Abstract
We have monitored L-type Ca2+ channel activity, local cytoplasmic Ca2+ transients, the distribution of insulin-containing secretory granules and exocytosis in individual mouse pancreatic B-cells. Subsequent to the opening of the Ca2+ channels, exocytosis is initiated with a latency < 100 ms. The entry of Ca2+ that precedes exocytosis is unevenly distributed over the cell and is concentrated to the region with the highest density of secretory granules. In this region, the cytoplasmic Ca2+ concentration is 5- to 10-fold higher than in the remainder of the cell reaching concentrations of several micromolar. Single-channel recordings confirm that the L-type Ca2+ channels are clustered in the part of the cell containing the secretory granules. This arrangement, which is obviously reminiscent of the 'active zones' in nerve terminals, can be envisaged as being favourable to the B-cell as it ensures that the Ca2+ transient is maximal and restricted to the part of the cell where it is required to rapidly initiate exocytosis whilst at the same time minimizing the expenditure of metabolic energy to subsequently restore the resting Ca2+ concentration.
Full text
PDF

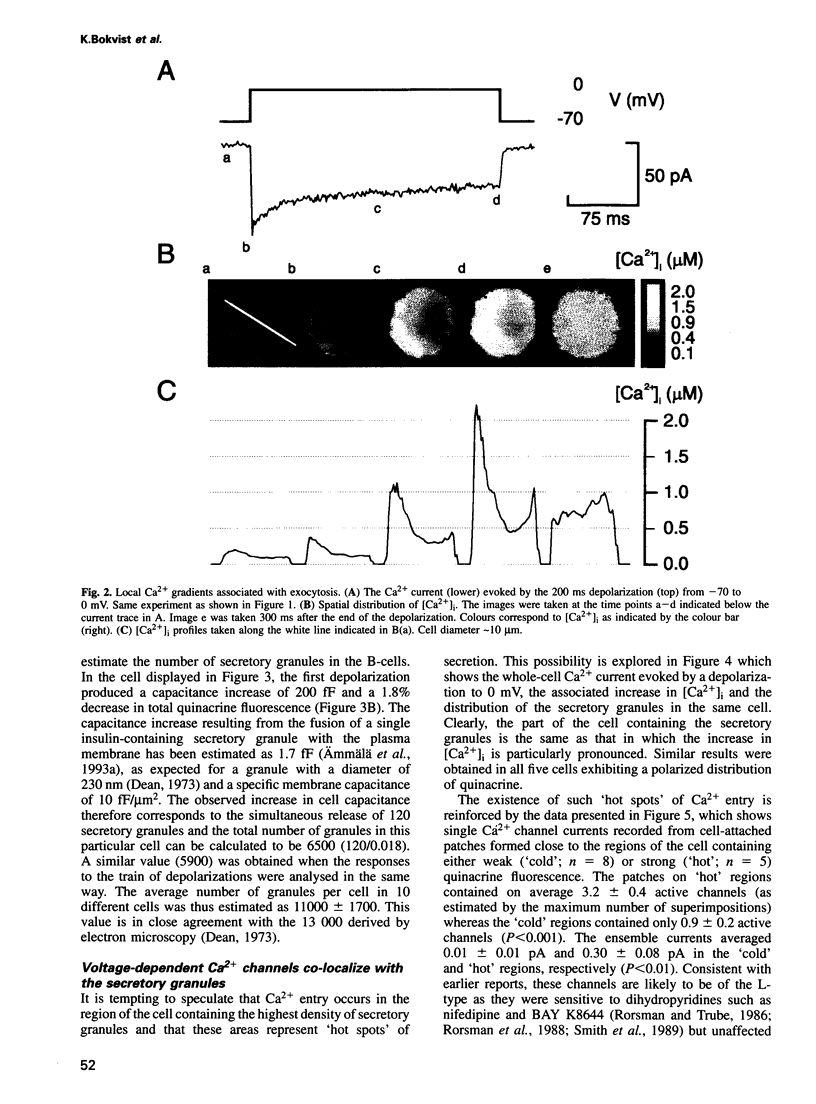

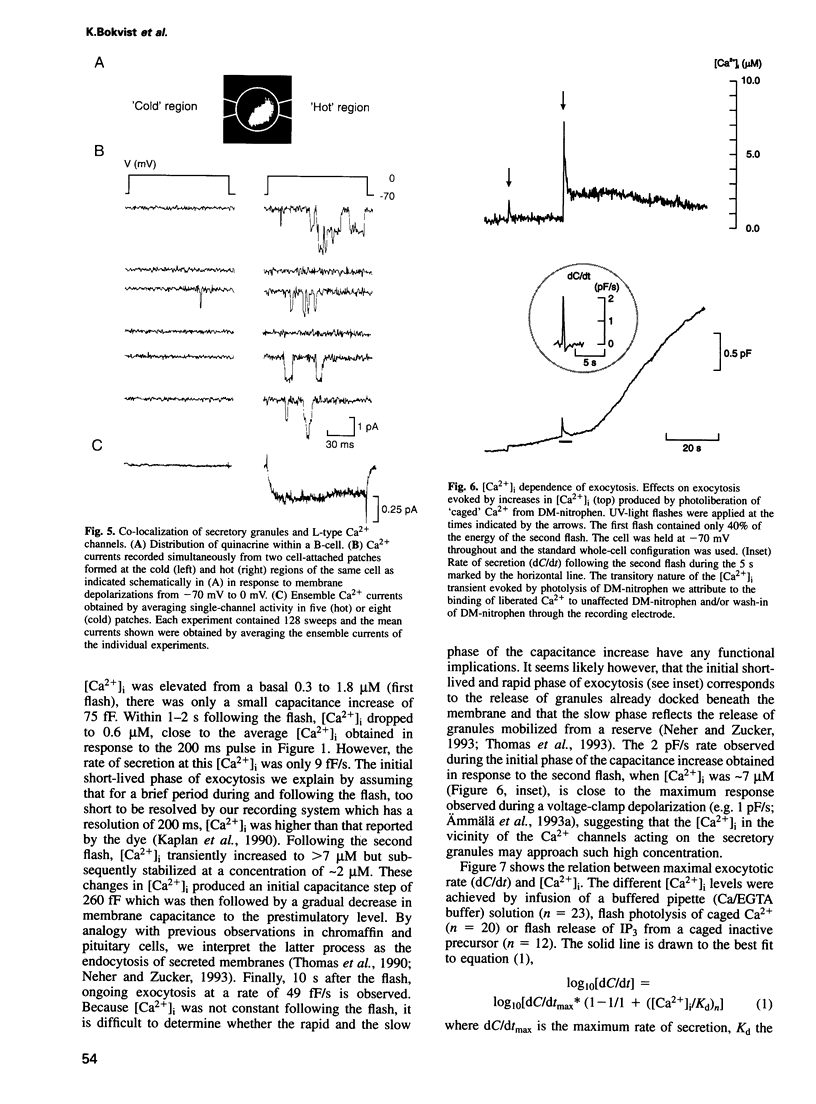
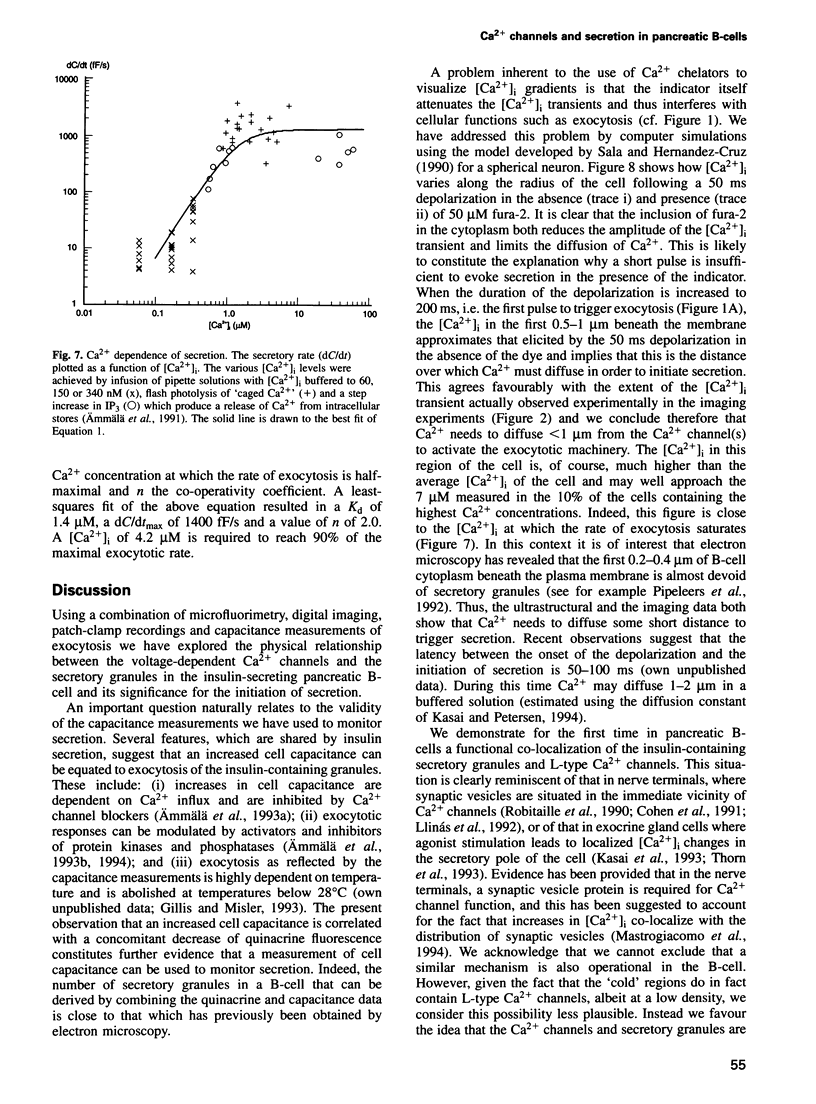


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamsson H., Gylfe E. Demonstration of a proton gradient across the insulin granule membrane. Acta Physiol Scand. 1980 May;109(1):113–114. doi: 10.1111/j.1748-1716.1980.tb06573.x. [DOI] [PubMed] [Google Scholar]
- Ammälä C., Ashcroft F. M., Rorsman P. Calcium-independent potentiation of insulin release by cyclic AMP in single beta-cells. Nature. 1993 May 27;363(6427):356–358. doi: 10.1038/363356a0. [DOI] [PubMed] [Google Scholar]
- Ammälä C., Eliasson L., Bokvist K., Berggren P. O., Honkanen R. E., Sjöholm A., Rorsman P. Activation of protein kinases and inhibition of protein phosphatases play a central role in the regulation of exocytosis in mouse pancreatic beta cells. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4343–4347. doi: 10.1073/pnas.91.10.4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammälä C., Eliasson L., Bokvist K., Larsson O., Ashcroft F. M., Rorsman P. Exocytosis elicited by action potentials and voltage-clamp calcium currents in individual mouse pancreatic B-cells. J Physiol. 1993 Dec;472:665–688. doi: 10.1113/jphysiol.1993.sp019966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammälä C., Larsson O., Berggren P. O., Bokvist K., Juntti-Berggren L., Kindmark H., Rorsman P. Inositol trisphosphate-dependent periodic activation of a Ca(2+)-activated K+ conductance in glucose-stimulated pancreatic beta-cells. Nature. 1991 Oct 31;353(6347):849–852. doi: 10.1038/353849a0. [DOI] [PubMed] [Google Scholar]
- Atwater I., Ribalet B., Rojas E. Cyclic changes in potential and resistance of the beta-cell membrane induced by glucose in islets of Langerhans from mouse. J Physiol. 1978 May;278:117–139. doi: 10.1113/jphysiol.1978.sp012296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Neher E. Calcium requirements for secretion in bovine chromaffin cells. J Physiol. 1992 May;450:247–271. doi: 10.1113/jphysiol.1992.sp019126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner-Weir S. Morphological evidence for pancreatic polarity of beta-cell within islets of Langerhans. Diabetes. 1988 May;37(5):616–621. doi: 10.2337/diab.37.5.616. [DOI] [PubMed] [Google Scholar]
- Breckenridge L. J., Almers W. Final steps in exocytosis observed in a cell with giant secretory granules. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1945–1949. doi: 10.1073/pnas.84.7.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. W., Jones O. T., Angelides K. J. Distribution of Ca2+ channels on frog motor nerve terminals revealed by fluorescent omega-conotoxin. J Neurosci. 1991 Apr;11(4):1032–1039. doi: 10.1523/JNEUROSCI.11-04-01032.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. M. Ultrastructural morphometry of the pancreatic -cell. Diabetologia. 1973 Apr;9(2):115–119. doi: 10.1007/BF01230690. [DOI] [PubMed] [Google Scholar]
- Gillis K. D., Misler S. Enhancers of cytosolic cAMP augment depolarization-induced exocytosis from pancreatic B-cells: evidence for effects distal to Ca2+ entry. Pflugers Arch. 1993 Jul;424(2):195–197. doi: 10.1007/BF00374612. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gylfe E., Grapengiesser E., Hellman B. Propagation of cytoplasmic Ca2+ oscillations in clusters of pancreatic beta-cells exposed to glucose. Cell Calcium. 1991 Feb-Mar;12(2-3):229–240. doi: 10.1016/0143-4160(91)90023-8. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iatridou H., Foukaraki E., Kuhn M. A., Marcus E. M., Haugland R. P., Katerinopoulos H. E. The development of a new family of intracellular calcium probes. Cell Calcium. 1994 Feb;15(2):190–198. doi: 10.1016/0143-4160(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Joshi C., Fernandez J. M. Capacitance measurements. An analysis of the phase detector technique used to study exocytosis and endocytosis. Biophys J. 1988 Jun;53(6):885–892. doi: 10.1016/S0006-3495(88)83169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. H. Photochemical manipulation of divalent cation levels. Annu Rev Physiol. 1990;52:897–914. doi: 10.1146/annurev.ph.52.030190.004225. [DOI] [PubMed] [Google Scholar]
- Kasai H., Li Y. X., Miyashita Y. Subcellular distribution of Ca2+ release channels underlying Ca2+ waves and oscillations in exocrine pancreas. Cell. 1993 Aug 27;74(4):669–677. doi: 10.1016/0092-8674(93)90514-q. [DOI] [PubMed] [Google Scholar]
- Kasai H., Petersen O. H. Spatial dynamics of second messengers: IP3 and cAMP as long-range and associative messengers. Trends Neurosci. 1994 Mar;17(3):95–101. doi: 10.1016/0166-2236(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Silver R. B. Microdomains of high calcium concentration in a presynaptic terminal. Science. 1992 May 1;256(5057):677–679. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- Lundquist I., Ahrén B., Håkanson R., Sundler F. Quinacrine accumulation in pancreatic islet cells of rat and mouse: relationship to functional activity and effects on basal and stimulated insulin secretion. Diabetologia. 1985 Mar;28(3):161–166. doi: 10.1007/BF00273865. [DOI] [PubMed] [Google Scholar]
- Mastrogiacomo A., Parsons S. M., Zampighi G. A., Jenden D. J., Umbach J. A., Gundersen C. B. Cysteine string proteins: a potential link between synaptic vesicles and presynaptic Ca2+ channels. Science. 1994 Feb 18;263(5149):981–982. doi: 10.1126/science.7906056. [DOI] [PubMed] [Google Scholar]
- Neher E., Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Zucker R. S. Multiple calcium-dependent processes related to secretion in bovine chromaffin cells. Neuron. 1993 Jan;10(1):21–30. doi: 10.1016/0896-6273(93)90238-m. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Petersen C. C., Kasai H. Calcium and hormone action. Annu Rev Physiol. 1994;56:297–319. doi: 10.1146/annurev.ph.56.030194.001501. [DOI] [PubMed] [Google Scholar]
- Pralong W. F., Bartley C., Wollheim C. B. Single islet beta-cell stimulation by nutrients: relationship between pyridine nucleotides, cytosolic Ca2+ and secretion. EMBO J. 1990 Jan;9(1):53–60. doi: 10.1002/j.1460-2075.1990.tb08079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki M., Matschinsky F. M. Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev. 1987 Oct;67(4):1185–1248. doi: 10.1152/physrev.1987.67.4.1185. [DOI] [PubMed] [Google Scholar]
- Robitaille R., Adler E. M., Charlton M. P. Strategic location of calcium channels at transmitter release sites of frog neuromuscular synapses. Neuron. 1990 Dec;5(6):773–779. doi: 10.1016/0896-6273(90)90336-e. [DOI] [PubMed] [Google Scholar]
- Rorsman P., Ashcroft F. M., Trube G. Single Ca channel currents in mouse pancreatic B-cells. Pflugers Arch. 1988 Oct;412(6):597–603. doi: 10.1007/BF00583760. [DOI] [PubMed] [Google Scholar]
- Rorsman P., Trube G. Calcium and delayed potassium currents in mouse pancreatic beta-cells under voltage-clamp conditions. J Physiol. 1986 May;374:531–550. doi: 10.1113/jphysiol.1986.sp016096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala F., Hernández-Cruz A. Calcium diffusion modeling in a spherical neuron. Relevance of buffering properties. Biophys J. 1990 Feb;57(2):313–324. doi: 10.1016/S0006-3495(90)82533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher E., Biancardi E., Pollo A., Carbone E., Li G., Wollheim C. B., Clementi F. omega-Conotoxin-sensitive, voltage-operated Ca2+ channels in insulin-secreting cells. Eur J Pharmacol. 1992 Jun 17;216(3):407–414. doi: 10.1016/0014-2999(92)90438-a. [DOI] [PubMed] [Google Scholar]
- Smith P. A., Rorsman P., Ashcroft F. M. Modulation of dihydropyridine-sensitive Ca2+ channels by glucose metabolism in mouse pancreatic beta-cells. Nature. 1989 Nov 30;342(6249):550–553. doi: 10.1038/342550a0. [DOI] [PubMed] [Google Scholar]
- Theler J. M., Mollard P., Guérineau N., Vacher P., Pralong W. F., Schlegel W., Wollheim C. B. Video imaging of cytosolic Ca2+ in pancreatic beta-cells stimulated by glucose, carbachol, and ATP. J Biol Chem. 1992 Sep 5;267(25):18110–18117. [PubMed] [Google Scholar]
- Thomas P., Surprenant A., Almers W. Cytosolic Ca2+, exocytosis, and endocytosis in single melanotrophs of the rat pituitary. Neuron. 1990 Nov;5(5):723–733. doi: 10.1016/0896-6273(90)90226-6. [DOI] [PubMed] [Google Scholar]
- Thomas P., Wong J. G., Lee A. K., Almers W. A low affinity Ca2+ receptor controls the final steps in peptide secretion from pituitary melanotrophs. Neuron. 1993 Jul;11(1):93–104. doi: 10.1016/0896-6273(93)90274-u. [DOI] [PubMed] [Google Scholar]
- Thorn P., Lawrie A. M., Smith P. M., Gallacher D. V., Petersen O. H. Local and global cytosolic Ca2+ oscillations in exocrine cells evoked by agonists and inositol trisphosphate. Cell. 1993 Aug 27;74(4):661–668. doi: 10.1016/0092-8674(93)90513-p. [DOI] [PubMed] [Google Scholar]