway.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amigorena S., Drake J. R., Webster P., Mellman I. Transient accumulation of new class II MHC molecules in a novel endocytic compartment in B lymphocytes. Nature. 1994 May 12;369(6476):113–120. doi: 10.1038/369113a0. [DOI] [PubMed] [Google Scholar]
- Bakke O., Dobberstein B. MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell. 1990 Nov 16;63(4):707–716. doi: 10.1016/0092-8674(90)90137-4. [DOI] [PubMed] [Google Scholar]
- Banta L. M., Robinson J. S., Klionsky D. J., Emr S. D. Organelle assembly in yeast: characterization of yeast mutants defective in vacuolar biogenesis and protein sorting. J Cell Biol. 1988 Oct;107(4):1369–1383. doi: 10.1083/jcb.107.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barasch J., Kiss B., Prince A., Saiman L., Gruenert D., al-Awqati Q. Defective acidification of intracellular organelles in cystic fibrosis. Nature. 1991 Jul 4;352(6330):70–73. doi: 10.1038/352070a0. [DOI] [PubMed] [Google Scholar]
- Bijlmakers M. J., Benaroch P., Ploegh H. L. Assembly of HLA DR1 molecules translated in vitro: binding of peptide in the endoplasmic reticulum precludes association with invariant chain. EMBO J. 1994 Jun 1;13(11):2699–2707. doi: 10.1002/j.1460-2075.1994.tb06560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijlmakers M. J., Benaroch P., Ploegh H. L. Mapping functional regions in the lumenal domain of the class II-associated invariant chain. J Exp Med. 1994 Aug 1;180(2):623–629. doi: 10.1084/jem.180.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikoff E. K., Huang L. Y., Episkopou V., van Meerwijk J., Germain R. N., Robertson E. J. Defective major histocompatibility complex class II assembly, transport, peptide acquisition, and CD4+ T cell selection in mice lacking invariant chain expression. J Exp Med. 1993 Jun 1;177(6):1699–1712. doi: 10.1084/jem.177.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum J. S., Cresswell P. Role for intracellular proteases in the processing and transport of class II HLA antigens. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3975–3979. doi: 10.1073/pnas.85.11.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman E. J., Siebers A., Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun M., Waheed A., von Figura K. Lysosomal acid phosphatase is transported to lysosomes via the cell surface. EMBO J. 1989 Dec 1;8(12):3633–3640. doi: 10.1002/j.1460-2075.1989.tb08537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke B., Matlin K., Bause E., Legler G., Peyrieras N., Ploegh H. Inhibition of N-linked oligosaccharide trimming does not interfere with surface expression of certain integral membrane proteins. EMBO J. 1984 Mar;3(3):551–556. doi: 10.1002/j.1460-2075.1984.tb01845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. E., Munro S. Retrieval of TGN proteins from the cell surface requires endosomal acidification. EMBO J. 1994 May 15;13(10):2305–2312. doi: 10.1002/j.1460-2075.1994.tb06514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicz R. M., Urban R. G., Lane W. S., Gorga J. C., Stern L. J., Vignali D. A., Strominger J. L. Predominant naturally processed peptides bound to HLA-DR1 are derived from MHC-related molecules and are heterogeneous in size. Nature. 1992 Aug 27;358(6389):764–768. doi: 10.1038/358764a0. [DOI] [PubMed] [Google Scholar]
- Clague M. J., Urbé S., Aniento F., Gruenberg J. Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J Biol Chem. 1994 Jan 7;269(1):21–24. [PubMed] [Google Scholar]
- Cresswell P. Assembly, transport, and function of MHC class II molecules. Annu Rev Immunol. 1994;12:259–293. doi: 10.1146/annurev.iy.12.040194.001355. [DOI] [PubMed] [Google Scholar]
- Davidson H. W., Reid P. A., Lanzavecchia A., Watts C. Processed antigen binds to newly synthesized MHC class II molecules in antigen-specific B lymphocytes. Cell. 1991 Oct 4;67(1):105–116. doi: 10.1016/0092-8674(91)90575-j. [DOI] [PubMed] [Google Scholar]
- Davis J. E., Cresswell P. Lack of detectable endocytosis of B lymphocyte MHC class II antigens using an antibody-independent technique. J Immunol. 1990 Feb 1;144(3):990–997. [PubMed] [Google Scholar]
- Elliott E. A., Drake J. R., Amigorena S., Elsemore J., Webster P., Mellman I., Flavell R. A. The invariant chain is required for intracellular transport and function of major histocompatibility complex class II molecules. J Exp Med. 1994 Feb 1;179(2):681–694. doi: 10.1084/jem.179.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein A. L., Marder R. J., Winter J. N., Fox R. I. Two new monoclonal antibodies (LN-1, LN-2) reactive in B5 formalin-fixed, paraffin-embedded tissues with follicular center and mantle zone human B lymphocytes and derived tumors. J Immunol. 1984 Aug;133(2):1028–1036. [PubMed] [Google Scholar]
- Fukuda M. Lysosomal membrane glycoproteins. Structure, biosynthesis, and intracellular trafficking. J Biol Chem. 1991 Nov 15;266(32):21327–21330. [PubMed] [Google Scholar]
- Fukuda M., Viitala J., Matteson J., Carlsson S. R. Cloning of cDNAs encoding human lysosomal membrane glycoproteins, h-lamp-1 and h-lamp-2. Comparison of their deduced amino acid sequences. J Biol Chem. 1988 Dec 15;263(35):18920–18928. [PubMed] [Google Scholar]
- Germain R. N., Hendrix L. R. MHC class II structure, occupancy and surface expression determined by post-endoplasmic reticulum antigen binding. Nature. 1991 Sep 12;353(6340):134–139. doi: 10.1038/353134a0. [DOI] [PubMed] [Google Scholar]
- Geuze H. J., Stoorvogel W., Strous G. J., Slot J. W., Bleekemolen J. E., Mellman I. Sorting of mannose 6-phosphate receptors and lysosomal membrane proteins in endocytic vesicles. J Cell Biol. 1988 Dec;107(6 Pt 2):2491–2501. doi: 10.1083/jcb.107.6.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S., Anderson R. G., Russell D. W., Schneider W. J. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Simons K. The trans Golgi network: sorting at the exit site of the Golgi complex. Science. 1986 Oct 24;234(4775):438–443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- Harding C. V., Geuze H. J. Class II MHC molecules are present in macrophage lysosomes and phagolysosomes that function in the phagocytic processing of Listeria monocytogenes for presentation to T cells. J Cell Biol. 1992 Nov;119(3):531–542. doi: 10.1083/jcb.119.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter C., Mellman I. Transport of the lysosomal membrane glycoprotein lgp120 (lgp-A) to lysosomes does not require appearance on the plasma membrane. J Cell Biol. 1992 Apr;117(2):311–325. doi: 10.1083/jcb.117.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinashi H., Someno K., Sakaguchi K. Isolation and characterization of concanamycins A, B and C. J Antibiot (Tokyo) 1984 Nov;37(11):1333–1343. doi: 10.7164/antibiotics.37.1333. [DOI] [PubMed] [Google Scholar]
- Klionsky D. J., Emr S. D. Membrane protein sorting: biosynthesis, transport and processing of yeast vacuolar alkaline phosphatase. EMBO J. 1989 Aug;8(8):2241–2250. doi: 10.1002/j.1460-2075.1989.tb08348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D. J., Herman P. K., Emr S. D. The fungal vacuole: composition, function, and biogenesis. Microbiol Rev. 1990 Sep;54(3):266–292. doi: 10.1128/mr.54.3.266-292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D. J., Nelson H., Nelson N. Compartment acidification is required for efficient sorting of proteins to the vacuole in Saccharomyces cerevisiae. J Biol Chem. 1992 Feb 15;267(5):3416–3422. [PubMed] [Google Scholar]
- Knudsen P. J., Strominger J. L. A monoclonal antibody that recognizes the alpha chain of HLA-DR antigens. Hum Immunol. 1986 Feb;15(2):150–163. doi: 10.1016/0198-8859(86)90023-6. [DOI] [PubMed] [Google Scholar]
- Koch N., Moldenhauer G., Hofmann W. J., Möller P. Rapid intracellular pathway gives rise to cell surface expression of the MHC class II-associated invariant chain (CD74). J Immunol. 1991 Oct 15;147(8):2643–2651. [PubMed] [Google Scholar]
- Kornfeld S., Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Kvist S., Wiman K., Claesson L., Peterson P. A., Dobberstein B. Membrane insertion and oligomeric assembly of HLA-DR histocompatibility antigens. Cell. 1982 May;29(1):61–69. doi: 10.1016/0092-8674(82)90090-3. [DOI] [PubMed] [Google Scholar]
- Lamb C. A., Cresswell P. Assembly and transport properties of invariant chain trimers and HLA-DR-invariant chain complexes. J Immunol. 1992 Jun 1;148(11):3478–3482. [PubMed] [Google Scholar]
- Lampson L. A., Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980 Jul;125(1):293–299. [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Fambrough D. M. Lysosomal membrane dynamics: structure and interorganellar movement of a major lysosomal membrane glycoprotein. J Cell Biol. 1986 May;102(5):1593–1605. doi: 10.1083/jcb.102.5.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loss G. E., Jr, Sant A. J. Invariant chain retains MHC class II molecules in the endocytic pathway. J Immunol. 1993 Apr 15;150(8 Pt 1):3187–3197. [PubMed] [Google Scholar]
- Lotteau V., Teyton L., Peleraux A., Nilsson T., Karlsson L., Schmid S. L., Quaranta V., Peterson P. A. Intracellular transport of class II MHC molecules directed by invariant chain. Nature. 1990 Dec 13;348(6302):600–605. doi: 10.1038/348600a0. [DOI] [PubMed] [Google Scholar]
- Machamer C. E., Cresswell P. Biosynthesis and glycosylation of the invariant chain associated with HLA-DR antigens. J Immunol. 1982 Dec;129(6):2564–2569. [PubMed] [Google Scholar]
- Mathews P. M., Martinie J. B., Fambrough D. M. The pathway and targeting signal for delivery of the integral membrane glycoprotein LEP100 to lysosomes. J Cell Biol. 1992 Sep;118(5):1027–1040. doi: 10.1083/jcb.118.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I., Simons K. The Golgi complex: in vitro veritas? Cell. 1992 Mar 6;68(5):829–840. doi: 10.1016/0092-8674(92)90027-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama Y., Nelson N. H+-translocating ATPase in Golgi apparatus. Characterization as vacuolar H+-ATPase and its subunit structures. J Biol Chem. 1989 Nov 5;264(31):18445–18450. [PubMed] [Google Scholar]
- Nabi I. R., Le Bivic A., Fambrough D., Rodriguez-Boulan E. An endogenous MDCK lysosomal membrane glycoprotein is targeted basolaterally before delivery to lysosomes. J Cell Biol. 1991 Dec;115(6):1573–1584. doi: 10.1083/jcb.115.6.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neefjes J. J., Doxiadis I., Stam N. J., Beckers C. J., Ploegh H. L. An analysis of class I antigens of man and other species by one-dimensional IEF and immunoblotting. Immunogenetics. 1986;23(3):164–171. doi: 10.1007/BF00373817. [DOI] [PubMed] [Google Scholar]
- Neefjes J. J., Ploegh H. L. Inhibition of endosomal proteolytic activity by leupeptin blocks surface expression of MHC class II molecules and their conversion to SDS resistance alpha beta heterodimers in endosomes. EMBO J. 1992 Feb;11(2):411–416. doi: 10.1002/j.1460-2075.1992.tb05069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neefjes J. J., Stollorz V., Peters P. J., Geuze H. J., Ploegh H. L. The biosynthetic pathway of MHC class II but not class I molecules intersects the endocytic route. Cell. 1990 Apr 6;61(1):171–183. doi: 10.1016/0092-8674(90)90224-3. [DOI] [PubMed] [Google Scholar]
- Nelson H., Nelson N. Disruption of genes encoding subunits of yeast vacuolar H(+)-ATPase causes conditional lethality. Proc Natl Acad Sci U S A. 1990 May;87(9):3503–3507. doi: 10.1073/pnas.87.9.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N. Structure and pharmacology of the proton-ATPases. Trends Pharmacol Sci. 1991 Feb;12(2):71–75. doi: 10.1016/0165-6147(91)90501-i. [DOI] [PubMed] [Google Scholar]
- Newcomb J. R., Cresswell P. Characterization of endogenous peptides bound to purified HLA-DR molecules and their absence from invariant chain-associated alpha beta dimers. J Immunol. 1993 Jan 15;150(2):499–507. [PubMed] [Google Scholar]
- Nowell J., Quaranta V. Chloroquine affects biosynthesis of Ia molecules by inhibiting dissociation of invariant (gamma) chains from alpha-beta dimers in B cells. J Exp Med. 1985 Oct 1;162(4):1371–1376. doi: 10.1084/jem.162.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi C. G., Trowbridge I. S., Xue L., Hopkins C. R., Davis C. D., Collawn J. F. Sorting signals in the MHC class II invariant chain cytoplasmic tail and transmembrane region determine trafficking to an endocytic processing compartment. J Cell Biol. 1994 Jul;126(2):317–330. doi: 10.1083/jcb.126.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham P., Barnstable C. J., Bodmer W. F. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J Immunol. 1979 Jul;123(1):342–349. [PubMed] [Google Scholar]
- Peters C., Braun M., Weber B., Wendland M., Schmidt B., Pohlmann R., Waheed A., von Figura K. Targeting of a lysosomal membrane protein: a tyrosine-containing endocytosis signal in the cytoplasmic tail of lysosomal acid phosphatase is necessary and sufficient for targeting to lysosomes. EMBO J. 1990 Nov;9(11):3497–3506. doi: 10.1002/j.1460-2075.1990.tb07558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters P. J., Neefjes J. J., Oorschot V., Ploegh H. L., Geuze H. J. Segregation of MHC class II molecules from MHC class I molecules in the Golgi complex for transport to lysosomal compartments. Nature. 1991 Feb 21;349(6311):669–676. doi: 10.1038/349669a0. [DOI] [PubMed] [Google Scholar]
- Pieters J., Bakke O., Dobberstein B. The MHC class II-associated invariant chain contains two endosomal targeting signals within its cytoplasmic tail. J Cell Sci. 1993 Nov;106(Pt 3):831–846. doi: 10.1242/jcs.106.3.831. [DOI] [PubMed] [Google Scholar]
- Qiu Y., Xu X., Wandinger-Ness A., Dalke D. P., Pierce S. K. Separation of subcellular compartments containing distinct functional forms of MHC class II. J Cell Biol. 1994 May;125(3):595–605. doi: 10.1083/jcb.125.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaranta V., Majdic O., Stingl G., Liszka K., Honigsmann H., Knapp W. A human Ia cytoplasmic determinant located on multiple forms of invariant chain (gamma, gamma 2, gamma 3). J Immunol. 1984 Apr;132(4):1900–1905. [PubMed] [Google Scholar]
- Riberdy J. M., Newcomb J. R., Surman M. J., Barbosa J. A., Cresswell P. HLA-DR molecules from an antigen-processing mutant cell line are associated with invariant chain peptides. Nature. 1992 Dec 3;360(6403):474–477. doi: 10.1038/360474a0. [DOI] [PubMed] [Google Scholar]
- Roche P. A., Cresswell P. Proteolysis of the class II-associated invariant chain generates a peptide binding site in intracellular HLA-DR molecules. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3150–3154. doi: 10.1073/pnas.88.8.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche P. A., Marks M. S., Cresswell P. Formation of a nine-subunit complex by HLA class II glycoproteins and the invariant chain. Nature. 1991 Dec 5;354(6352):392–394. doi: 10.1038/354392a0. [DOI] [PubMed] [Google Scholar]
- Roche P. A., Teletski C. L., Stang E., Bakke O., Long E. O. Cell surface HLA-DR-invariant chain complexes are targeted to endosomes by rapid internalization. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8581–8585. doi: 10.1073/pnas.90.18.8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval I. V., Bakke O. Targeting of membrane proteins to endosomes and lysosomes. Trends Cell Biol. 1994 Aug;4(8):292–297. doi: 10.1016/0962-8924(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Shaw S., Ziegler A., DeMars R. Specificity of monoclonal antibodies directed against human and murine class II histocompatibility antigens as analyzed by binding to HLA-deletion mutant cell lines. Hum Immunol. 1985 Apr;12(4):191–211. doi: 10.1016/0198-8859(85)90336-2. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Kaufman J. F., Siddoway L. A., Mann D. L., Strominger J. L. Purification of HLA-linked B lymphocyte alloantigens in immunologically active form by preparative sodium dodecyl sulfate-gel electrophoresis and studies on their subunit association. J Biol Chem. 1977 Sep 10;252(17):6201–6207. [PubMed] [Google Scholar]
- Stern L. J., Wiley D. C. The human class II MHC protein HLA-DR1 assembles as empty alpha beta heterodimers in the absence of antigenic peptide. Cell. 1992 Feb 7;68(3):465–477. doi: 10.1016/0092-8674(92)90184-e. [DOI] [PubMed] [Google Scholar]
- Teyton L., O'Sullivan D., Dickson P. W., Lotteau V., Sette A., Fink P., Peterson P. A. Invariant chain distinguishes between the exogenous and endogenous antigen presentation pathways. Nature. 1990 Nov 1;348(6296):39–44. doi: 10.1038/348039a0. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Collawn J. F., Hopkins C. R. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- Tulp A., Verwoerd D., Dobberstein B., Ploegh H. L., Pieters J. Isolation and characterization of the intracellular MHC class II compartment. Nature. 1994 May 12;369(6476):120–126. doi: 10.1038/369120a0. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Cellular studies on antigen presentation by class II MHC molecules. Curr Opin Immunol. 1992 Feb;4(1):63–69. doi: 10.1016/0952-7915(92)90127-z. [DOI] [PubMed] [Google Scholar]
- Viville S., Neefjes J., Lotteau V., Dierich A., Lemeur M., Ploegh H., Benoist C., Mathis D. Mice lacking the MHC class II-associated invariant chain. Cell. 1993 Feb 26;72(4):635–648. doi: 10.1016/0092-8674(93)90081-z. [DOI] [PubMed] [Google Scholar]
- West M. A., Lucocq J. M., Watts C. Antigen processing and class II MHC peptide-loading compartments in human B-lymphoblastoid cells. Nature. 1994 May 12;369(6476):147–151. doi: 10.1038/369147a0. [DOI] [PubMed] [Google Scholar]
- Woo J. T., Shinohara C., Sakai K., Hasumi K., Endo A. Inhibition of the acidification of endosomes and lysosomes by the antibiotic concanamycin B in macrophage J774. Eur J Biochem. 1992 Jul 1;207(1):383–389. doi: 10.1111/j.1432-1033.1992.tb17061.x. [DOI] [PubMed] [Google Scholar]
- Wraight C. J., van Endert P., Möller P., Lipp J., Ling N. R., MacLennan I. C., Koch N., Moldenhauer G. Human major histocompatibility complex class II invariant chain is expressed on the cell surface. J Biol Chem. 1990 Apr 5;265(10):5787–5792. [PubMed] [Google Scholar]
- Yilla M., Tan A., Ito K., Miwa K., Ploegh H. L. Involvement of the vacuolar H(+)-ATPases in the secretory pathway of HepG2 cells. J Biol Chem. 1993 Sep 5;268(25):19092–19100. [PubMed] [Google Scholar]
- Zachgo S., Dobberstein B., Griffiths G. A block in degradation of MHC class II-associated invariant chain correlates with a reduction in transport from endosome carrier vesicles to the prelysosome compartment. J Cell Sci. 1992 Nov;103(Pt 3):811–822. doi: 10.1242/jcs.103.3.811. [DOI] [PubMed] [Google Scholar]
- Zeuzem S., Feick P., Zimmermann P., Haase W., Kahn R. A., Schulz I. Intravesicular acidification correlates with binding of ADP-ribosylation factor to microsomal membranes. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6619–6623. doi: 10.1073/pnas.89.14.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn M., Geurts van Kessel A. H., Kroezen V., van Agthoven A. J., Verstijnen K., Terhorst C., Hilgers J. Localization of a gene controlling the expression of the human transferrin receptor to the region q12 leads to qter of chromosome 3. Cytogenet Cell Genet. 1983;36(3):525–531. doi: 10.1159/000131967. [DOI] [PubMed] [Google Scholar]