Abstract
Signal transducer and activator of transcription 3 (STAT3) plays critical roles in tumorigenesis and malignant evolution and has been intensively studied as a therapeutic target for cancer. A number of STAT3 inhibitors have been evaluated for their antitumor activity in vitro and in vivo in experimental tumor models and several approved therapeutic agents have been reported to function as STAT3 inhibitors. Nevertheless, most STAT3 inhibitors have yet to be translated to clinical evaluation for cancer treatment, presumably because of pharmacokinetic, efficacy, and safety issues. In fact, a major cause of failure of anticancer drug development is lack of efficacy. Genetic interactions among various cancer-related pathways often provide redundant input from parallel and/or cooperative pathways that drives and maintains survival environments for cancer cells, leading to low efficacy of single-target agents. Exploiting genetic interactions of STAT3 with other cancer-related pathways may provide molecular insight into mechanisms of cancer resistance to pathway-targeted therapies and strategies for development of more effective anticancer agents and treatment regimens. This review focuses on functional regulation of STAT3 activity; possible interactions of the STAT3, RAS, epidermal growth factor receptor, and reduction-oxidation pathways; and molecular mechanisms that modulate therapeutic efficacies of STAT3 inhibitors.
Keywords: genetic interaction, cancer, drug development, STAT3, Ras, EGFR, redox, reactive oxygen species, synthetic lethality
1. Introduction
Signal transducer and activator of transcription 3 (STAT3) is known to promote tumor cell proliferation, survival, and invasion [1,2], mediate procarcinogenic inflammation while suppressing the host’s antitumor immunity [3], induce cancer stem cell renewal [4,5,6], enhance epithelial-mesenchymal transition [7,8] and angiogenesis [9,10], and generate positive autocrine and paracrine feedback loops between tumor cells and their microenvironments [11,12] by regulating expression of a number of cancer-related key proteins, cytokines, and growth factors. Ectopic expression of constitutive STAT3 is sufficient to induce transformation of rodent cells in vitro and tumor formation in vivo [3,13]. Constitutive activation of STAT3 has been reported in many human cancer cell lines and primary tumors, and this activation is associated with poor outcomes of a number of cancers. Inhibiting STAT3 expression or phosphorylation using antisense oligonucleotides and small-molecule inhibitors suppressed the growth of human and murine tumors in animal models [14,15,16], demonstrating that STAT3 is a potential target for cancer therapy. Substantial efforts have been devoted to developing strategies for pharmaceutical intervention directed toward STAT3 functions, including interrupting STAT3 dimerization and inhibiting its interaction with its upstream activating kinases or downstream DNA targets using oligonucleotides [17], peptides [18], and small-molecule inhibitors [19,20,21,22]. A number of STAT3 inhibitors have been identified and evaluated their antitumor activity in vitro and in vivo in experimental tumor models [23,24,25,26]. Moreover, several U.S. Food and Drug Administration (FDA)-approved therapeutic agents are reported to function as STAT3 inhibitors. For example, pyrimethamine, an antimalarial drug [27,28], inhibits STAT3 phosphorylation and is in clinical investigation for treatment of leukemia [26,29]. In addition, sorafenib, an inhibitor of RAF and multiple other kinases [30,31] approved for the treatment of advanced renal and liver cancer, inhibits STAT3 phosphorylation, possibly by activating the phosphatase shatterproof 2 (SHP2), as knockdown of SHP2 expression inhibited sorafenib-induced STAT3 phosphorylated Y705 (pY705) dephosphorylation [32,33]. Arsenic trioxide, an inorganic compound used to treat leukemia, inhibits STAT3 phosphorylation possibly by inhibiting its upstream kinases [34,35]. Furthermore, auranofin, a thioredoxin inhibitor that is used to treat rheumatoid arthritis [36], inhibits Janus kinase 1 (JAK1)/STAT3 phosphorylation [37,38]. However, most STAT3 inhibitors have yet to be translated to clinical trials for cancer treatment, presumably because of pharmacokinetic, efficacy, and safety issues.
Lack of therapeutic efficacy may be caused by low potency of the candidate drug in inhibiting its proposed target. Nevertheless, mutation analyses of primary cancer cells for genes encoding kinases or known to be associated with cancers have revealed that individual tumors may harbor multiple changes in such genes [39,40,41,42]. Several important signaling pathways are often cooperatively involved in tumorigenesis and malignant evolution of cancers [39,40,41,42,43]. As a result, interrupting just one of these pathways is often insufficient to induce cancer cell death in most cases because redundant input from different pathways drives and maintains downstream signaling, leading to low therapeutic efficacy because of inhibition of a single target [44,45]. Conceivably, agents that can modulate the functions of multiple cancer-related targets and/or pathways will improve the efficacy of cancer therapy because they are more likely to have a broad anticancer spectrum and less likely to induce therapy resistance than single-target anticancer agents. Indeed, multitarget agents such as sorafenib and sunitinib, which block several kinases, have proven to be useful clinically for cancer treatment and have a broader spectrum of activity than single-target agents such as erlotinib and gefitinib [46]. The knowledge on genetic interactions among cancer-associated pathways may facilitate development of multitarget agents or rational design of combinatorial therapy using single-target agents to enhance therapeutic efficacy. This review describes potential interactions of STAT3 with other cancer-associated pathways and molecular mechanisms that modulate therapeutic efficacies of STAT3 inhibitors.
2. STAT3-Associated Single-Gene Diseases
The human STAT3 gene is located on chromosome 17q21.31 and encodes two major isoforms of STAT3 proteins via alternative mRNA splicing: STAT3α (p92) and STAT3β (p83). A 55-residue C-terminal transactivation domain of STAT3α is deleted in STAT3β and replaced by seven unique C-terminal residues (CT7) whose functions remain undefined [47]. Both isoforms contain STAT protein interaction, DNA-binding, and Src homology 2 (SH2) domains, but only STAT3α contains a transactivation domain at the C-terminus.
Targeted disruption of the murine Stat3 gene leads to early embryonic lethality. Stat3-deficient embryos have exhibited rapid degeneration from embryonic day 6.5 to embryonic day 7.5 [48]. Ablation of isoform-specific gene expression demonstrated that Stat3β is not required for viability of mice but is involved in inflammation because mice with deficiency of Stat3β were viable and fertile; in contrast, Stat3α-deficient mice died within 24 h after birth [49]. In comparison with Stat3−/− mice that die at early stages of embryonic development [48], expression of Stat3β rescues the embryonic lethality of a Stat3-null mutation, and Stat3β alone can induce transient transcription of acute-phase genes, suggesting that although Stat3β does not have a transactivation domain, it may induce the expression of specific Stat3 target genes by interacting with other transcriptional factors. A study of green fluorescent protein-tagged Stat3α and Stat3β demonstrated that the two isoforms have distinct intracellular dynamics, with Stat3β exhibiting prolonged nuclear retention and reduced intranuclear mobility, especially following ligand stimulation, and prolonged nuclear retention but not reduced intranuclear mobility mapping to the CT7 domain of Stat3β [50].
De novo dominant-negative mutations in the DNA-binding domain of STAT3 have been identified in autosomal dominant or sporadic cases of hyperimmunoglobulinemia syndrome (HIES; or Job syndrome) in humans [51]. These mutations rendered patients’ peripheral blood cells defective in responding to interleukin (IL)-6 and IL-10 stimulation. De novo deficiency mutations of STAT3 have also occurred in the SH2 domain [52,53] and the transactivation domain [53,54] of STAT3 in patients with HIES from different ethnic groups [55]. These dominant-negative mutations in STAT3 impaired the development of IL-17-producing T cells, which is critical to the clearance of fungal and extracellular bacterial infections and may be the underlying mechanism of susceptibility to recurrent infections commonly seen in HIES patients [56,57,58].
There are only a few reports of STAT3 gene mutation in human cancer cells. In one report, a patient with HIES owing to STAT3 mutation had a subsequent primary parotid gland diffuse large B-cell lymphoma [59]. Because HIES patients are predisposed to lymphoma [60], STAT3 mutations may occur with other types of lymphoma in HIES patients. Mutations in the SH2 domain that lead to constitutive activation of STAT3 were recently reported in patients with human inflammatory hepatocellular adenoma, a benign liver tumor, suggesting that STAT3-activating mutations play roles in human tumorigenesis [61]. In contrast with STAT3 gene mutations, constitutive activation of STAT3 and/or STAT5 at the protein level has occurred in many human cancer cell lines and primary tumors. For example, persistent activation of STAT3 and STAT5 has been reported in breast cancer, lung cancer [62], glioma [63], liver cancer [15], pancreatic cancer [64,65], and nasopharyngeal carcinoma [66] cases. STAT3 also is activated in 77% of lymph node metastases and 67% of bone metastases of prostate cancer [67]. In addition, constitutive JAK3 and STAT5 activation has been observed in patients with T-cell leukemia caused by human T-cell leukemia virus type 1 [68]. High levels of STAT3 protein expression have been associated with poor tumor differentiation and/or development of metastasis and poor survival rates in leukemia [69], lymphoma [70], osteosarcoma [71,72], glioma [63], gastric adenocarcinoma [73,74], colorectal cancer [75,76], bladder cancer [77], and cervical squamous cell carcinoma [78] cases. These data demonstrate the critical roles of the STAT3 pathway in malignant progression.
3. Genetic Interactions and STAT3 Activation
Genetic interactions are functional cross-talks among genes, which regulate or compensate for one another in many signaling and/or metabolic pathways, leading to phenotypic changes, including disease status (synthetic sickness) and viability (synthetic lethality or semilethality) alterations. Genetic interactions have been used by investigators to identify genes that are crucial to the survival of certain oncogene-transformed cells [79,80,81,82] or that sensitize cells to chemotherapy [83,84] or to find small molecules that selectively induce the death of oncogene-transformed isogenic cells [85,86,87]. Several models have been proposed to account for these genetic interactions [88,89,90], including the components of parallel pathways that combine to regulate an essential biological function, subunits of an essential multiprotein complex, or components of a linear essential pathway (Figure 1) [91]. Thus, key components in the signaling pathways that regulate STAT3 functions or regulate parallel pathways involved in similar biological processes or common downstream targets as STAT3 may be partners of genetic interactions with STAT3. The status and functionality of these partners are critical determinants of cellular fate when the functionality of STAT3 is disrupted.
Figure 1.
Diagram of genetic interactions. (A) The essential biological function E is regulated by pathways A and B. A functional change in either of these pathways, such as a mutation in A1 or B1, is insufficient to induce dysfunction of E. However, the simultaneous presence of a mutation in A1 and a mutation in any of B1, B2, or B3 induces dysfunction of E (or phenotype changes). Thus, A1 has genetic interaction with B1, B2 and B3, and vice versa; (B) The essential biological function E is regulated by pathway A alone, in which A2 is a multiprotein complex composed of X, Y, and Z, while A3 has homologues of α, β and γ. Genetic interaction may exist among X, Y and Z, and among A3α, β and γ.
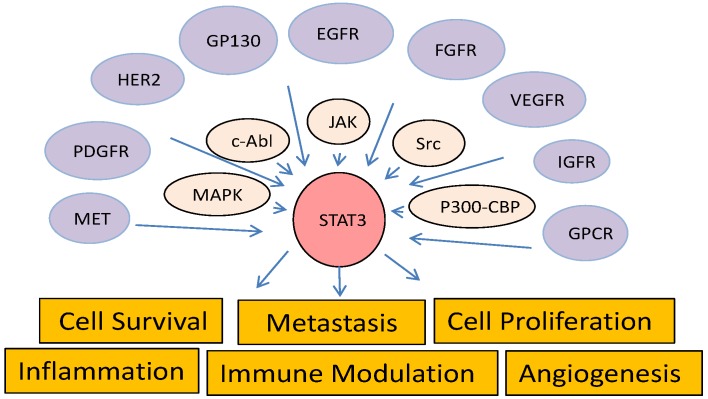
STAT3 is activated by tyrosine phosphorylation in response to stimulation of a variety of cytokines, such as IL-6, leptin, prolactin, erythropoietin, and thrombopoietin, and growth factors, such as epidermal growth factor (EGF), fibroblast growth factor, insulin-like growth factor, hepatocyte growth factor, platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF) [92,93,94,95,96]. Interaction of those ligands with their receptors in a cell triggers receptor phosphorylation by intrinsic receptor tyrosine kinases or non-receptor protein tyrosine kinases such as JAK and Src family members, resulting in translocation of STAT3 protein from the cytoplasm to the phosphorylated receptors and further STAT3 phosphorylation at Y705 by these kinases (Figure 2). Once phosphorylated, STAT3 forms dimers and translocates to the nucleus, where it activates expression of its target genes [97,98]. In addition, STAT3 has been identified in mitochondria [99] and some endosomes in the cytoplasm [100]. Mitochondrial localized STAT3 was independent of Y705 phosphorylation and DNA-binding activity but required for Ras-mediated oncogenic transformation. In contrast, enrichment of STAT3 in some endosomes is stimulated by IL-6 and dependent on pY705, co-localized with myeloid differentiation primary response gene 88 (MYD88), and enhanced by dominant-negative mutants of GTPase dynamin II [100]. Interestingly, a gain-of-function mutation of MYD88 that promotes cell survival via activation of IL-1 receptor-associated kinase 1, nuclear factor (NF)-κB, and JAK/STAT3 signaling is frequently found in cases of a subtype of B-cell lymphoma [101]. Knockdown of MYD88 expression has significantly inhibited secretion of IL-6 and phosphorylation of STAT3 in B-cell lymphoma cells.
Figure 2.
Diagram of STAT3 pathways. STAT3 is activated by upstream receptor tyrosine kinases, intracellular kinases, or histone acetyltransferases and regulates a diverse biological functions.
The activity of STAT3 is also regulated by serine phosphorylation and lysine acetylation. Phosphorylation of S727 in STAT3 by c-Jun N-terminal kinase (JNK) and extracellular signal-regulated kinase (ERK) may enhance or be required for STAT3/DNA interaction [102,103] and promote tumorigenesis independently of pY705 [102,104]. Blockage of S727 phosphorylation via substitution of the this serine with an alanine led to death soon after birth or growth retardation in transgenic mice [105]. Nevertheless, S727 phosphorylation also has negatively regulated Y705 phosphorylation [106,107]. Acetylation of K685 in STAT3 by CREB-binding protein/p300 is stimulated by IL-6 and interferon-α independently of Y705 and S727 phosphorylation and is required for STAT3 activation and nuclear accumulation [108,109]. This acetylation also mediates cross-talk between the STAT3 and NF-κB pathways [110]. A recent study demonstrated that multiple lysine-acetylation sites adjacent to Y705 are essential for pY705 phosphorylation in STAT3 [111].
Within cells, STAT3 activity is also regulated by several inhibitory molecules that prevent its continued activation. For example, tyrosine dephosphorylation of receptors and JAK receptor-associated phosphatases lead to inactivation of JAKs and prevent further STAT activation [112,113,114]. Histone deacetylases and NAD-dependent deacetylase sirtuin-1 inactivate STAT3 by reversing lysine acetylation [109,111,115]. Moreover, expression of STAT3-activated suppressor of cytokine signaling (SOCS) proteins [116,117] or cytokine-induced SH2 protein [118] provides negative feedback for JAK/STAT activation. SOCS proteins bind directly to and inhibit the activity of tyrosine-phosphorylated JAKs and cytokine receptors. Also, phosphorylated STAT proteins can be inactivated in the nucleus via dephosphorylation [119] or by interaction with the nuclear protein inhibitor of activated STAT [120,121] or cytoplasmic protein aplasia Ras homolog member I [122]. The latter protein was found to form a complex specifically with STAT3 in the cytoplasm, preventing IL-6-induced STAT3 accumulation in the nucleus and inhibiting STAT3-dependent promoter activity while only moderately affecting STAT3 phosphorylation [122]. In addition, tripartite motif 8 (TRIM8) interacts with protein inhibitor of activated STAT3 (PIAS3), which inhibits IL-6-ependent activation of STAT3. Ectopic expression of TRIM8 cancels the negative effect of PIAS3 on STAT3 activation via either degradation of PIAS3 in the ubiquitin/proteasome pathway or exclusion of PIAS3 from the nucleus.
The complexity of STAT3 activity regulation networks suggests that pharmaceutical inhibition of STAT3 activity can be achieved with a variety of mechanisms. Small-molecule inhibitors of upstream kinases JAK, Src, and Bcr-Abl are predicted to inhibit STAT3 activation. Indeed, the JAK inhibitors INCB16562, AZD1480, and tofacitinib (CP-690550) have potently blocked STAT3 signaling, suppressed cancer cell proliferation, or induced apoptosis in vitro and tumor growth in vivo [123,124,125]. Additionally, the JAK inhibitors ruxolitinib and tofacitinib have been approved for treatment of myelofibrosis [126,127,128] and rheumatoid arthritis [129,130], respectively. However, depletion of c-Src by small interfering RNA (siRNA) and sustained inhibition of Src by dasatinib led to JAK-dependent STAT3 activation in lung cancer cells in vitro and in vivo [131]. Phosphorylated STAT3 levels were initially decreased but strongly increased after sustained treatment with dasatinib, suggesting the existence of a compensatory feedback pathway that supports cancer cell survival by regulating STAT3 activity [132].
4. Cross-Talk of STAT3 with Other Cancer-Associated Pathways
4.1. RAS Pathway
As members of a subfamily of small guanine nucleotide-binding proteins, Ras proteins (HRAS, KRAS, and NRAS) cycle between active GTP-bound and inactive GDP-bound forms [133,134]. Binding of Ras with GTP is facilitated by guanine nucleotide exchange factors (GEFs) via catalysis of the release of GDP and is required for the interaction of Ras with target proteins [135]. Intrinsic GTPase activity enhanced by GTPase-activating proteins [136] converts GTP to GDP, leading to inactive GDP-bound Ras. Ras mutations that diminish GTPase activity or decrease GDP-binding capacity render Ras in a constitutively active GTP-bound status. Activating mutations in RAS genes are among the most frequently observed oncogenic mutations in human cancer cases. In the absence of a Ras mutation, increased Ras activity in human cancer cells frequently results from gene amplification [137,138], gene overexpression [139], or an increase in activity of upstream signals from tyrosine kinase growth factor receptors such as Her2 and EGF receptor (EGFR) [140,141]. Activation of EGFR results in the assembly of Grb2 and the Son of Sevenless (SOS) complex; SOS is one of the guanine nucleotide exchange factors that activate RAS [141,142]. RAS activation has resulted in stimulation of a wide range of downstream signaling pathways, most notably the RAF/mitogen-activated protein kinase (MAPK) kinase (MEK)/ERK [143,144] and phosphoinositide 3-kinase (PI3K)/AKT/mammalian target of rapamycin pathways. GTP-RAS binds directly to and activates the catalytic subunit of PI3K p110 independently of the regulatory subunit PI3K p85 [145,146].
Both Ras and STAT3 proteins are activated by EGFR [96,141,147] and suppressed by the microRNA let-7 [148,149,150,151], and both of them regulate the common downstream targets, such as cyclin D1 [152,153,154], Bcl2 family proteins [155,156,157,158], Rho family GTPases [159,160,161], hypoxia-inducible factor-1α [162,163], and VEGF [9,164], suggesting that Ras and STAT3 mediate certain parallel, complementary, or coordinated biological processes (Figure 3). In fact, STAT3 has been found to be an important factor in Ras-mediated oncogenic transformation. Ras transduction in different cell types has induced secretion of the cytokine IL-6, whereas knockdown of IL-6 expression, genetic ablation of the IL-6 gene, and treatment with a neutralizing anti-IL-6 antibody has retarded Ras-driven tumorigenesis [65,165]. A recent study demonstrated that mitochondrial STAT3 is required for Ras-induced malignant transformation [99]. This function of STAT3 may be involved in glucose and energy metabolism and does not require Y705 phosphorylation or the presence of intact SH2 and DNA-binding domains but does require S727 phosphorylation.
Figure 3.
Ras and STAT3 mediated parallel pathways for EGFR and let-7. Ras and STAT3 has common upstream regulators and downstream targets.
Studies of K-ras-transgenic mice demonstrated that Stat3 phosphorylation occurs in tumors at multiple stages of K-ras-induced pancreatic tumorigenesis but not in normal pancreatic tissue [65,166]. Disruption of the IL-6 gene and conditional inactivation of STAT3 reduced progressive pancreatic intraepithelial neoplasia (PanIn) lesion and pancreatic ductal adenocarcinoma formation in mutant K-ras-transgenic mice [65,166], suggesting that STAT3 activity is required for the development of the earliest premalignant pancreatic lesions, acinar-to-ductal metaplasia, and PanIN and for the progression from PanIN to invasive pancreatic ductal adenocarcinoma [65]. Disruption of Socs3, an endogenous feedback inhibitor of the STAT3 signaling pathway, in mutant K-ras-transgenic mice resulted in early, robust activation of STAT3 and high-grade PanIn lesions [166]. Also, Ras- and Rac1-mediated p38 and JNK signals are required for STAT3 transcriptional activity induced by the Src oncoprotein [167]. STAT3-mediated gene regulation by v-Src is strictly Ras-dependent in NIH 3T3 cells, as STAT3 function is completely abrogated by expression of dominant-negative Ras or Rac1.
Both Ras and STAT3 regulate the activity of Rho-family small GTPases. STAT3 regulates Rac1 activity by interacting with the Rac1 activator betaPIX in the cytoplasm, thereby modulating the organization of the actin cytoskeleton and cell migration [159]. Although evidence of Rho GTPase-mediated Ras activation is lacking, researchers have shown that Rho GTPases such as RhoA, Rac1, and Cdc42 can regulate STAT3 phosphorylation and nuclear translocation [168]. Rho GTPases are required for G protein-coupled receptor (GPCR)-mediated JAK/STAT signaling [169]. GPCR-stimulated Rac activation resulted in generation of reactive oxygen species (ROS), which in turn activated the JAK/STAT pathway. Rho GTPase can also activate STAT3 through direct interaction with it [170], via the IL-6 autocrine pathway [171,172] or Rho-associated kinase [173]. STAT3 mediates RhoA-induced NF-κB and cyclin D1 expression and NF-κB nuclear translocation [173]. In addition, Rac1 is a key mediator of IL-6/gp130-induced STAT3 S727 phosphorylation by SEK-1/MKK-4 [174].
The interaction between STAT3 and Ras signaling pathways suggests that the functional status of STAT3 affects responses of cancer to treatment with agents targeting these signaling pathways. Indeed, a study of response to treatment with the MEK inhibitor AZD6244 using a panel of lung cancer cell lines revealed that activation of the STAT3 pathway is associated with resistance to AZD6244 [175]. Inhibiting STAT3 activity with siRNA or a small-molecule inhibitor dramatically sensitized lung cancer cells to treatment with AZD6244 both in vitro and in vivo. Similarly, investigators observed a synergistic effect between a PI3K inhibitor and a STAT3 inhibitor in human gastric cancer cells harboring KRAS mutations [176]. Moreover, small molecules that induced synthetic lethality in KRAS mutant cancer cells [87,177] were found to be a novel class of STAT3 inhibitors [178,179], demonstrating that genetic interaction between Ras and STAT3 pathways can be explored for genotype-specific anticancer therapeutics.
4.2. EGFR Pathway
EGFR-mediated signaling pathways are known to be a driving force in lung tumorigenesis and have been extensively investigated as targets for cancer therapy. Activating EGFR mutations are detected in about 10%–17% of human lung adenocarcinoma cases, with higher percentages in women and patients with no smoking history [39,180,181,182]. In addition, EGFR gene amplification and overexpression have been reported in 20%–60% of primary non-small cell lung cancer tumors [183,184]. Amplification of the EGFR gene has been observed in colorectal cancer [185,186], pancreatic cancer [187,188], head and neck cancer [189], and glioma [190] cases. Inducible expression of human lung cancer-related mutant EGFR genes in transgenic mice caused the development of lung adenocarcinoma, whereas stopping inducible expression of the mutant EGFR genes led to lung tumor regression [191,192], demonstrating that activating EGFR mutations are required and sufficient for lung cancer tumorigenesis and malignancy maintenance. Small-molecule inhibitors (erlotinib, gefitinib, and afatinib) and a monoclonal antibody (cetuximab) targeted to EGFR have been used for treatment of lung, colorectal, head and neck, and pancreatic cancers.
STAT3 is identified because of its activation by tyrosine phosphorylation in response to exposure to EGF and IL-6 [96]. Activation mutations of EGFR have been reported to activate STAT3, which is required for the oncogenic effects of EGFR mutations [193,194]. EGFR can activate STAT3 via direct recruitment and activation of STAT3 [195], upregulation of IL-6 expression, and activation of the gp130/JAK/STAT3 pathway [194] or activation of STAT3 by activating non-receptor tyrosine kinases such as Src and Pyk2 [196,197]. An in vitro study using recombinant proteins demonstrated that STAT3α and STAT3β formed stable complexes with EGFR and were phosphorylated in tyrosine by the EGFR and activated for binding to DNA [198]. Activation of EGFR results in autophosphorylation of several tyrosine residues in EGFR, which provide docking sites for direct recruitment of downstream substrates. In EGFR, pY1068 and pY1086 are the docking sites for STAT3 [195] and Grb2, an SH2 domain-containing adaptor protein [199,200]. In contrast, Shc, another SH2 domain-containing adaptor protein, binds to Y1148 and Y1173 in EGFR [190,201], whereas phospholipase Cγ binds to Y992 [202] and the protein tyrosine phosphatase SHP-1 binds to Y1173 in EGFR [203].
As a key downstream mediator of the EGFR signaling pathways, STAT3 is crucial to EGFR-mediated cell growth in vitro. Inhibition of STAT3 expression by an antisense oligonucleotide dramatically suppressed the transforming growth factor-α/EGFR-mediated growth of transformed epithelial cells [204]. STAT3 is critically involved in EGFR-induced cancer cell migration and invasion [205,206]. Moreover, EGFR physically interacts with STAT3 in the nucleus, leading to transcriptional activation of inducible nitric oxide synthase [207], suggesting that STAT3 functions not only as a downstream mediator of EGFR but also as a partner of EGFR in regulating diverse biological functions. In addition to EGFR, STAT3 is activated by many other growth factor receptors, such as PDGF receptor [208,209] and MET [210,211] (Figure 2). STAT3 may be a mediator of MET-induced resistance to anti-EGFR therapy. In cancer cells with high MET activity, inhibition of EGFR activity alone likely is not sufficient to abrogate STAT3 activity. Synthetic lethal screening of an EGFR-centered signaling network using a siRNA library revealed that STAT3 is one of the targets that synergize with EGFR antagonists to reduce cancer cell viability and tumor size [84]. Inhibition of the STAT3 pathway has been shown to overcome resistance of lung cancer [212,213], head and neck cancer [214], pancreatic cancer [215], and glioma [216] to anti-EGFR therapy. Furthermore, treatment of cancer cells with EGFR inhibitors such as afatinib and dacomitinib can activate the IL-6/JAK/STAT3 signaling pathway, which in turn induces resistance to these agents [217]. Inhibiting IL-6/JAK/STAT3 signaling or STAT3 activity alone with antisense oligonucleotides, siRNA, or small-molecule inhibitors can dramatically sensitize cancer cells to treatment with EGFR inhibitors [212,214,217].
4.3. Reduction-Oxidation Pathways
In reduction-oxidation (redox) reactions, a molecule’s oxidation state changes because of a gain or loss of electrons. Inside a cell, redox metabolism is balanced by production and elimination of ROS, a group of oxygen- or nitrogen-containing molecules that are highly active in redox reactions. Produced in living organisms via a wide range of physiological process, ROS can serve as second messengers in response to exposure to growth factors, hormones, and cytokines. For example, H2O2 participates in essentially all receptor tyrosine kinase-mediated signal transduction, including EGF, PDGF, insulin, and cytokines [218,219]. Also, ligand-receptor interactions can induce the production of ROS, which regulates the intracellular activity of key signaling components, including protein kinases and protein phosphatases [218], and is required for cell proliferation [220], adhesion [221], migration [222,223], differentiation [224], oncogenic transformation [225], and apoptosis [226,227,228]. ROS also are involved in several critical steps in cancer initiation and progression, including somatic mutations and genome instability in cancer cells [229,230,231], epithelial-mesenchymal transition [230,232], metastasis [233,234,235], angiogenesis [236,237], and maintenance of stem cell status [238,239]. Nevertheless, deregulation of redox metabolism in cancer cells caused by overexpression of oncogenes such as Ras [225], c-Myc [231], c-Abl [240], and Src [241] and growth factor receptors such as c-MET [230], insulin-like growth factor receptor [242], EGFR [243], and VEGF receptor [244] may render cancer cells more vulnerable than normal cells to oxidative stress-induced death [245,246]. Indeed, ROS generation and consequent oxidative damage are among the major mechanisms by which radiotherapy [247,248] and chemotherapy [249] induce apoptosis.
Cellular ROS may have either a stimulatory or inhibitory effect on STAT3 signaling depending on the cellular context or level or duration of ROS generation in a cell. Oxidative stress has been reported to stimulate the JAK/STAT pathway [250,251,252]. Also, H2O2 stimulates the activity of the STAT kinases JAK2 and TYK2 and activates STAT1 and STAT3 in fibroblasts, lymphocytes, and cancer cells. Activation of STATs by PDGF is markedly inhibited by the ROS scavenger N-acetyl-L-cysteine and diphenylene iodonium, indicating that ROS production contributes to STAT activation in response to PDGF exposure. These findings demonstrate that the JAK/STAT pathway responds to intracellular ROS induction and that PDGF uses ROS as second messengers to regulate STAT activation [250]. Stimulation of the JAK/STAT cascade by angiotensin II requires O2− anions generated by the NAD(P)H oxidase system [253]. ROS are mediators of GPCR-stimulated Rac activity and subsequent activation of the JAK/STAT pathway [169]. Increasing evidence suggests that the cellular redox state is involved in regulating tyrosine phosphatase activity via reversible oxidization of catalytic cysteine to sulfenic acid (Cys–SOH) [254,255,256]. Moreover, cell death caused by oxidative stress triggers secretion of IL-11 from dying cells because of ERK2-mediated activation of the transcriptional factor Fra-1, leading to STAT3 activation and compensatory proliferation of neighboring cells [257]. Interestingly, ROS do not induce tyrosine phosphorylation of STAT3 in SYK-deficient human leukemia cells. Inhibition of SYK activity by a small molecule prevents ROS-induced activation of STAT3 and overcomes the resistance of human B-lineage leukemia and lymphoma cells to ROS-induced apoptosis [258], indicating that SYK plays an indispensable role in oxidative stress-induced STAT3 activation in B-cell leukemia and lymphoma cells.
Evidence also suggests that ROS attenuate cytokine-induced JAK/STAT activation [259,260,261]. Four cysteine residues in the catalytic domain of JAK2 (Cys866, Cys917, Cys1094, and Cys1105) provide a mechanism for redox regulation in JAK2 via oxidation and reduction of these residues [262]. JAK2 is catalytically inactive when oxidized; its activity can be restored via reduction to the thiol state. In addition, nitric oxide can markedly suppress endogenous tyrosine phosphorylation of JAK3 and STAT5 [257] and leptin-mediated activation of STAT3 [260]. Nitric oxide and other thiol oxidants can inhibit the autokinase activity of murine JAK2 in vitro, presumably via oxidation of crucial dithiols to disulfides in JAK2. The autokinase activity of JAK3 responds in a similar fashion to exposure to thiol redox reagents in vitro and nitric oxide donors in vivo. Treatment with parthenolide, an anti-inflammatory compound, increases intracellular ROS level and inhibits JAK1 and STAT3 activation but stimulates the MAPK pathways. Pretreatment with the antioxidant N-acetyl-L-cysteine completely suppressed the inhibitory effect of parthenolide on JAK1 and STAT3 [259]. The cysteine residues in STAT3 can be modified by S-glutathionylation in response to mild oxidative stress, attenuating IL-6-mediated STAT3 activation, as glutathionylated STAT3 is a poor substrate for JAKs [263].
In contrast to many reports on ROS-mediated changes in STAT3 activity, only a few reported studies have examined regulation of ROS levels by STAT3. In cardiomyocytes, constitutively active STAT3 protects against hypoxia- and/or reoxygenation-induced injury by scavenging ROS via upregulation of expression of manganese superoxide dismutase and its enzyme activity [264] and via upregulation of expression of the ROS scavengers metallothioneins [265]. Depletion of mitochondrial STAT3 has resulted in decreased ATP production and triggered ROS production [266,267]. This cross-talk between STAT3 and redox pathways suggests that STAT3 plays a critical role in oxidative stress-mediated cancer therapy. In fact, the STAT3 inhibitors developed in our laboratory [178,179] drastically induced ROS generation in the susceptible cancer cells [179,268,269]. Scavenging of ROS using antioxidants such as nordihydroguaiaretic acid, aesculetin, baicalein, caffeic acid, and flavonoids abolished their apoptosis-induction activity [268,269], demonstrating the critical roles of oxidative stress in antitumor activity induced by this class of STAT3 inhibitors.
5. Conclusions and Perspectives
Efficacy and toxicity are the two major issues in drug development. A survey of anticancer agents evaluated in human studies from 1991 to 2000 demonstrated that only about 5% of those entering clinical trials were approved for clinical use, and the majority of treatment failures in late-phase clinical trials of candidate anticancer agents resulted from a lack of efficacy of the drugs [270,271,272,273]. Genetic interactions among pathways cooperatively involved in initiation and maintenance of malignancy indicate that the therapeutic efficacy of single-target therapy for cancer is highly dependent on the cellular context of signaling networks. Single-target therapy may be highly effective for cancers that are addicted to certain oncogenes. For example, treatment of various cancers in humans with the EGFR inhibitors erlotinib and gefitinib [274] and the BCR-Abl inhibitor imatinib [275] already has been successful. Nevertheless, the success of such single-target therapeutics relies on the identification of potential responders in patient populations. The use of EGFR mutations as biomarkers to identify responders has greatly contributed to the success of gefitinib [181,274,276] and erlotinib [181], because both gefitinib [277] and erlotinib [278,279] failed to be beneficial in randomized phase 3 trials in unselected patient populations.
The advances in knowledge about networks of genetic interactions are expected to impact strategies for enhancing the therapeutic efficacy of anticancer therapy. The interactions of the STAT3 pathway with other cancer-related pathways, such as the Ras, EGFR, and redox pathways, indicate that simultaneous targeting of several key molecules in these pathways using either combination therapy or agents capable of modulating the functions of multiple targets will be required for effective cancer therapy. Our own experience in anticancer drug development demonstrated that genetic interactions can be exploited for the development of anticancer agents targeting multiple pathways and that performing robust compound optimization once a lead compound is identified is critical, as compounds with similar chemical structures and in vitro activity may have dramatically different in vivo toxicity and efficacy profiles.
Acknowledgment
The author thanks Donald R. Norwood from the MD Anderson Department of Scientific Publications for editorial review of this manuscript. This research is supported in part by National Cancer Institute Grant R01 CA 124951 (to B. Fang).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Barbieri I., Pensa S., Pannellini T., Quaglino E., Maritano D., Demaria M., Voster A., Turkson J., Cavallo F., Watson C.J., et al. Constitutively active Stat3 enhances neu-mediated migration and metastasis in mammary tumors via upregulation of Cten. Cancer Res. 2010;70:2558–2567. doi: 10.1158/0008-5472.CAN-09-2840. [DOI] [PubMed] [Google Scholar]
- 2.Ranger J.J., Levy D.E., Shahalizadeh S., Hallett M., Muller W.J. Identification of a Stat3-dependent transcription regulatory network involved in metastatic progression. Cancer Res. 2009;69:6823–6830. doi: 10.1158/0008-5472.CAN-09-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y., Du H., Qin Y., Roberts J., Cummings O.W., Yan C. Activation of the signal transducers and activators of the transcription 3 pathway in alveolar epithelial cells induces inflammation and adenocarcinomas in mouse lung. Cancer Res. 2007;67:8494–8503. doi: 10.1158/0008-5472.CAN-07-0647. [DOI] [PubMed] [Google Scholar]
- 4.Okamoto N., Yasukawa M., Nguyen C., Kasim V., Maida Y., Possemato R., Shibata T., Ligon K.L., Fukami K., Hahn W.C., et al. Maintenance of tumor initiating cells of defined genetic composition by nucleostemin. Proc. Natl. Acad. Sci. USA. 2011;108:20388–20393. doi: 10.1073/pnas.1015171108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marotta L.L., Almendro V., Marusyk A., Shipitsin M., Schemme J., Walker S.R., Bloushtain-Qimron N., Kim J.J., Choudhury S.A., Maruyama R., et al. The JAK2/STAT3 signaling pathway is required for growth of CD44+CD24− stem cell-like breast cancer cells in human tumors. J. Clin. Investig. 2011;121:2723–2735. doi: 10.1172/JCI44745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guryanova O.A., Wu Q., Cheng L., Lathia J.D., Huang Z., Yang J., MacSwords J., Eyler C.E., McLendon R.E., Heddleston J.M., et al. Nonreceptor tyrosine kinase BMX maintains self-renewal and tumorigenic potential of glioblastoma stem cells by activating STAT3. Cancer Cell. 2011;19:498–511. doi: 10.1016/j.ccr.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng G.Z., Zhang W.Z., Sun M., Wang Q., Coppola D., Mansour M., Xu L.M., Costanzo C., Cheng J.Q., Wang L.H. Twist is transcriptionally induced by activation of STAT3 and mediates STAT3 oncogenic function. J. Biol. Chem. 2008;283:14665–14673. doi: 10.1074/jbc.M707429200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lo H.W., Hsu S.C., Xia W., Cao X., Shih J.Y., Wei Y., Abbruzzese J.L., Hortobagyi G.N., Hung M.C. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res. 2007;67:9066–9076. doi: 10.1158/0008-5472.CAN-07-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niu G., Wright K.L., Huang M., Song L., Haura E., Turkson J., Zhang S., Wang T., Sinibaldi D., Coppola D., et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 10.Wei D., Le X., Zheng L., Wang L., Frey J.A., Gao A.C., Peng Z., Huang S., Xiong H.Q., Abbruzzese J.L., et al. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene. 2003;22:319–329. doi: 10.1038/sj.onc.1206122. [DOI] [PubMed] [Google Scholar]
- 11.Yu H., Pardoll D., Jove R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grivennikov S.I., Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21:11–19. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bromberg J.F., Wrzeszczynska M.H., Devgan G., Zhao Y., Pestell R.G., Albanese C., Darnell J.E., Jr. STAT3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/S0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 14.Chiarle R., Simmons W.J., Cai H., Dhall G., Zamo A., Raz R., Karras J.G., Levy D.E., Inghirami G. Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nat. Med. 2005;11:623–629. doi: 10.1038/nm1249. [DOI] [PubMed] [Google Scholar]
- 15.Lin L., Amin R., Gallicano G.I., Glasgow E., Jogunoori W., Jessup J.M., Zasloff M., Marshall J.L., Shetty K., Johnson L., et al. The STAT3 inhibitor NSC 74859 is effective in hepatocellular cancers with disrupted TGF-beta signaling. Oncogene. 2009;28:961–972. doi: 10.1038/onc.2008.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X., Yue P., Fletcher S., Zhao W., Gunning P.T., Turkson J. A novel small-molecule disrupts Stat3 SH2 domain-phosphotyrosine interactions and Stat3-dependent tumor processes. Biochem. Pharmacol. 2010;79:1398–1409. doi: 10.1016/j.bcp.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leong P.L., Andrews G.A., Johnson D.E., Dyer K.F., Xi S., Mai J.C., Robbins P.D., Gadiparthi S., Burke N.A., Watkins S.F., et al. Targeted inhibition of Stat3 with a decoy oligonucleotide abrogates head and neck cancer cell growth. Proc. Natl. Acad. Sci. USA. 2003;100:4138–4143. doi: 10.1073/pnas.0534764100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao W., Jaganathan S., Turkson J. A cell-permeable Stat3 SH2 domain mimetic inhibits Stat3 activation and induces antitumor cell effects in vitro. J. Biol. Chem. 2010;285:35855–35865. doi: 10.1074/jbc.M110.154088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schust J., Sperl B., Hollis A., Mayer T.U., Berg T. Stattic: A small-molecule inhibitor of STAT3 activation and dimerization. Chem. Biol. 2006;13:1235–1242. doi: 10.1016/j.chembiol.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Siddiquee K., Zhang S., Guida W.C., Blaskovich M.A., Greedy B., Lawrence H.R., Yip M.L., Jove R., McLaughlin M.M., Lawrence N.J., et al. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc. Natl. Acad. Sci. USA. 2007;104:7391–7396. doi: 10.1073/pnas.0609757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song H., Wang R., Wang S., Lin J. A low-molecular-weight compound discovered through virtual database screening inhibits Stat3 function in breast cancer cells. Proc. Natl. Acad. Sci. USA. 2005;102:4700–4705. doi: 10.1073/pnas.0409894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhasin D., Cisek K., Pandharkar T., Regan N., Li C., Pandit B., Lin J., Li P.K. Design, synthesis, and studies of small molecule STAT3 inhibitors. Bioorg. Med. Chem. Lett. 2008;18:391–395. doi: 10.1016/j.bmcl.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 23.Deng J., Grande F., Neamati N. Small molecule inhibitors of Stat3 signaling pathway. Curr. Cancer Drug Targets. 2007;7:91–107. doi: 10.2174/156800907780006922. [DOI] [PubMed] [Google Scholar]
- 24.Yue P., Turkson J. Targeting STAT3 in cancer: How successful are we? Exp. Opin. Investig. Drugs. 2009;18:45–56. doi: 10.1517/13543780802565791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Debnath B., Xu S., Neamati N. Small molecule inhibitors of signal transducer and activator of transcription 3 (Stat3) protein. J. Med. Chem. 2012;55:6645–6668. doi: 10.1021/jm300207s. [DOI] [PubMed] [Google Scholar]
- 26.Sansone P., Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J. Clin. Oncol. 2012;30:1005–1014. doi: 10.1200/JCO.2010.31.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kayentao K., Garner P., van Eijk A.M., Naidoo I., Roper C., Mulokozi A., MacArthur J.R., Luntamo M., Ashorn P., Doumbo O.K., et al. Intermittent preventive therapy for malaria during pregnancy using 2 vs. 3 or more doses of sulfadoxine-pyrimethamine and risk of low birth weight in Africa: Systematic review and meta-analysis. JAMA. 2013;309:594–604. doi: 10.1001/jama.2012.216231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aponte J.J., Schellenberg D., Egan A., Breckenridge A., Carneiro I., Critchley J., Danquah I., Dodoo A., Kobbe R., Lell B., et al. Efficacy and safety of intermittent preventive treatment with sulfadoxine-pyrimethamine for malaria in African infants: A pooled analysis of six randomised, placebo-controlled trials. Lancet. 2009;374:1533–1542. doi: 10.1016/S0140-6736(09)61258-7. [DOI] [PubMed] [Google Scholar]
- 29.Takakura A., Nelson E.A., Haque N., Humphreys B.D., Zandi-Nejad K., Frank D.A., Zhou J. Pyrimethamine inhibits adult polycystic kidney disease by modulating STAT signaling pathways. Hum. Mol. Genet. 2011;20:4143–4154. doi: 10.1093/hmg/ddr338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowinger T.B., Riedl B., Dumas J., Smith R.A. Design and discovery of small molecules targeting raf-1 kinase. Curr. Pharm. Design. 2002;8:2269–2278. doi: 10.2174/1381612023393125. [DOI] [PubMed] [Google Scholar]
- 31.Wilhelm S.M., Carter C., Tang L., Wilkie D., McNabola A., Rong H., Chen C., Zhang X., Vincent P., McHugh M., et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 32.Blechacz B.R., Smoot R.L., Bronk S.F., Werneburg N.W., Sirica A.E., Gores G.J. Sorafenib inhibits signal transducer and activator of transcription-3 signaling in cholangiocarcinoma cells by activating the phosphatase shatterproof 2. Hepatology. 2009;50:1861–1870. doi: 10.1002/hep.23214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang F., Brown C., Buettner R., Hedvat M., Starr R., Scuto A., Schroeder A., Jensen M., Jove R. Sorafenib induces growth arrest and apoptosis of human glioblastoma cells through the dephosphorylation of signal transducers and activators of transcription 3. Mol. Cancer Ther. 2010;9:953–962. doi: 10.1158/1535-7163.MCT-09-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wetzler M., Brady M.T., Tracy E., Li Z.R., Donohue K.A., O’Loughlin K.L., Cheng Y., Mortazavi A., McDonald A.A., Kunapuli P., et al. Arsenic trioxide affects signal transducer and activator of transcription proteins through alteration of protein tyrosine kinase phosphorylation. Clin. Cancer Res. 2006;12:6817–6825. doi: 10.1158/1078-0432.CCR-06-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jia Y., Liu D., Xiao D., Ma X., Han S., Zheng Y., Sun S., Zhang M., Gao H., Cui X., et al. Expression of AFP and STAT3 is involved in arsenic trioxide-induced apoptosis and inhibition of proliferation in AFP-producing gastric cancer cells. PLoS One. 2013;8:e54774. doi: 10.1371/journal.pone.0054774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernhard G.C. Auranofin therapy in rheumatoid arthritis. J. Lab. Clin. Med. 1982;100:167–177. [PubMed] [Google Scholar]
- 37.Kim N.H., Lee M.Y., Park S.J., Choi J.S., Oh M.K., Kim I.S. Auranofin blocks interleukin-6 signalling by inhibiting phosphorylation of JAK1 and STAT3. Immunology. 2007;122:607–614. doi: 10.1111/j.1365-2567.2007.02679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schuh E., Pfluger C., Citta A., Folda A., Rigobello M.P., Bindoli A., Casini A., Mohr F. Gold(I) carbene complexes causing thioredoxin 1 and thioredoxin 2 oxidation as potential anticancer agents. J. Med. Chem. 2012;55:5518–5528. doi: 10.1021/jm300428v. [DOI] [PubMed] [Google Scholar]
- 39.Ding L., Getz G., Wheeler D.A., Mardis E.R., McLellan M.D., Cibulskis K., Sougnez C., Greulich H., Muzny D.M., Morgan M.B., et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenman C., Stephens P., Smith R., Dalgliesh G.L., Hunter C., Bignell G., Davies H., Teague J., Butler A., Stevens C., et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153–158. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kan Z., Jaiswal B.S., Stinson J., Janakiraman V., Bhatt D., Stern H.M., Yue P., Haverty P.M., Bourgon R., Zheng J., et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature. 2010;466:869–873. doi: 10.1038/nature09208. [DOI] [PubMed] [Google Scholar]
- 42.Stephens P., Edkins S., Davies H., Greenman C., Cox C., Hunter C., Bignell G., Teague J., Smith R., Stevens C., et al. A screen of the complete protein kinase gene family identifies diverse patterns of somatic mutations in human breast cancer. Nat. Genet. 2005;37:590–592. doi: 10.1038/ng1571. [DOI] [PubMed] [Google Scholar]
- 43.Pleasance E.D., Cheetham R.K., Stephens P.J., McBride D.J., Humphray S.J., Greenman C.D., Varela I., Lin M.L., Ordonez G.R., Bignell G.R., et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2010;463:191–196. doi: 10.1038/nature08658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stommel J.M., Kimmelman A.C., Ying H., Nabioullin R., Ponugoti A.H., Wiedemeyer R., Stegh A.H., Bradner J.E., Ligon K.L., Brennan C., et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–290. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- 45.Grant S. Is the focus moving toward a combination of targeted drugs? Bailliere’s Best Pract. Clin. Haematol. 2008;21:629–637. doi: 10.1016/j.beha.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faivre S., Demetri G., Sargent W., Raymond E. Molecular basis for sunitinib efficacy and future clinical development. Nat. Rev. Drug Disc. 2007;6:734–745. doi: 10.1038/nrd2380. [DOI] [PubMed] [Google Scholar]
- 47.Schaefer T.S., Sanders L.K., Nathans D. Cooperative transcriptional activity of Jun and Stat3 beta, a short form of Stat3. Proc. Natl. Acad. Sci. USA. 1995;92:9097–9101. doi: 10.1073/pnas.92.20.9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takeda K., Noguchi K., Shi W., Tanaka T., Matsumoto M., Yoshida N., Kishimoto T., Akira S. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc. Natl. Acad. Sci. USA. 1997;94:3801–3804. doi: 10.1073/pnas.94.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maritano D., Sugrue M.L., Tininini S., Dewilde S., Strobl B., Fu X., Murray-Tait V., Chiarle R., Poli V. The STAT3 isoforms alpha and beta have unique and specific functions. Nat. Immunol. 2004;5:401–409. doi: 10.1038/ni1052. [DOI] [PubMed] [Google Scholar]
- 50.Huang Y., Qiu J., Dong S., Redell M.S., Poli V., Mancini M.A., Tweardy D.J. Stat3 isoforms, alpha and beta, demonstrate distinct intracellular dynamics with prolonged nuclear retention of Stat3beta mapping to its unique C-terminal end. J. Biol. Chem. 2007;282:34958–34967. doi: 10.1074/jbc.M704548200. [DOI] [PubMed] [Google Scholar]
- 51.Minegishi Y., Saito M., Tsuchiya S., Tsuge I., Takada H., Hara T., Kawamura N., Ariga T., Pasic S., Stojkovic O., et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–1062. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 52.Liu J.Y., Li Q., Chen T.T., Guo X., Ge J., Yuan L.X. Destructive pulmonary staphylococcal infection in a boy with hyper-IgE syndrome: A novel mutation in the signal transducer and activator of transcription 3 (STAT3) gene (p.Y657S) Eur. J. Pediat. 2011;170:661–666. doi: 10.1007/s00431-010-1349-6. [DOI] [PubMed] [Google Scholar]
- 53.Renner E.D., Rylaarsdam S., Anover-Sombke S., Rack A.L., Reichenbach J., Carey J.C., Zhu Q., Jansson A.F., Barboza J., Schimke L.F., et al. Novel signal transducer and activator of transcription 3 (STAT3) mutations, reduced T(H)17 cell numbers, and variably defective STAT3 phosphorylation in hyper-IgE syndrome. J. Allergy Clin. Immunol. 2008;122:181–187. doi: 10.1016/j.jaci.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miyazaki K., Miyazawa T., Sugimoto K., Fujita S., Yanagida H., Okada M., Takemura T. An adolescent with marked hyperimmuno-globulinemia E showing minimal change nephrotic syndrome and a STAT3 gene mutation. Clin. Nephrol. 2011;75:369–373. doi: 10.5414/CN106548. [DOI] [PubMed] [Google Scholar]
- 55.Jiao H., Toth B., Erdos M., Fransson I., Rakoczi E., Balogh I., Magyarics Z., Derfalvi B., Csorba G., Szaflarska A., et al. Novel and recurrent STAT3 mutations in hyper-IgE syndrome patients from different ethnic groups. Mol. Immunol. 2008;46:202–206. doi: 10.1016/j.molimm.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 56.Milner J.D., Brenchley J.M., Laurence A., Freeman A.F., Hill B.J., Elias K.M., Kanno Y., Spalding C., Elloumi H.Z., Paulson M.L., et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma C.S., Chew G.Y., Simpson N., Priyadarshi A., Wong M., Grimbacher B., Fulcher D.A., Tangye S.G., Cook M.C. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J. Exp. Med. 2008;205:1551–1557. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Beaucoudrey L., Puel A., Filipe-Santos O., Cobat A., Ghandil P., Chrabieh M., Feinberg J., von B.H., Samarina A., Janniere L., et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J. Exp. Med. 2008;205:1543–1550. doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumanovics A., Perkins S.L., Gilbert H., Cessna M.H., Augustine N.H., Hill H.R. Diffuse large B cell lymphoma in hyper-IgE syndrome due to STAT3 mutation. J. Clin. Immunol. 2010;30:886–893. doi: 10.1007/s10875-010-9452-z. [DOI] [PubMed] [Google Scholar]
- 60.Leonard G.D., Posadas E., Herrmann P.C., Anderson V.L., Jaffe E.S., Holland S.M., Wilson W.H. Non-Hodgkin’s lymphoma in Job’s syndrome: A case report and literature review. Leuk. Lymphoma. 2004;45:2521–2525. doi: 10.1080/10428190400004463. [DOI] [PubMed] [Google Scholar]
- 61.Pilati C., Amessou M., Bihl M.P., Balabaud C., Nhieu J.T., Paradis V., Nault J.C., Izard T., Bioulac-Sage P., Couchy G., et al. Somatic mutations activating STAT3 in human inflammatory hepatocellular adenomas. J. Exp. Med. 2011;208:1359–1366. doi: 10.1084/jem.20110283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanchez-Ceja S.G., Reyes-Maldonado E., Vazquez-Manriquez M.E., Lopez-Luna J.J., Belmont A., Gutierrez-Castellanos S. Differential expression of STAT5 and Bcl-xL, and high expression of Neu and STAT3 in non-small-cell lung carcinoma. Lung Cancer. 2006;54:163–168. doi: 10.1016/j.lungcan.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 63.Abou-Ghazal M., Yang D.S., Qiao W., Reina-Ortiz C., Wei J., Kong L.Y., Fuller G.N., Hiraoka N., Priebe W., Sawaya R., et al. The incidence, correlation with tumor-infiltrating inflammation, and prognosis of phosphorylated STAT3 expression in human gliomas. Clin. Cancer Res. 2008;14:8228–8235. doi: 10.1158/1078-0432.CCR-08-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scholz A., Heinze S., Detjen K.M., Peters M., Welzel M., Hauff P., Schirner M., Wiedenmann B., Rosewicz S. Activated signal transducer and activator of transcription 3 (STAT3) supports the malignant phenotype of human pancreatic cancer. Gastroenterology. 2003;125:891–905. doi: 10.1016/S0016-5085(03)01064-3. [DOI] [PubMed] [Google Scholar]
- 65.Corcoran R.B., Contino G., Deshpande V., Tzatsos A., Conrad C., Benes C.H., Levy D.E., Settleman J., Engelman J.A., Bardeesy N. STAT3 plays a critical role in kras-induced pancreatic tumorigenesis. Cancer Res. 2011;71:5020–5029. doi: 10.1158/0008-5472.CAN-11-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hsiao J.R., Jin Y.T., Tsai S.T., Shiau A.L., Wu C.L., Su W.C. Constitutive activation of STAT3 and STAT5 is present in the majority of nasopharyngeal carcinoma and correlates with better prognosis. Br. J. Cancer. 2003;89:344–349. doi: 10.1038/sj.bjc.6601003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abdulghani J., Gu L., Dagvadorj A., Lutz J., Leiby B., Bonuccelli G., Lisanti M.P., Zellweger T., Alanen K., Mirtti T., et al. Stat3 promotes metastatic progression of prostate cancer. Am. J. Pathol. 2008;172:1717–1728. doi: 10.2353/ajpath.2008.071054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Migone T.S., Lin J.X., Cereseto A., Mulloy J.C., O’Shea J.J., Franchini G., Leonard W.J. Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-I. Science. 1995;269:79–81. doi: 10.1126/science.7604283. [DOI] [PubMed] [Google Scholar]
- 69.Benekli M., Xia Z., Donohue K.A., Ford L.A., Pixley L.A., Baer M.R., Baumann H., Wetzler M. Constitutive activity of signal transducer and activator of transcription 3 protein in acute myeloid leukemia blasts is associated with short disease-free survival. Blood. 2002;99:252–257. doi: 10.1182/blood.V99.1.252. [DOI] [PubMed] [Google Scholar]
- 70.Stewart D.A., Bahlis N., Mansoor A. pY-STAT3 and p53 expression predict outcome for poor prognosis diffuse large B-cell lymphoma treated with high dose chemotherapy and autologous stem cell transplantation. Leuk. Lymphoma. 2009;50:1276–1282. doi: 10.1080/10428190903015628. [DOI] [PubMed] [Google Scholar]
- 71.Wang Y.C., Zheng L.H., Ma B.A., Zhou Y., Zhang M.H., Zhang D.Z., Fan Q.Y. Clinical value of signal transducers and activators of transcription 3 (STAT3) gene expression in human osteosarcoma. Acta Histochem. 2011;113:402–408. doi: 10.1016/j.acthis.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 72.Ryu K., Choy E., Yang C., Susa M., Hornicek F.J., Mankin H., Duan Z. Activation of signal transducer and activator of transcription 3 (Stat3) pathway in osteosarcoma cells and overexpression of phosphorylated-Stat3 correlates with poor prognosis. J. Orthopaed. Res. 2010;28:971–978. doi: 10.1002/jor.21088. [DOI] [PubMed] [Google Scholar]
- 73.Chatterjee D., Sabo E., Tavares R., Resnick M.B. Inverse association between Raf Kinase Inhibitory Protein and signal transducers and activators of transcription 3 expression in gastric adenocarcinoma patients: Implications for clinical outcome. Clin. Cancer Res. 2008;14:2994–3001. doi: 10.1158/1078-0432.CCR-07-4496. [DOI] [PubMed] [Google Scholar]
- 74.Yakata Y., Nakayama T., Yoshizaki A., Kusaba T., Inoue K., Sekine I. Expression of p-STAT3 in human gastric carcinoma: Significant correlation in tumour invasion and prognosis. Int. J. Oncol. 2007;30:437–442. [PubMed] [Google Scholar]
- 75.Morikawa T., Baba Y., Yamauchi M., Kuchiba A., Nosho K., Shima K., Tanaka N., Huttenhower C., Frank D.A., Fuchs C.S., et al. STAT3 expression, molecular features, inflammation patterns, and prognosis in a database of 724 colorectal cancers. Clin. Cancer Res. 2011;17:1452–1462. doi: 10.1158/1078-0432.CCR-10-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kusaba T., Nakayama T., Yamazumi K., Yakata Y., Yoshizaki A., Inoue K., Nagayasu T., Sekine I. Activation of STAT3 is a marker of poor prognosis in human colorectal cancer. Oncol. Rep. 2006;15:1445–1451. [PubMed] [Google Scholar]
- 77.Guo S., Sun F., Guo Z., Li W., Alfano A., Chen H., Magyar C.E., Huang J., Chai T.C., Qiu S., et al. Tyrosine kinase ETK/BMX is up-regulated in bladder cancer and predicts poor prognosis in patients with cystectomy. PLoS One. 2011;6:e17778. doi: 10.1371/journal.pone.0017778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takemoto S., Ushijima K., Kawano K., Yamaguchi T., Terada A., Fujiyoshi N., Nishio S., Tsuda N., Ijichi M., Kakuma T., et al. Expression of activated signal transducer and activator of transcription-3 predicts poor prognosis in cervical squamous-cell carcinoma. Br. J. Cancer. 2009;101:967–972. doi: 10.1038/sj.bjc.6605212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barbie D.A., Tamayo P., Boehm J.S., Kim S.Y., Moody S.E., Dunn I.F., Schinzel A.C., Sandy P., Meylan E., Scholl C., et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature. 2009;462:108–112. doi: 10.1038/nature08460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Licciulli S., Kissil J.L. WT1: A weak spot in KRAS-induced transformation. J. Clin. Investig. 2010;120:3804–3807. doi: 10.1172/JCI44901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luo J., Emanuele M.J., Li D., Creighton C.J., Schlabach M.R., Westbrook T.F., Wong K.K., Elledge S.J. A genome-wide RNAi screen identifies multiple synthetic lethal interactions with the Ras oncogene. Cell. 2009;137:835–848. doi: 10.1016/j.cell.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scholl C., Frohling S., Dunn I.F., Schinzel A.C., Barbie D.A., Kim S.Y., Silver S.J., Tamayo P., Wadlow R.C., Ramaswamy S., et al. Synthetic lethal interaction between oncogenic KRAS dependency and STK33 suppression in human cancer cells. Cell. 2009;137:821–834. doi: 10.1016/j.cell.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 83.Whitehurst A.W., Bodemann B.O., Cardenas J., Ferguson D., Girard L., Peyton M., Minna J.D., Michnoff C., Hao W., Roth M.G., et al. Synthetic lethal screen identification of chemosensitizer loci in cancer cells. Nature. 2007;446:815–819. doi: 10.1038/nature05697. [DOI] [PubMed] [Google Scholar]
- 84.Astsaturov I., Ratushny V., Sukhanova A., Einarson M.B., Bagnyukova T., Zhou Y., Devarajan K., Silverman J.S., Tikhmyanova N., Skobeleva N., et al. Synthetic lethal screen of an EGFR-centered network to improve targeted therapies. Sci. Signal. 2010;3 doi: 10.1126/scisignal.2001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Torrance C.J., Agrawal V., Vogelstein B., Kinzler K.W. Use of isogenic human cancer cells for high-throughput screening and drug discovery. Nat. Biotechnol. 2001;19:940–945. doi: 10.1038/nbt1001-940. [DOI] [PubMed] [Google Scholar]
- 86.Dolma S., Lessnick S.L., Hahn W.C., Stockwell B.R. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3:285–296. doi: 10.1016/S1535-6108(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 87.Guo W., Wu S., Liu J., Fang B. Identification of a small molecule with synthetic lethality for K-ras and protein kinase C iota. Cancer Res. 2008;68:7403–7408. doi: 10.1158/0008-5472.CAN-08-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaelin W.G., Jr. The concept of synthetic lethality in the context of anticancer therapy. Nat. Rev. Cancer. 2005;5:689–698. doi: 10.1038/nrc1691. [DOI] [PubMed] [Google Scholar]
- 89.Le Meur N., Gentleman R. Modeling synthetic lethality. Genome Biol. 2008;9 doi: 10.1186/gb-2008-9-9-r135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ooi S.L., Pan X., Peyser B.D., Ye P., Meluh P.B., Yuan D.S., Irizarry R.A., Bader J.S., Spencer F.A., Boeke J.D. Global synthetic-lethality analysis and yeast functional profiling. Trends Genet. 2006;22:56–63. doi: 10.1016/j.tig.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 91.Fang B. Genetic interactions in translational research on cancer. World J. Med. Genet. 2011;1:14–22. doi: 10.5496/wjmg.v1.i1.14. [DOI] [Google Scholar]
- 92.Argetsinger L.S., Campbell G.S., Yang X., Witthuhn B.A., Silvennoinen O., Ihle J.N., Carter-Su C. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell. 1993;74:237–244. doi: 10.1016/0092-8674(93)90415-M. [DOI] [PubMed] [Google Scholar]
- 93.Parganas E., Wang D., Stravopodis D., Topham D.J., Marine J.C., Teglund S., Vanin E.F., Bodner S., Colamonici O.R., van Deursen J.M., et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–395. doi: 10.1016/S0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 94.Bromberg J.F. Activation of STAT proteins and growth control. Bioessays. 2001;23:161–169. doi: 10.1002/1521-1878(200102)23:2<161::AID-BIES1023>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 95.Bowman T., Garcia R., Turkson J., Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 96.Zhong Z., Wen Z., Darnell J.E., Jr. Stat3: A STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 97.Darnell J.E., Jr., Kerr I.M., Stark G.R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 98.Levy D.E., Darnell J.E., Jr. Stats: Transcriptional control and biological impact. Nat. Rev. Mol. Cell. Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 99.Gough D.J., Corlett A., Schlessinger K., Wegrzyn J., Larner A.C., Levy D.E. Mitochondrial STAT3 supports Ras-dependent oncogenic transformation. Science. 2009;324:1713–1716. doi: 10.1126/science.1171721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu F., Mukhopadhyay S., Sehgal P.B. Live cell imaging of interleukin-6-induced targeting of “transcription factor” STAT3 to sequestering endosomes in the cytoplasm. Am. J. Physiol. 2007;293:C1374–C1382. doi: 10.1152/ajpcell.00220.2007. [DOI] [PubMed] [Google Scholar]
- 101.Ngo V.N., Young R.M., Schmitz R., Jhavar S., Xiao W., Lim K.H., Kohlhammer H., Xu W., Yang Y., Zhao H., et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hazan-Halevy I., Harris D., Liu Z., Liu J., Li P., Chen X., Shanker S., Ferrajoli A., Keating M.J., Estrov Z. STAT3 is constitutively phosphorylated on serine 727 residues, binds DNA, and activates transcription in CLL cells. Blood. 2010;115:2852–2863. doi: 10.1182/blood-2009-10-230060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang X., Blenis J., Li H.C., Schindler C., Chen-Kiang S. Requirement of serine phosphorylation for formation of STAT-promoter complexes. Science. 1995;267:1990–1994. doi: 10.1126/science.7701321. [DOI] [PubMed] [Google Scholar]
- 104.Qin H.R., Kim H.J., Kim J.Y., Hurt E.M., Klarmann G.J., Kawasaki B.T., Duhagon Serrat M.A., Farrar W.L. Activation of signal transducer and activator of transcription 3 through a phosphomimetic serine 727 promotes prostate tumorigenesis independent of tyrosine 705 phosphorylation. Cancer Res. 2008;68:7736–7741. doi: 10.1158/0008-5472.CAN-08-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shen Y., Schlessinger K., Zhu X., Meffre E., Quimby F., Levy D.E., Darnell J.E., Jr. Essential role of STAT3 in postnatal survival and growth revealed by mice lacking STAT3 serine 727 phosphorylation. Mol. Cell. Biol. 2004;24:407–419. doi: 10.1128/MCB.24.1.407-419.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chung J., Uchida E., Grammer T.C., Blenis J. STAT3 serine phosphorylation by ERK-dependent and -independent pathways negatively modulates its tyrosine phosphorylation. Mol. Cell. Biol. 1997;17:6508–6516. doi: 10.1128/mcb.17.11.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lim C.P., Cao X. Serine phosphorylation and negative regulation of Stat3 by JNK. J. Biol. Chem. 1999;274:31055–31061. doi: 10.1074/jbc.274.43.31055. [DOI] [PubMed] [Google Scholar]
- 108.Wang R., Cherukuri P., Luo J. Activation of Stat3 sequence-specific DNA binding and transcription by p300/CREB-binding protein-mediated acetylation. J. Biol. Chem. 2005;280:11528–11534. doi: 10.1074/jbc.M413930200. [DOI] [PubMed] [Google Scholar]
- 109.Yuan Z.L., Guan Y.J., Chatterjee D., Chin Y.E. Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science. 2005;307:269–273. doi: 10.1126/science.1105166. [DOI] [PubMed] [Google Scholar]
- 110.Nadiminty N., Lou W., Lee S.O., Lin X., Trump D.L., Gao A.C. Stat3 activation of NFkappaB p100 processing involves CBP/p300-mediated acetylation. Proc. Natl. Acad. Sci. USA. 2006;103:7264–7269. doi: 10.1073/pnas.0509808103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nie Y., Erion D.M., Yuan Z., Dietrich M., Shulman G.I., Horvath T.L., Gao Q. STAT3 inhibition of gluconeogenesis is downregulated by SirT1. Nat. Cell. Biol. 2009;11:492–500. doi: 10.1038/ncb1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Klingmuller U., Lorenz U., Cantley L.C., Neel B.G., Lodish H.F. Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell. 1995;80:729–738. doi: 10.1016/0092-8674(95)90351-8. [DOI] [PubMed] [Google Scholar]
- 113.Zabolotny J.M., Bence-Hanulec K.K., Stricker-Krongrad A., Haj F., Wang Y., Minokoshi Y., Kim Y.B., Elmquist J.K., Tartaglia L.A., Kahn B.B., et al. PTP1B regulates leptin signal transduction in vivo. Dev. Cell. 2002;2:489–495. doi: 10.1016/S1534-5807(02)00148-X. [DOI] [PubMed] [Google Scholar]
- 114.Lee C.K., Bluyssen H.A., Levy D.E. Regulation of interferon-alpha responsiveness by the duration of Janus kinase activity. J. Biol. Chem. 1997;272:21872–21877. doi: 10.1074/jbc.272.35.21872. [DOI] [PubMed] [Google Scholar]
- 115.Ray S., Lee C., Hou T., Boldogh I., Brasier A.R. Requirement of histone deacetylase1 (HDAC1) in signal transducer and activator of transcription 3 (STAT3) nucleocytoplasmic distribution. Nucleic Acids Res. 2008;36:4510–4520. doi: 10.1093/nar/gkn419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Endo T.A., Masuhara M., Yokouchi M., Suzuki R., Sakamoto H., Mitsui K., Matsumoto A., Tanimura S., Ohtsubo M., Misawa H., et al. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387:921–924. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- 117.Naka T., Narazaki M., Hirata M., Matsumoto T., Minamoto S., Aono A., Nishimoto N., Kajita T., Taga T., Yoshizaki K., et al. Structure and function of a new STAT-induced STAT inhibitor. Nature. 1997;387:924–929. doi: 10.1038/43219. [DOI] [PubMed] [Google Scholar]
- 118.Yoshimura A., Ohkubo T., Kiguchi T., Jenkins N.A., Gilbert D.J., Copeland N.G., Hara T., Miyajima A. A novel cytokine-inducible gene CIS encodes an SH2-containing protein that binds to tyrosine-phosphorylated interleukin 3 and erythropoietin receptors. EMBO J. 1995;14:2816–2826. doi: 10.1002/j.1460-2075.1995.tb07281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Haspel R.L., Darnell J.E., Jr. A nuclear protein tyrosine phosphatase is required for the inactivation of Stat1. Proc. Natl. Acad. Sci. USA. 1999;96:10188–10193. doi: 10.1073/pnas.96.18.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chung C.D., Liao J., Liu B., Rao X., Jay P., Berta P., Shuai K. Specific inhibition of Stat3 signal transduction by PIAS3. Science. 1997;278:1803–1805. doi: 10.1126/science.278.5344.1803. [DOI] [PubMed] [Google Scholar]
- 121.Betz A., Lampen N., Martinek S., Young M.W., Darnell J.E., Jr. A Drosophila PIAS homologue negatively regulates stat92E. Proc. Natl. Acad. Sci. USA. 2001;98:9563–9568. doi: 10.1073/pnas.171302098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nishimoto A., Yu Y., Lu Z., Mao X., Ren Z., Watowich S.S., Mills G.B., Liao W.S., Chen X., Bast R.C., Jr., et al. A Ras homologue member I directly inhibits signal transducers and activators of transcription 3 translocation and activity in human breast and ovarian cancer cells. Cancer Res. 2005;65:6701–6710. doi: 10.1158/0008-5472.CAN-05-0130. [DOI] [PubMed] [Google Scholar]
- 123.Hedvat M., Huszar D., Herrmann A., Gozgit J.M., Schroeder A., Sheehy A., Buettner R., Proia D., Kowolik C.M., Xin H., et al. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell. 2009;16:487–497. doi: 10.1016/j.ccr.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li J., Favata M., Kelley J.A., Caulder E., Thomas B., Wen X., Sparks R.B., Arvanitis A., Rogers J.D., Combs A.P., et al. INCB16562, a JAK1/2 selective inhibitor, is efficacious against multiple myeloma cells and reverses the protective effects of cytokine and stromal cell support. Neoplasia. 2010;12:28–38. doi: 10.1593/neo.91192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Manshouri T., Quintas-Cardama A., Nussenzveig R.H., Gaikwad A., Estrov Z., Prchal J., Cortes J.E., Kantarjian H.M., Verstovsek S. The JAK kinase inhibitor CP-690,550 suppresses the growth of human polycythemia vera cells carrying the JAK2V617F mutation. Cancer Sci. 2008;99:1265–1273. doi: 10.1111/j.1349-7006.2008.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Deisseroth A., Kaminskas E., Grillo J., Chen W., Saber H., Lu H.L., Rothmann M.D., Brar S., Wang J., Garnett C., et al. Food and Drug Administration approval: Ruxolitinib for the treatment of patients with intermediate and high-risk myelofibrosis. Clin. Cancer Res. 2012;18:3212–3217. doi: 10.1158/1078-0432.CCR-12-0653. [DOI] [PubMed] [Google Scholar]
- 127.Mascarenhas J., Hoffman R. Ruxolitinib: The first FDA approved therapy for the treatment of myelofibrosis. Clin. Cancer Res. 2012;18:3008–3014. doi: 10.1158/1078-0432.CCR-11-3145. [DOI] [PubMed] [Google Scholar]
- 128.Verstovsek S., Mesa R.A., Gotlib J., Levy R.S., Gupta V., DiPersio J.F., Catalano J.V., Deininger M., Miller C., Silver R.T., et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N. Engl. J. Med. 2012;366:799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Burmester G.R., Blanco R., Charles-Schoeman C., Wollenhaupt J., Zerbini C., Benda B., Gruben D., Wallenstein G., Krishnaswami S., Zwillich S.H., et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: A randomised phase 3 trial. Lancet. 2013;381:451–460. doi: 10.1016/S0140-6736(12)61424-X. [DOI] [PubMed] [Google Scholar]
- 130.Sandborn W.J., Ghosh S., Panes J., Vranic I., Su C., Rousell S., Niezychowski W., Study A. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N. Engl. J. Med. 2012;367:616–624. doi: 10.1056/NEJMoa1112168. [DOI] [PubMed] [Google Scholar]
- 131.Byers L.A., Sen B., Saigal B., Diao L., Wang J., Nanjundan M., Cascone T., Mills G.B., Heymach J.V., Johnson F.M. Reciprocal regulation of c-Src and STAT3 in non-small cell lung cancer. Clin. Cancer Res. 2009;15:6852–6861. doi: 10.1158/1078-0432.CCR-09-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sen B., Saigal B., Parikh N., Gallick G., Johnson F.M. Sustained Src inhibition results in signal transducer and activator of transcription 3 (STAT3) activation and cancer cell survival via altered Janus-activated kinase-STAT3 binding. Cancer Res. 2009;69:1958–1965. doi: 10.1158/0008-5472.CAN-08-2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bar-Sagi D., Hall A. Ras and Rho GTPases: A family reunion. Cell. 2000;103:227–238. doi: 10.1016/S0092-8674(00)00115-X. [DOI] [PubMed] [Google Scholar]
- 134.Colicelli J. Human RAS superfamily proteins and related GTPases. Sci. STKE. 2004;2004 doi: 10.1126/stke.2502004re13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rossman K.L., Der C.J., Sondek J. GEF means go: Turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell. Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 136.Bernards A., Settleman J. GAP control: Regulating the regulators of small GTPases. Trends Cell Biol. 2004;14:377–385. doi: 10.1016/j.tcb.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 137.Hoa M., Davis S.L., Ames S.J., Spanjaard R.A. Amplification of wild-type K-ras promotes growth of head and neck squamous cell carcinoma. Cancer Res. 2002;62:7154–7156. [PubMed] [Google Scholar]
- 138.Filmus J.E., Buick R.N. Stability of c-K-ras amplification during progression in a patient with adenocarcinoma of the ovary. Cancer Res. 1985;45:4468–4472. [PubMed] [Google Scholar]
- 139.Coleman W.B., Throneburg D.B., Grisham J.W., Smith G.J. Overexpression of c-K-ras, c-N-ras and transforming growth factor beta co-segregate with tumorigenicity in morphologically transformed C3H 10T1/2 cell lines. Carcinogenesis. 1994;15:1005–1012. doi: 10.1093/carcin/15.5.1005. [DOI] [PubMed] [Google Scholar]
- 140.Ehrhardt A., David M.D., Ehrhardt G.R., Schrader J.W. Distinct mechanisms determine the patterns of differential activation of H-Ras, N-Ras, K-Ras 4B, and M-Ras by receptors for growth factors or antigen. Mol. Cell Biol. 2004;24:6311–6323. doi: 10.1128/MCB.24.14.6311-6323.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Buday L., Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 1993;73:611–620. doi: 10.1016/0092-8674(93)90146-H. [DOI] [PubMed] [Google Scholar]
- 142.Egan S.E., Giddings B.W., Brooks M.W., Buday L., Sizeland A.M., Weinberg R.A. Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature. 1993;363:45–51. doi: 10.1038/363045a0. [DOI] [PubMed] [Google Scholar]
- 143.Moodie S.A., Willumsen B.M., Weber M.J., Wolfman A. Complexes of Ras.GTP with Raf-1 and mitogen-activated protein kinase kinase. Science. 1993;260:1658–1661. doi: 10.1126/science.8503013. [DOI] [PubMed] [Google Scholar]
- 144.Vojtek A.B., Hollenberg S.M., Cooper J.A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993;74:205–214. doi: 10.1016/0092-8674(93)90307-C. [DOI] [PubMed] [Google Scholar]
- 145.Rodriguez-Viciana P., Warne P.H., Dhand R., Vanhaesebroeck B., Gout I., Fry M.J., Waterfield M.D., Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 146.Pacold M.E., Suire S., Perisic O., Lara-Gonzalez S., Davis C.T., Walker E.H., Hawkins P.T., Stephens L., Eccleston J.F., Williams R.L. Crystal structure and functional analysis of Ras binding to its effector phosphoinositide 3-kinase gamma. Cell. 2000;103:931–943. doi: 10.1016/S0092-8674(00)00196-3. [DOI] [PubMed] [Google Scholar]
- 147.Lowenstein E.J., Daly R.J., Batzer A.G., Li W., Margolis B., Lammers R., Ullrich A., Skolnik E.Y., Bar-Sagi D., Schlessinger J. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 1992;70:431–442. doi: 10.1016/0092-8674(92)90167-B. [DOI] [PubMed] [Google Scholar]
- 148.Iliopoulos D., Hirsch H.A., Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Johnson S.M., Grosshans H., Shingara J., Byrom M., Jarvis R., Cheng A., Labourier E., Reinert K.L., Brown D., Slack F.J. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 150.Kumar M.S., Erkeland S.J., Pester R.E., Chen C.Y., Ebert M.S., Sharp P.A., Jacks T. Suppression of non-small cell lung tumor development by the let-7 microRNA family. Proc. Natl. Acad. Sci. USA. 2008;105:3903–3908. doi: 10.1073/pnas.0712321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sugimura K., Miyata H., Tanaka K., Hamano R., Takahashi T., Kurokawa Y., Yamasaki M., Nakajima K., Takiguchi S., Mori M., et al. Let-7 expression is a significant determinant of response to chemotherapy through the regulation of IL-6/STAT3 pathway in esophageal squamous cell carcinoma. Clin. Cancer Res. 2012;18:5144–5153. doi: 10.1158/1078-0432.CCR-12-0701. [DOI] [PubMed] [Google Scholar]
- 152.Filmus J., Robles A.I., Shi W., Wong M.J., Colombo L.L., Conti C.J. Induction of cyclin D1 overexpression by activated ras. Oncogene. 1994;9:3627–3633. [PubMed] [Google Scholar]
- 153.Liu J.J., Chao J.R., Jiang M.C., Ng S.Y., Yen J.J., Yang-Yen H.F. Ras transformation results in an elevated level of cyclin D1 and acceleration of G1 progression in NIH 3T3 cells. Mol. Cell Biol. 1995;15:3654–3663. doi: 10.1128/mcb.15.7.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Leslie K., Lang C., Devgan G., Azare J., Berishaj M., Gerald W., Kim Y.B., Paz K., Darnell J.E., Albanese C., et al. Cyclin D1 is transcriptionally regulated by and required for transformation by activated signal transducer and activator of transcription 3. Cancer Res. 2006;66:2544–2552. doi: 10.1158/0008-5472.CAN-05-2203. [DOI] [PubMed] [Google Scholar]
- 155.Rosen K., Rak J., Leung T., Dean N.M., Kerbel R.S., Filmus J. Activated Ras prevents downregulation of Bcl-X(L) triggered by detachment from the extracellular matrix. A mechanism of Ras-induced resistance to anoikis in intestinal epithelial cells. J. Cell Biol. 2000;149:447–456. doi: 10.1083/jcb.149.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zushi S., Shinomura Y., Kiyohara T., Miyazaki Y., Kondo S., Sugimachi M., Higashimoto Y., Kanayama S., Matsuzawa Y. STAT3 mediates the survival signal in oncogenic ras-transfected intestinal epithelial cells. Int. J. Cancer. 1998;78:326–330. doi: 10.1002/(SICI)1097-0215(19981029)78:3<326::AID-IJC12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 157.Epling-Burnette P.K., Liu J.H., Catlett-Falcone R., Turkson J., Oshiro M., Kothapalli R., Li Y., Wang J.M., Yang-Yen H.F., Karras J., et al. Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J. Clin. Investig. 2001;107:351–362. doi: 10.1172/JCI9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nagaoka H., Takahashi Y., Hayashi R., Nakamura T., Ishii K., Matsuda J., Ogura A., Shirakata Y., Karasuyama H., Sudo T., et al. Ras mediates effector pathways responsible for pre-B cell survival, which is essential for the developmental progression to the late pre-B cell stage. J. Exp. Med. 2000;192:171–182. doi: 10.1084/jem.192.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Teng T.S., Lin B., Manser E., Ng D.C., Cao X. Stat3 promotes directional cell migration by regulating Rac1 activity via its activator betaPIX. J. Cell Sci. 2009;122:4150–4159. doi: 10.1242/jcs.057109. [DOI] [PubMed] [Google Scholar]
- 160.Khosravi-Far R., Solski P.A., Clark G.J., Kinch M.S., Der C.J. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol. Cell Biol. 1995;15:6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Scita G., Tenca P., Frittoli E., Tocchetti A., Innocenti M., Giardina G., di Fiore P.P. Signaling from Ras to Rac and beyond: Not just a matter of GEFs. EMBO J. 2000;19:2393–2398. doi: 10.1093/emboj/19.11.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Blancher C., Moore J.W., Robertson N., Harris A.L. Effects of ras and von Hippel-Lindau (VHL) gene mutations on hypoxia-inducible factor (HIF)-1alpha, HIF-2alpha, and vascular endothelial growth factor expression and their regulation by the phosphatidylinositol 3'-kinase/Akt signaling pathway. Cancer Res. 2001;61:7349–7355. [PubMed] [Google Scholar]
- 163.Xu Q., Briggs J., Park S., Niu G., Kortylewski M., Zhang S., Gritsko T., Turkson J., Kay H., Semenza G.L., et al. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene. 2005;24:5552–5560. doi: 10.1038/sj.onc.1208719. [DOI] [PubMed] [Google Scholar]
- 164.Rak J., Mitsuhashi Y., Bayko L., Filmus J., Shirasawa S., Sasazuki T., Kerbel R.S. Mutant ras oncogenes upregulate VEGF/VPF expression: Implications for induction and inhibition of tumor angiogenesis. Cancer Res. 1995;55:4575–4580. [PubMed] [Google Scholar]
- 165.Ancrile B., Lim K.H., Counter C.M. Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Gene Dev. 2007;21:1714–1719. doi: 10.1101/gad.1549407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lesina M., Kurkowski M.U., Ludes K., Rose-John S., Treiber M., Kloppel G., Yoshimura A., Reindl W., Sipos B., Akira S., et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19:456–469. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 167.Turkson J., Bowman T., Adnane J., Zhang Y., Djeu J.Y., Sekharam M., Frank D.A., Holzman L.B., Wu J., Sebti S., et al. Requirement for Ras/Rac1-mediated p38 and c-Jun N-terminal kinase signaling in Stat3 transcriptional activity induced by the Src oncoprotein. Mol. Cell Biol. 1999;19:7519–7528. doi: 10.1128/mcb.19.11.7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Raptis L., Arulanandam R., Geletu M., Turkson J. The R(h)oads to Stat3: Stat3 activation by the Rho GTPases. Exp. Cell Res. 2011;317:1787–1795. doi: 10.1016/j.yexcr.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pelletier S., Duhamel F., Coulombe P., Popoff M.R., Meloche S. Rho family GTPases are required for activation of Jak/STAT signaling by G protein-coupled receptors. Mol. Cell Biol. 2003;23:1316–1333. doi: 10.1128/MCB.23.4.1316-1333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Simon A.R., Vikis H.G., Stewart S., Fanburg B.L., Cochran B.H., Guan K.L. Regulation of STAT3 by direct binding to the Rac1 GTPase. Science. 2000;290:144–147. doi: 10.1126/science.290.5489.144. [DOI] [PubMed] [Google Scholar]
- 171.Faruqi T.R., Gomez D., Bustelo X.R., Bar-Sagi D., Reich N.C. Rac1 mediates STAT3 activation by autocrine IL-6. Proc. Natl. Acad. Sci. USA. 2001;98:9014–9019. doi: 10.1073/pnas.161281298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Arulanandam R., Geletu M., Feracci H., Raptis L. Activated Rac1 requires gp130 for Stat3 activation, cell proliferation and migration. Exp. Cell Res. 2010;316:875–886. doi: 10.1016/j.yexcr.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 173.Debidda M., Wang L., Zang H., Poli V., Zheng Y. A role of STAT3 in Rho GTPase-regulated cell migration and proliferation. J. Biol. Chem. 2005;280:17275–17285. doi: 10.1074/jbc.M413187200. [DOI] [PubMed] [Google Scholar]
- 174.Schuringa J.J., Jonk L.J., Dokter W.H., Vellenga E., Kruijer W. Interleukin-6-induced STAT3 transactivation and Ser727 phosphorylation involves Vav, Rac-1 and the kinase SEK-1/MKK-4 as signal transduction components. Biochem. J. 2000;347:89–96. doi: 10.1042/0264-6021:3470089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dai B., Meng J., Peyton M., Girard L., Bornmann W.G., Ji L., Minna J.D., Fang B., Roth J.A. STAT3 mediates resistance to MEK inhibitor through microRNA miR-17. Cancer Res. 2011;71:3658–3668. doi: 10.1158/0008-5472.CAN-10-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Park E., Park J., Han S.W., Im S.A., Kim T.Y., Oh D.Y., Bang Y.J. NVP-BKM120, a novel PI3K inhibitor, shows synergism with a STAT3 inhibitor in human gastric cancer cells harboring KRAS mutations. Int. J. Oncol. 2012;40:1259–1266. doi: 10.3892/ijo.2011.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Guo W., Wu S., Wang L., Wang R., Wei L., Liu J., Fang B. Interruption of RNA processing machinery by a small compound 1-[(4-chlorophenyl) methyl]-1H-indole-3-carboxaldehyde (oncrasin-1) Mol. Cancer Ther. 2009;8:441–448. doi: 10.1158/1535-7163.MCT-08-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Guo W., Wu S., Wang L., Wei X., Liu X., Wang J., Lu Z., Hollingshead M., Fang B. Antitumor activity of a novel oncrasin analogue is mediated by JNK activation and STAT3 inhibition. PLoS One. 2011;6:e28487. doi: 10.1371/journal.pone.0028487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liu X., Guo W., Wu S., Wang L., Wang J., Dai B., Kim E.S., Heymach J.V., Wang M., Girard L., et al. Antitumor activity of a novel STAT3 inhibitor and redox modulator in non-small cell lung cancer cells. Biochem. Pharmacol. 2012;83:1456–1464. doi: 10.1016/j.bcp.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rosell R., Moran T., Queralt C., Porta R., Cardenal F., Camps C., Majem M., Lopez-Vivanco G., Isla D., Provencio M., et al. Screening for epidermal growth factor receptor mutations in lung cancer. N. Engl. J. Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 181.Pao W., Miller V., Zakowski M., Doherty J., Politi K., Sarkaria I., Singh B., Heelan R., Rusch V., Fulton L., et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc. Natl. Acad. Sci. USA. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Marchetti A., Martella C., Felicioni L., Barassi F., Salvatore S., Chella A., Camplese P.P., Iarussi T., Mucilli F., Mezzetti A., et al. EGFR mutations in non-small-cell lung cancer: Analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J. Clin. Oncol. 2005;23:857–865. doi: 10.1200/JCO.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 183.Hirsch F.R., Varella-Garcia M., Bunn P.A., Jr., di Maria M.V., Veve R., Bremmes R.M., Baron A.E., Zeng C., Franklin W.A. Epidermal growth factor receptor in non-small-cell lung carcinomas: Correlation between gene copy number and protein expression and impact on prognosis. J. Clin. Oncol. 2003;21:3798–3807. doi: 10.1200/JCO.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 184.Cappuzzo F., Hirsch F.R., Rossi E., Bartolini S., Ceresoli G.L., Bemis L., Haney J., Witta S., Danenberg K., Domenichini I., et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J. Natl. Cancer Inst. 2005;97:643–655. doi: 10.1093/jnci/dji112. [DOI] [PubMed] [Google Scholar]
- 185.Laurent-Puig P., Cayre A., Manceau G., Buc E., Bachet J.B., Lecomte T., Rougier P., Lievre A., Landi B., Boige V., et al. Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J. Clin. Oncol. 2009;27:5924–5930. doi: 10.1200/JCO.2008.21.6796. [DOI] [PubMed] [Google Scholar]
- 186.Shia J., Klimstra D.S., Li A.R., Qin J., Saltz L., Teruya-Feldstein J., Akram M., Chung K.Y., Yao D., Paty P.B., et al. Epidermal growth factor receptor expression and gene amplification in colorectal carcinoma: An immunohistochemical and chromogenic in situ hybridization study. Mod. Pathol. 2005;18:1350–1356. doi: 10.1038/modpathol.3800417. [DOI] [PubMed] [Google Scholar]
- 187.Boeck S., Jung A., Laubender R.P., Neumann J., Egg R., Goritschan C., Vehling-Kaiser U., Winkelmann C., Fischer von W.L., Clemens M.R., et al. EGFR pathway biomarkers in erlotinib-treated patients with advanced pancreatic cancer: Translational results from the randomised, crossover phase 3 trial AIO-PK0104. Br. J. Cancer. 2013;108:469–476. doi: 10.1038/bjc.2012.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lee J., Jang K.T., Ki C.S., Lim T., Park Y.S., Lim H.Y., Choi D.W., Kang W.K., Park K., Park J.O. Impact of epidermal growth factor receptor (EGFR) kinase mutations, EGFR gene amplifications, and KRAS mutations on survival of pancreatic adenocarcinoma. Cancer. 2007;109:1561–1569. doi: 10.1002/cncr.22559. [DOI] [PubMed] [Google Scholar]
- 189.Chung C.H., Ely K., McGavran L., Varella-Garcia M., Parker J., Parker N., Jarrett C., Carter J., Murphy B.A., Netterville J., et al. Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. J. Clin. Oncol. 2006;24:4170–4176. doi: 10.1200/JCO.2006.07.2587. [DOI] [PubMed] [Google Scholar]
- 190.Yang L., Luquette L.J., Gehlenborg N., Xi R., Haseley P.S., Hsieh C.H., Zhang C., Ren X., Protopopov A., Chin L., et al. Diverse mechanisms of somatic structural variations in human cancer genomes. Cell. 2013;153:919–929. doi: 10.1016/j.cell.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ji H., Li D., Chen L., Shimamura T., Kobayashi S., McNamara K., Mahmood U., Mitchell A., Sun Y., Al-Hashem R., et al. The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapies. Cancer Cell. 2006;9:485–495. doi: 10.1016/j.ccr.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 192.Politi K., Zakowski M.F., Fan P.D., Schonfeld E.A., Pao W., Varmus H.E. Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down-regulation of the receptors. Gene Dev. 2006;20:1496–1510. doi: 10.1101/gad.1417406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Alvarez J.V., Greulich H., Sellers W.R., Meyerson M., Frank D.A. Signal transducer and activator of transcription 3 is required for the oncogenic effects of non-small-cell lung cancer-associated mutations of the epidermal growth factor receptor. Cancer Res. 2006;66:3162–3168. doi: 10.1158/0008-5472.CAN-05-3757. [DOI] [PubMed] [Google Scholar]
- 194.Gao S.P., Mark K.G., Leslie K., Pao W., Motoi N., Gerald W.L., Travis W.D., Bornmann W., Veach D., Clarkson B., et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J. Clin. Investig. 2007;117:3846–3856. doi: 10.1172/JCI31871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Shao H., Cheng H.Y., Cook R.G., Tweardy D.J. Identification and characterization of signal transducer and activator of transcription 3 recruitment sites within the epidermal growth factor receptor. Cancer Res. 2003;63:3923–3930. [PubMed] [Google Scholar]
- 196.Shi C.S., Kehrl J.H. Pyk2 amplifies epidermal growth factor and c-Src-induced Stat3 activation. J. Biol. Chem. 2004;279:17224–17231. doi: 10.1074/jbc.M311875200. [DOI] [PubMed] [Google Scholar]
- 197.Goi T., Shipitsin M., Lu Z., Foster D.A., Klinz S.G., Feig L.A. An EGF receptor/Ral-GTPase signaling cascade regulates c-Src activity and substrate specificity. EMBO J. 2000;19:623–630. doi: 10.1093/emboj/19.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Park O.K., Schaefer T.S., Nathans D. In vitro activation of Stat3 by epidermal growth factor receptor kinase. Proc. Natl. Acad. Sci. USA. 1996;93:13704–13708. doi: 10.1073/pnas.93.24.13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Batzer A.G., Rotin D., Urena J.M., Skolnik E.Y., Schlessinger J. Hierarchy of binding sites for Grb2 and Shc on the epidermal growth factor receptor. Mol. Cell. Biol. 1994;14:5192–5201. doi: 10.1128/mcb.14.8.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Okutani T., Okabayashi Y., Kido Y., Sugimoto Y., Sakaguchi K., Matuoka K., Takenawa T., Kasuga M. Grb2/Ash binds directly to tyrosines 1068 and 1086 and indirectly to tyrosine 1148 of activated human epidermal growth factor receptors in intact cells. J. Biol. Chem. 1994;269:31310–31314. [PubMed] [Google Scholar]
- 201.Okabayashi Y., Kido Y., Okutani T., Sugimoto Y., Sakaguchi K., Kasuga M. Tyrosines 1148 and 1173 of activated human epidermal growth factor receptors are binding sites of Shc in intact cells. J. Biol. Chem. 1994;269:18674–18678. [PubMed] [Google Scholar]
- 202.Rotin D., Margolis B., Mohammadi M., Daly R.J., Daum G., Li N., Fischer E.H., Burgess W.H., Ullrich A., Schlessinger J. SH2 domains prevent tyrosine dephosphorylation of the EGF receptor: Identification of Tyr992 as the high-affinity binding site for SH2 domains of phospholipase C gamma. EMBO J. 1992;11:559–567. doi: 10.1002/j.1460-2075.1992.tb05087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Keilhack H., Tenev T., Nyakatura E., Godovac-Zimmermann J., Nielsen L., Seedorf K., Bohmer F.D. Phosphotyrosine 1173 mediates binding of the protein-tyrosine phosphatase SHP-1 to the epidermal growth factor receptor and attenuation of receptor signaling. J. Biol. Chem. 1998;273:24839–24846. doi: 10.1074/jbc.273.38.24839. [DOI] [PubMed] [Google Scholar]
- 204.Grandis J.R., Drenning S.D., Chakraborty A., Zhou M.Y., Zeng Q., Pitt A.S., Tweardy D.J. Requirement of Stat3 but not Stat1 activation for epidermal growth factor receptor- mediated cell growth In vitro. J. Clin. Investig. 1998;102:1385–1392. doi: 10.1172/JCI3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wheeler S.E., Suzuki S., Thomas S.M., Sen M., Leeman-Neill R.J., Chiosea S.I., Kuan C.T., Bigner D.D., Gooding W.E., Lai S.Y., et al. Epidermal growth factor receptor variant III mediates head and neck cancer cell invasion via STAT3 activation. Oncogene. 2010;29:5135–5145. doi: 10.1038/onc.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhou W., Grandis J.R., Wells A. STAT3 is required but not sufficient for EGF receptor-mediated migration and invasion of human prostate carcinoma cell lines. Br. J. Cancer. 2006;95:164–171. doi: 10.1038/sj.bjc.6603234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lo H.W., Hsu S.C., Ali-Seyed M., Gunduz M., Xia W., Wei Y., Bartholomeusz G., Shih J.Y., Hung M.C. Nuclear interaction of EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer Cell. 2005;7:575–589. doi: 10.1016/j.ccr.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 208.Kim Y., Kim E., Wu Q., Guryanova O., Hitomi M., Lathia J.D., Serwanski D., Sloan A.E., Weil R.J., Lee J., et al. Platelet-derived growth factor receptors differentially inform intertumoral and intratumoral heterogeneity. Gene Dev. 2012;26:1247–1262. doi: 10.1101/gad.193565.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Vignais M.L., Gilman M. Distinct mechanisms of activation of Stat1 and Stat3 by platelet-derived growth factor receptor in a cell-free system. Mol. Cell. Biol. 1999;19:3727–3735. doi: 10.1128/mcb.19.5.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhang Y.W., Wang L.M., Jove R., Vande Woude G.F. Requirement of Stat3 signaling for HGF/SF-Met mediated tumorigenesis. Oncogene. 2002;21:217–226. doi: 10.1038/sj.onc.1205004. [DOI] [PubMed] [Google Scholar]
- 211.Kitade M., Factor V.M., Andersen J.B., Tomokuni A., Kaji K., Akita H., Holczbauer A., Seo D., Marquardt J.U., Conner E.A., et al. Specific fate decisions in adult hepatic progenitor cells driven by MET and EGFR signaling. Gene Dev. 2013;27:1706–1717. doi: 10.1101/gad.214601.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chiu H.C., Chou D.L., Huang C.T., Lin W.H., Lien T.W., Yen K.J., Hsu J.T. Suppression of Stat3 activity sensitizes gefitinib-resistant non small cell lung cancer cells. Biochem. Pharmacol. 2011;81:1263–1270. doi: 10.1016/j.bcp.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 213.Harada D., Takigawa N., Ochi N., Ninomiya T., Yasugi M., Kubo T., Takeda H., Ichihara E., Ohashi K., Takata S., et al. JAK2-related pathway induces acquired erlotinib resistance in lung cancer cells harboring an epidermal growth factor receptor-activating mutation. Cancer Sci. 2012;103:1795–1802. doi: 10.1111/j.1349-7006.2012.02363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sen M., Joyce S., Panahandeh M., Li C., Thomas S.M., Maxwell J., Wang L., Gooding W.E., Johnson D.E., Grandis J.R. Targeting Stat3 abrogates EGFR inhibitor resistance in cancer. Clin. Cancer Res. 2012;18:4986–4996. doi: 10.1158/1078-0432.CCR-12-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Nagaraj N.S., Washington M.K., Merchant N.B. Combined blockade of Src kinase and epidermal growth factor receptor with gemcitabine overcomes STAT3-mediated resistance of inhibition of pancreatic tumor growth. Clin. Cancer Res. 2011;17:483–493. doi: 10.1158/1078-0432.CCR-10-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lo H.W., Cao X., Zhu H., Ali-Osman F. Constitutively activated STAT3 frequently coexpresses with epidermal growth factor receptor in high-grade gliomas and targeting STAT3 sensitizes them to Iressa and alkylators. Clin. Cancer Res. 2008;14:6042–6054. doi: 10.1158/1078-0432.CCR-07-4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kim S.M., Kwon O.J., Hong Y.K., Kim J.H., Solca F., Ha S.J., Soo R.A., Christensen J.G., Lee J.H., Cho B.C. Activation of IL-6R/JAK1/STAT3 signaling induces de novo resistance to irreversible EGFR inhibitors in non-small cell lung cancer with T790M resistance mutation. Mol. Cancer Ther. 2012;11:2254–2264. doi: 10.1158/1535-7163.MCT-12-0311. [DOI] [PubMed] [Google Scholar]
- 218.Rhee S.G., Bae Y.S., Lee S.R., Kwon J. Hydrogen peroxide: A key messenger that modulates protein phosphorylation through cysteine oxidation. Sci. STKE. 2000;2000:E1. doi: 10.1126/stke.2000.53.pe1. [DOI] [PubMed] [Google Scholar]
- 219.Droge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 220.Menon S.G., Sarsour E.H., Spitz D.R., Higashikubo R., Sturm M., Zhang H., Goswami P.C. Redox regulation of the G1 to S phase transition in the mouse embryo fibroblast cell cycle. Cancer Res. 2003;63:2109–2117. [PubMed] [Google Scholar]
- 221.Chiarugi P., Pani G., Giannoni E., Taddei L., Colavitti R., Raugei G., Symons M., Borrello S., Galeotti T., Ramponi G. Reactive oxygen species as essential mediators of cell adhesion: The oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J. Cell Biol. 2003;161:933–944. doi: 10.1083/jcb.200211118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sundaresan M., Yu Z.X., Ferrans V.J., Irani K., Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 223.Jagadeeswaran R., Jagadeeswaran S., Bindokas V.P., Salgia R. Activation of HGF/c-Met pathway contributes to the reactive oxygen species generation and motility of small cell lung cancer cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;292:L1488–L1494. doi: 10.1152/ajplung.00147.2006. [DOI] [PubMed] [Google Scholar]
- 224.Smith J., Ladi E., Mayer-Proschel M., Noble M. Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proc. Natl. Acad. Sci. USA. 2000;97:10032–10037. doi: 10.1073/pnas.170209797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Irani K., Xia Y., Zweier J.L., Sollott S.J., Der C.J., Fearon E.R., Sundaresan M., Finkel T., Goldschmidt-Clermont P.J. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 226.Giorgio M., Migliaccio E., Orsini F., Paolucci D., Moroni M., Contursi C., Pelliccia G., Luzi L., Minucci S., Marcaccio M., et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 227.Gu Z., Kaul M., Yan B., Kridel S.J., Cui J., Strongin A., Smith J.W., Liddington R.C., Lipton S.A. S-Nitrosylation of matrix metalloproteinases: Signaling pathway to neuronal cell death. Science. 2002;297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 228.Johnson T.M., Yu Z.X., Ferrans V.J., Lowenstein R.A., Finkel T. Reactive oxygen species are downstream mediators of p53-dependent apoptosis. Proc. Natl. Acad. Sci. USA. 1996;93:11848–11852. doi: 10.1073/pnas.93.21.11848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hussain S.P., Hofseth L.J., Harris C.C. Radical causes of cancer. Nat. Rev. Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 230.Radisky D.C., Levy D.D., Littlepage L.E., Liu H., Nelson C.M., Fata J.E., Leake D., Godden E.L., Albertson D.G., Nieto M.A., et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Vafa O., Wade M., Kern S., Beeche M., Pandita T.K., Hampton G.M., Wahl G.M. c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: A mechanism for oncogene-induced genetic instability. Mol. Cell. 2002;9:1031–1044. doi: 10.1016/S1097-2765(02)00520-8. [DOI] [PubMed] [Google Scholar]
- 232.Zhang K.H., Tian H.Y., Gao X., Lei W.W., Hu Y., Wang D.M., Pan X.C., Yu M.L., Xu G.J., Zhao F.K., et al. Ferritin heavy chain-mediated iron homeostasis and subsequent increased reactive oxygen species production are essential for epithelial-mesenchymal transition. Cancer Res. 2009;69:5340–5348. doi: 10.1158/0008-5472.CAN-09-0112. [DOI] [PubMed] [Google Scholar]
- 233.Ferraro D., Corso S., Fasano E., Panieri E., Santangelo R., Borrello S., Giordano S., Pani G., Galeotti T. Pro-metastatic signaling by c-Met through RAC-1 and reactive oxygen species (ROS) Oncogene. 2006;25:3689–3698. doi: 10.1038/sj.onc.1209409. [DOI] [PubMed] [Google Scholar]
- 234.Ishikawa K., Takenaga K., Akimoto M., Koshikawa N., Yamaguchi A., Imanishi H., Nakada K., Honma Y., Hayashi J. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320:661–664. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- 235.Qu Y., Wang J., Ray P.S., Guo H., Huang J., Shin-Sim M., Bukoye B.A., Liu B., Lee A.V., Lin X., et al. Thioredoxin-like 2 regulates human cancer cell growth and metastasis via redox homeostasis and NF-B signaling. J. Clin. Investig. 2011;121:212–225. doi: 10.1172/JCI43144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Arbiser J.L., Petros J., Klafter R., Govindajaran B., McLaughlin E.R., Brown L.F., Cohen C., Moses M., Kilroy S., Arnold R.S., et al. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc. Natl. Acad. Sci. USA. 2002;99:715–720. doi: 10.1073/pnas.022630199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Xia C., Meng Q., Liu L.Z., Rojanasakul Y., Wang X.R., Jiang B.H. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007;67:10823–10830. doi: 10.1158/0008-5472.CAN-07-0783. [DOI] [PubMed] [Google Scholar]
- 238.Diehn M., Cho R.W., Lobo N.A., Kalisky T., Dorie M.J., Kulp A.N., Qian D., Lam J.S., Ailles L.E., Wong M., et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ito K., Hirao A., Arai F., Matsuoka S., Takubo K., Hamaguchi I., Nomiyama K., Hosokawa K., Sakurada K., Nakagata N., et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 240.Ushio-Fukai M., Zuo L., Ikeda S., Tojo T., Patrushev N.A., Alexander R.W. cAbl tyrosine kinase mediates reactive oxygen species- and caveolin-dependent AT1 receptor signaling in vascular smooth muscle: Role in vascular hypertrophy. Circul. Res. 2005;97:829–836. doi: 10.1161/01.RES.0000185322.46009.F5. [DOI] [PubMed] [Google Scholar]
- 241.Chowdhury A.K., Watkins T., Parinandi N.L., Saatian B., Kleinberg M.E., Usatyuk P.V., Natarajan V. Src-mediated tyrosine phosphorylation of p47phox in hyperoxia-induced activation of NADPH oxidase and generation of reactive oxygen species in lung endothelial cells. J. Biol. Chem. 2005;280:20700–20711. doi: 10.1074/jbc.M411722200. [DOI] [PubMed] [Google Scholar]
- 242.Meng D., Shi X., Jiang B.H., Fang J. Insulin-like growth factor-I (IGF-I) induces epidermal growth factor receptor transactivation and cell proliferation through reactive oxygen species. Free Rad. Biol. Med. 2007;42:1651–1660. doi: 10.1016/j.freeradbiomed.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 243.Chen C.H., Cheng T.H., Lin H., Shih N.L., Chen Y.L., Chen Y.S., Cheng C.F., Lian W.S., Meng T.C., Chiu W.T., et al. Reactive oxygen species generation is involved in epidermal growth factor receptor transactivation through the transient oxidization of Src homology 2-containing tyrosine phosphatase in endothelin-1 signaling pathway in rat cardiac fibroblasts. Mol. Pharmacol. 2006;69:1347–1355. doi: 10.1124/mol.105.017558. [DOI] [PubMed] [Google Scholar]
- 244.Colavitti R., Pani G., Bedogni B., Anzevino R., Borrello S., Waltenberger J., Galeotti T. Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/KDR. J. Biol. Chem. 2002;277:3101–3108. doi: 10.1074/jbc.M107711200. [DOI] [PubMed] [Google Scholar]
- 245.Szatrowski T.P., Nathan C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 246.Zhou Y., Hileman E.O., Plunkett W., Keating M.J., Huang P. Free radical stress in chronic lymphocytic leukemia cells and its role in cellular sensitivity to ROS-generating anticancer agents. Blood. 2003;101:4098–4104. doi: 10.1182/blood-2002-08-2512. [DOI] [PubMed] [Google Scholar]
- 247.Mishra K.P. Cell membrane oxidative damage induced by gamma-radiation and apoptotic sensitivity. J. Environ. Pathol. Toxicol. Oncol. 2004;23:61–66. doi: 10.1615/JEnvPathToxOncol.v23.i1.60. [DOI] [PubMed] [Google Scholar]
- 248.Bhosle S.M., Pandey B.N., Huilgol N.G., Mishra K.P., Bhosle S.M., Pandey B.N., Huilgol N.G., Mishra K.P. Membrane oxidative damage and apoptosis in cervical carcinoma cells of patients after radiation therapy. Methods Cell Sci. 2002;24:65–68. doi: 10.1023/A:1024145931652. [DOI] [PubMed] [Google Scholar]
- 249.Davis W., Jr., Ronai Z., Tew K.D. Cellular thiols and reactive oxygen species in drug-induced apoptosis. J. Pharmacol. Exp. Ther. 2001;296:1–6. [PubMed] [Google Scholar]
- 250.Simon A.R., Rai U., Fanburg B.L., Cochran B.H. Activation of the JAK-STAT pathway by reactive oxygen species. Am. J. Physiol. 1998;275:C1640–C1652. doi: 10.1152/ajpcell.1998.275.6.C1640. [DOI] [PubMed] [Google Scholar]
- 251.Shaw S., Wang X., Redd H., Alexander G.D., Isales C.M., Marrero M.B. High glucose augments the angiotensin II-induced activation of JAK2 in vascular smooth muscle cells via the polyol pathway. J. Biol. Chem. 2003;278:30634–30641. doi: 10.1074/jbc.M305008200. [DOI] [PubMed] [Google Scholar]
- 252.Carballo M., Conde M., El Bekay R., Martin-Nieto J., Camacho M.J., Monteseirin J., Conde J., Bedoya F.J., Sobrino F. Oxidative stress triggers STAT3 tyrosine phosphorylation and nuclear translocation in human lymphocytes. J. Biol. Chem. 1999;274:17580–17586. doi: 10.1074/jbc.274.25.17580. [DOI] [PubMed] [Google Scholar]
- 253.Schieffer B., Luchtefeld M., Braun S., Hilfiker A., Hilfiker-Kleiner D., Drexler H. Role of NAD(P)H oxidase in angiotensin II-induced JAK/STAT signaling and cytokine induction. Circul. Res. 2000;87:1195–1201. doi: 10.1161/01.RES.87.12.1195. [DOI] [PubMed] [Google Scholar]
- 254.Van Montfort R.L., Congreve M., Tisi D., Carr R., Jhoti H. Oxidation state of the active-site cysteine in protein tyrosine phosphatase 1B. Nature. 2003;423:773–777. doi: 10.1038/nature01681. [DOI] [PubMed] [Google Scholar]
- 255.Meng T.C., Fukada T., Tonks N.K. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol. Cell. 2002;9:387–399. doi: 10.1016/S1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 256.Bingisser R.M., Tilbrook P.A., Holt P.G., Kees U.R. Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. J. Immunol. 1998;160:5729–5734. [PubMed] [Google Scholar]
- 257.Nishina T., Komazawa-Sakon S., Yanaka S., Piao X., Zheng D.M., Piao J.H., Kojima Y., Yamashina S., Sano E., Putoczki T., et al. Interleukin-11 links oxidative stress and compensatory proliferation. Sci. Signal. 2012;5:ra5. doi: 10.1126/scisignal.2002056. [DOI] [PubMed] [Google Scholar]
- 258.Uckun F.M., Qazi S., Ma H., Tuel-Ahlgren L., Ozer Z. STAT3 is a substrate of SYK tyrosine kinase in B-lineage leukemia/lymphoma cells exposed to oxidative stress. Proc. Natl. Acad. Sci. USA. 2010;107:2902–2907. doi: 10.1073/pnas.0909086107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kurdi M., Booz G.W. Evidence that IL-6-type cytokine signaling in cardiomyocytes is inhibited by oxidative stress: Parthenolide targets JAK1 activation by generating ROS. J. Cell Physiol. 2007;212:424–431. doi: 10.1002/jcp.21033. [DOI] [PubMed] [Google Scholar]
- 260.Jang E.H., Park C.S., Lee S.K., Pie J.E., Kang J.H. Excessive nitric oxide attenuates leptin-mediated signal transducer and activator of transcription 3 activation. Life Sci. 2007;80:609–617. doi: 10.1016/j.lfs.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 261.Duhe R.J., Evans G.A., Erwin R.A., Kirken R.A., Cox G.W., Farrar W.L. Nitric oxide and thiol redox regulation of Janus kinase activity. Proc. Natl. Acad. Sci. USA. 1998;95:126–131. doi: 10.1073/pnas.95.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Mamoon N.M., Smith J.K., Chatti K., Lee S., Kundrapu K., Duhe R.J. Multiple cysteine residues are implicated in Janus kinase 2-mediated catalysis. Biochemistry. 2007;46:14810–14818. doi: 10.1021/bi701118u. [DOI] [PubMed] [Google Scholar]
- 263.Xie Y., Kole S., Precht P., Pazin M.J., Bernier M. S-Glutathionylation impairs signal transducer and activator of transcription 3 activation and signaling. Endocrinology. 2009;150:1122–1131. doi: 10.1210/en.2008-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Negoro S., Kunisada K., Fujio Y., Funamoto M., Darville M.I., Eizirik D.L., Osugi T., Izumi M., Oshima Y., Nakaoka Y., et al. Activation of signal transducer and activator of transcription 3 protects cardiomyocytes from hypoxia/reoxygenation-induced oxidative stress through the upregulation of manganese superoxide dismutase. Circulation. 2001;104:979–981. doi: 10.1161/hc3401.095947. [DOI] [PubMed] [Google Scholar]
- 265.Oshima Y., Fujio Y., Nakanishi T., Itoh N., Yamamoto Y., Negoro S., Tanaka K., Kishimoto T., Kawase I., Azuma J. STAT3 mediates cardioprotection against ischemia/reperfusion injury through metallothionein induction in the heart. Cardiovasc. Res. 2005;65:428–435. doi: 10.1016/j.cardiores.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 266.Sarafian T.A., Montes C., Imura T., Qi J., Coppola G., Geschwind D.H., Sofroniew M.V. Disruption of astrocyte STAT3 signaling decreases mitochondrial function and increases oxidative stress in vitro. PLoS One. 2010;5:e9532. doi: 10.1371/journal.pone.0009532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Szczepanek K., Chen Q., Derecka M., Salloum F.N., Zhang Q., Szelag M., Cichy J., Kukreja R.C., Dulak J., Lesnefsky E.J., et al. Mitochondrial-targeted Signal transducer and activator of transcription 3 (STAT3) protects against ischemia-induced changes in the electron transport chain and the generation of reactive oxygen species. J. Biol. Chem. 2011;286:29610–29620. doi: 10.1074/jbc.M111.226209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Guo W., Wei X., Wu S., Wang L., Peng H., Wang J., Fang B. Antagonistic effect of flavonoids on NSC-741909-mediated antitumor activity via scavenging of reactive oxygen species. Eur. J. Pharmacol. 2010;649:51–58. doi: 10.1016/j.ejphar.2010.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Wei X., Guo W., Wu S., Wang L., Huang P., Liu J., Fang B. Oxidative stress in NSC-741909-induced apoptosis of cancer cells. J. Transl. Med. 2010;8 doi: 10.1186/1479-5876-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Kola I. The state of innovation in drug development. Clin. Pharmacol. Ther. 2008;83:227–230. doi: 10.1038/sj.clpt.6100479. [DOI] [PubMed] [Google Scholar]
- 271.Kola I., Landis J. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Disc. 2004;3:711–715. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 272.Arrowsmith J. Trial watch: Phase III and submission failures: 2007–2010. Nat. Rev. Drug Disc. 2011;10 doi: 10.1038/nrd3375. [DOI] [PubMed] [Google Scholar]
- 273.Arrowsmith J. Trial watch: Phase II failures: 2008–2010. Nat. Rev. Drug Disc. 2011;10:328–329. doi: 10.1038/nrd3439. [DOI] [PubMed] [Google Scholar]
- 274.Lynch T.J., Bell D.W., Sordella R., Gurubhagavatula S., Okimoto R.A., Brannigan B.W., Harris P.L., Haserlat S.M., Supko J.G., Haluska F.G., et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 275.Druker B.J., Tamura S., Buchdunger E., Ohno S., Segal G.M., Fanning S., Zimmermann J., Lydon N.B. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat. Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 276.Paez J.G., Janne P.A., Lee J.C., Tracy S., Greulich H., Gabriel S., Herman P., Kaye F.J., Lindeman N., Boggon T.J., et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 277.Thatcher N., Chang A., Parikh P., Rodrigues P.J., Ciuleanu T., von Pawel J., Thongprasert S., Tan E.H., Pemberton K., Archer V., et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: Results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366:1527–1537. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 278.Gatzemeier U., Pluzanska A., Szczesna A., Kaukel E., Roubec J., De R.F., Milanowski J., Karnicka-Mlodkowski H., Pesek M., Serwatowski P., et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: The Tarceva Lung Cancer Investigation Trial. J. Clin. Oncol. 2007;25:1545–1552. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- 279.Herbst R.S., Prager D., Hermann R., Fehrenbacher L., Johnson B.E., Sandler A., Kris M.G., Tran H.T., Klein P., Li X., et al. TRIBUTE: A phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J. Clin. Oncol. 2005;23:5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]