Abstract
Nanomaterials hold great promise for medical, technological and economical benefits. Knowledge concerning the toxicological properties of these novel materials is typically lacking. At the same time, it is becoming evident that some nanomaterials could have a toxic potential in humans and the environment. Animal based systems lack the needed capacity to cope with the abundance of novel nanomaterials being produced, and thus we have to employ in vitro methods with high throughput to manage the rush logistically and use high content readouts wherever needed in order to gain more depth of information. Towards this end, high throughput screening (HTS) and high content screening (HCS) approaches can be used to speed up the safety analysis on a scale that commensurate with the rate of expansion of new materials and new properties. The insights gained from HTS/HCS should aid in our understanding of the tenets of nanomaterial hazard at biological level as well as asset the development of safe-by-design approaches. This review aims to provide a comprehensive introduction to the HTS/HCS methodology employed for safety assessment of engineered nanomaterials (ENMs), including data analysis and prediction of potentially hazardous material properties. Given the current pace of nanomaterial development, HTS/HCS is a potentially effective means of keeping up with the rapid progress in this field – we have literally no time to lose.
Introduction
The production of engineered nano-materials (ENMs) represents a scientific breakthrough in material design and the development of new consumer products that are expected to impact almost every industry1–4. ENMs can be used for a host of applications: ranging from building materials, clothes, aircraft and motor vehicle parts and sports goods. ENMs are also anticipated to contribute significantly to the development of the next generation of electronics and will also play a key role in the development of new nano-therapeutics and diagnostics in the field of medicine 5–14.
Although nanotechnology is still considered a young science there is a rapid expansion of the number and identity of novel ENMs. This includes material compositions such as metals, metal oxides, metal chalcogenides (“quantum dots”), fullerenes, single walled nanotubes (SWNT), multi-walled nanotubes (MWNCT), dendrimers etc. Moreover, individual ENMs can be produced to exhibit different sizes, shapes, aspect ratios, crystallinity, surface modifications or hybridization with other nanomaterials. Thus, the number of commercial products that include ENMs has exceeded 1000 consumer products in 2009 and could grow to 104 materials within a decade15.
At the same time, it has to be acknowledged that nanomaterials are not new. The history of ultrafine materials can be traced to ~ 2000 BC, including Chinese ink that contained ultrafine pigment particles or the colloidal gold suspensions that were used for the bright colors in painted church windows. Moreover, since the 50s, companies such as Cabot, Cristal (formerly SCM and Millenium Chemicals), DuPont, and Evonik (formerly Degussa) have been producing materials such as carbon black, SiO2, TiO2, Al2O3 in millions of tons per year for use in paints, catalysts and surfactants. Currently, it is not only the quantity but also the diversity and novelty of nanomaterials that are prompting us to consider the toxicological properties of nanomaterials.
Soft-chemistry routes were developed to synthesize metal oxides ENMs with well defined shape, size and composition 16–18. Around the same time, flame spray pyrolysis (FSP) enabled the synthesis of complex nanomaterial systems using a scalable process. Due to the broad range of available precursor materials, FSP allows for a single step synthesis of a broad class of binary, mixed binary, ternary and mixed ternary metal oxide nanoparticles 19. Furthermore, control of orientation of the particles and the capability to generate aligned and ordered nano and microcrystallites onto a substrate were achieved by masking/patterning techniques such as template, lithography or by epitaxial electrodeposition 20–22.
Almost all known ENM classes, including recently introduced materials, have been shown to pose some biological hazard under experimental conditions (Table 1). However, whether this is relevant from a health and environmental safety perspective is dependent on several additional factors such as the fate and transport of ENMs, exposure, bio-uptake and dose.
Table 1.
Overview of ENM classes and examples of properties which are associated with toxicity responses to the respective ENM class and the observed phenomenon or mode of action.
| ENM class | ENM Property associated with toxicity | Toxicological Phenomenon obrserved/Mode of action |
|---|---|---|
| Metal | Shedding heavy metal (eg. Ag, Cu, Pt) | DNA cleavage and damage leading to genotoxicity and mutation; heavy metal ions induced oxidative stress and inflammatory responses |
| Surface chemistries that affect the structure and function of proteins (e.g. Au) | Protein denaturation that may lead to inactivation of hormones or proteins, opening of cryptic epitopes that could lead to autoimmune diseases, protein fibrillation and accumulation of mis-folded protein could lead to disease conditions such as Alzheimers | |
| Metal oxide | Dissolution and heavy metal release (e.g. ZnO) | Heavy metal ions induced oxidative stress and inflammatory responses |
| Electron hole pair generation during photoactivation (e.g. TiO2) | Electron hole pair generation during photo-activation leading to free radical generation. | |
| Silica particles | Surface defects (e.g. SiO2) | Blood platelet, vascular endothelial and clotting abnormalities |
| Metal chalgenide | Heavy metal release (e.g. CdSe Qantumdots) | ROS generation, lipid peroxidation, DNA damage |
| Fullerenes and CNTs | Heavy metal contaminantion, Aspect ratio >5 | Fibrogenesis and tissue remodeling injury, oxygen radical production, GSH depletion, bio-catalytic mechanisms |
| Polymer | Cationic charge density | Membrane damage/leakage/thinning Protein binding or unfolding responses/, denaturation of proteins or fibrillation, lysosomal damage through proton pump inactivation or lysis of lysosomes |
Consequently, straightforward predictions about the toxicological behavior of the ENMs are not possible as one has to consider all the ENM properties that bring the materials in contact with the biophysical interface and all the biological properties that may contribute to hazard induction at the nano-bio interface 19. The inability to predict ENMs safety or hazard has raised considerable discussion about the safety of nanomaterials, including the best platform to use for material screening 23–26 in academia, industry and regulatory agencies. It is clear from the experience dealing with >60,000 industrial chemicals that it is not feasible to use animal testing as a primary screening platform because of cost, duration and ethical concerns associated with such a venture.
Additional approaches are required to conduct initial screening. One possible solution to this bottleneck is the use of high throughput screening (HTS), one of the leading paradigms for drug discovery since the late 80’s and early 90’s. HTS has since also been adopted by the toxicological community as a screening tool in place of the labor-intensive and descriptive toxicological approaches from the past 27,28. As a result, an arsenal of toxicology assays and platforms that can be used in HTS mode has been adopted for chemicals is now available for ENM toxicity screening. In fact, the National Academy of Sciences (NAS) has put forth a vision and strategy for using HTS approaches to assess multiple toxicants using a robust and mechanistic platform instead of using more descriptive and expensive animal studies29. Similarly, the European Union has implemented the REACH program that requires all chemicals to undergo toxicology testing and have taken note of the EPA ToxCast program that utilizes HTS approaches similar to the NAS vision 30.
Against this background, we will review the necessary characterization methods of nanomaterials, the current state of the art in high throughput toxicological approaches, its applications for ENM screening, as well as outlining the key steps for successful implementation of HTS for ENMs. We will also review the possible pitfalls that are specific to ENM toxicity screening and will present examples of successful HTS screening approaches that have been developed for ENMs generating oxidative stress. We will review how HTS data can be used for ENMs hazard scoring and the important role of data mining approaches that use heatmap and self-organizing maps for data display and are useful for generating of quantitative structure-activity relationships (QSAR).
HTS for ENM Toxicity Screening
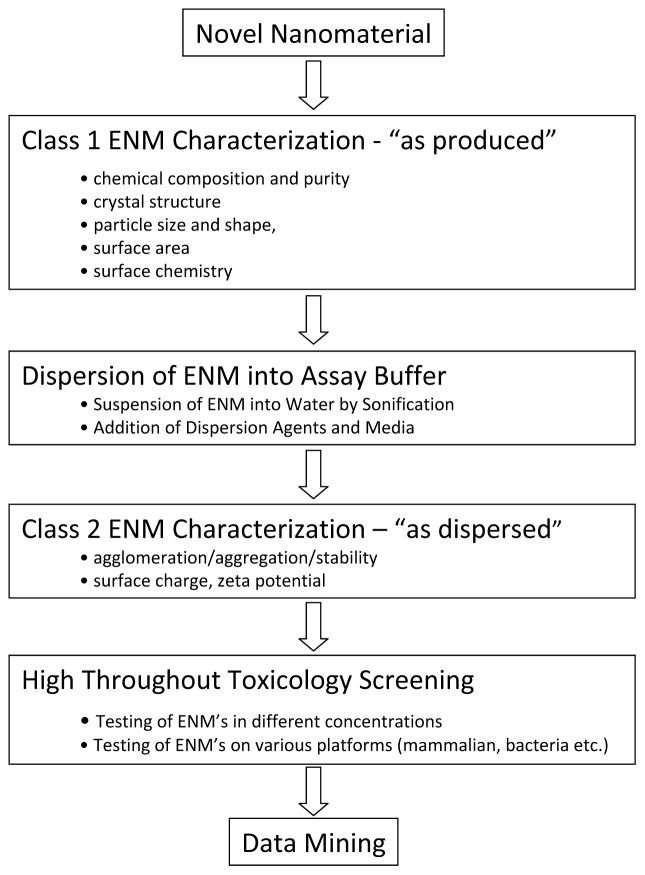
The workflow that is required for ENM toxicity screening is outlined in figure 1. Typically, commercial ENMs are received as dry powders, which are subjected to a detailed class 1 characterization of the physicochemical properties in the “as-produced” state. This includes the assessment of properties such as chemical composition, size, shape and phase. In the next step up to biological screening, the ENMs are suspended in deionized water and dispersed by sonification in the absence or presence of a dispersion agent. Class 2 characterization assesses the size, size distribution, state of dispersal/aggregation, stability and the zeta-potential of the material in water as well as tissue culture media or other relevant assay buffers. This is followed by the actual HTS and data mining, processing and analysis. As evident from the workflow diagram, ENM characterization is a centrepiece of the approach without which it is impossible to relate to HTS outcome to individual material properties 31,32. It is important to understand that the reported ENM characteristics (e.g., size and surface charge) may vary depending on the specific experimental protocol that is used to initially prepare the ENMs. This has sparked efforts such as the Minimum Information of Nanoparticle Characterization (MINChar) to develop standards that ensure ENMs are characterized sufficiently to allow informed interpretation of data and cross-comparison of experimental results in a community of researchers. The MINChar set includes the following nine parameters: agglomeration/aggregation, chemical composition, crystal structure, particle size, purity, shape, surface area, surface charge and surface chemistry33.
Figure 1.
Assay Development Workflow for high throughput toxicity screening of Nanomaterials.
It is important to mention that the absent or incomplete nomenclature to classify ENM poses a considerable problem for correlating material properties with biological outcomes, including the generation of nano-QSARs. For example, carbon nanotubes (CNT) at one point were assigned the same chemical abstract number (CAS number) as graphite, even though they differed in electrical conductivity, presence of metal impurities and different toxicological effects. Thus, while graphite tends to be biologically inert, CNTs are capable of eliciting granulomatous inflammation and fibrotic reactions in the lung. Not only is the ENM nomenclature made complex by differences in chemical composition, but a number of additional physicochemical properties could contribute to toxicological outcomes. It is therefore important to define a set of minimal class 1 parameters to understand the toxicological properties of ENMs in addition to defining more complicated class 2 parameters (e.g., dispersion, agglomeration and aggregation). The latter can be described as the acquirement of new properties due to the modification of the primary ENM properties in response to the composition of the suspending media, including the presence of mono- and divalent cations, pH, temperature and biological molecules, such as proteins. We will briefly review ENM characterization of class 1 and 2 properties (Table 2).
Table 2.
Overview of characterization of ENMs. While class 1 properties are intrinsic to a nanomaterial itself, the class 2 properties are dependent on the interplay between ENM and assay system.
| Nanomaterial property | Available analytical techniques |
|---|---|
| Class 1: “As-produced” -morphology and structure | |
| Primary particle size, shape, and size distribution | TEM, SEM, AFM, ESEM, FIB-SEM, cryo-TEM |
| Fractal structure | TEM, SAXS, SANS |
| Pore size, porosity, and surface area | BET, SAXS, SANS |
| Crystallinity, framework structure, and crystal size | XRD, Raman, SAXS, NMR |
| Chemical composition | Raman, NMR, EDAX, FTIR, XPS, ICP |
| Elemental speciation and redox state | Raman, XAFS, XANES, NMR |
| Electronic, magnetic, and photonic properties | SAXS, Mossbauer, ESR, Raman, UV-Vis |
| “Dustiness” or tendency to aerosolize | EMPS, SMPS |
| Class 2: “As Dispersed” - interfacial properties | |
| Dispersed size and size distribution | DLS, EMPS, SMPS, laser diffraction |
| Aggregate size | DLS, electro-acoustics |
| Charge density, pKa, PZC, ionization fraction | Direct titration in various suspending media |
| Surface (zeta) potential and IEP | EPM measurements in various suspending media |
| Surface tension components (LW, γ +, γ −) | Multiple probe liquid contact angle measurment |
| Roughness and chemical heterogeneity | AFM, CFM, FTIR, XPS, NMR, Raman |
Class 1 Characterization of ENMs – “As Produced”
A number of class 1 ENM properties have been reported to influence ENM hazard potential, including chemical composition, purity, size, shape, surface area, state of dispersion, crystallinity, and surface functionalization 25, 26, 34. The commonly used techniques and tools for assessing this class of physical-chemical properties include:
(i). Assessment of size and shape
A variety of techniques are available for particle size determination, both for primary as well as aggregated oral agglomerated particle size. In most cases, the primary particles size is determined in the “dry” phase. In contrast, the size of agglomerated particles is commonly assessed in various aqueous media or in the “wet phase”. Besides the transmission electron microscopy (TEM) measurements, some of the commonly used methods include Braunauer,-Emmett-Teller (BET) surface area measurements 35, X-ray diffraction (XRD), dynamic light scattering (DLS) 36, 37, static light scattering (SLS) 35, centrifugal sedimentation, atomic force microscopy (AFM), size exclusion chromatography 38, 39, and molecular sieving 35. BET measures the surface area of a given amount of the ENM by gas adsorption. The average size can be estimated based on nonporous spherical model. Additionally, the BET can also be used to analyze the surface area and porosity of samples 40. With particle sizes of < 50 nm, appreciable broadening in the x-ray diffraction might occur due to the non-Bragg scattering phenomenon; this can lead to the incorrect estimation of the average particle size 41. The selection and conduction of these measurements are guided by standard-setting organizations such as the International Standards Organization (ISO), American Society for Testing and Materials (ASTM), United States Pharmacopeia (USP), etc. It should be noted that a sufficient number of particles measurements are required to achieve statistically valid size distribution of nanomaterials, especially for aggregates and polydispersed dry particles.
(ii) Assessment of crystal structure and crystallinity
XRD is one of the classical techniques that can be used for revealing crystallographic structure, chemical composition, and physical properties of metal and metal oxide nanomaterials. This technique elicited the atomic structure of the samples based on the use of pattern, position, intensity and shape of the peaks. X-ray diffraction analysis has become a very important tool in characterizing metal and metal oxide nanoparticles with respect to the arrangements of atoms in the bulk as well as on the material surface. The Rietveld fitting of the X-ray diffraction patterns are important to model and obtain the crystal structure of the nanoparticles which can then be compared to the microscopy results 42. Moreover, small angle X-ray diffraction (SAXS) can be used to determine the crystallographic properties of nanoparticles 43,44. Thus, XRD is commonly used to identify unknown components (e.g. in the elemental composition) and provide structural information of new nanomaterials. Alternatively, X-ray powder diffraction patterns are also used in quantitative determination of the chemical composition of the nanomaterials using standard intensity ratio methods 45, 46.
Class 2 ENM Characterization – “As Dispersed”
While class 1 ENM properties are intrinsic material characteristics, class 2 properties (Table 2) arise from the interplay of the suspending medium (i.e. assay buffer) with the ENM surface. This could impact HTS analysis because ENMs may display altered or new properties, different from small molecules. While, for instance, most small molecules are easily dissoluble in the assay buffer, ENMs often form unstable suspensions and may therefore require dispersing agents to stabilize them in suspension. Even then, one has to be careful to ensure that the ENM suspension remains stable for the duration of the assay. This is not always possible and complicates data analysis as well as dosimetry calculations. Examples of dispersing agents that can be included in HTS assays include serum proteins, phospholipids, polyelectrolytes and a variety of surfactants.
Serum albumin, including bovine serum albumin (BSA), is the most abundant protein in blood plasma. It is believed that nanoparticles are coated by BSA immediately upon the material addition to tissue culture medium. This non-specific binding occurs either through an electrostatic process or binding via a Ca2+ bridge 107. Fetal bovine serum (FBS) is another example of a biologically relevant dispersant that is often used as growth supplement for various mammalian cell culture media. Studies have shown that FBS at a typical concentration of 10% (v/v) in the culture medium can effectively improve nanoparticle dispersion. Another dispersing agent is dipalmitoylphosphatidylcholine (DPPC), which contains a hydrophilic acidic phosphate headgroup and two hydrophobic fatty acid tails. Since DPPC is the major component of lung surfactant, this phospholipid has often been used as a simple mimic of lung surfactant in nanotoxicity studies. Various other surfactants such as poly-vinylpyrrolidone (PVP), Tween 80, and Pluronic F108 have been used for improving the dispersion of carbon allotropes (e.g., carbon nanotubes, carbon black, fullerenes). Special attention should be paid when using all of the aforementioned dispersing agents, as they modify the surface of the material and may therefore interfere in toxicity screening: For example, DPPC in relatively high doses has been shown to completely suppress the cytotoxicity and pro-apoptotic affects of quartz nanoparticles 108. This shielding can be avoided by using relatively low concentrations (e.g.160 μg/mL). The commonly used techniques and tools for assessing class 2 properties include:
(i) Surface charge and surface energy
The surface charge of nanomaterials is particularly interesting as it is one of the factors controlling the dispersion and aggregation of ENMs as well as affecting cellular uptake. From the characterization perspective, surface charge is usually reported as zeta potential, which represents the electric potential in the interfacial double layer 34 as well as the pKa of the particle. While pKa can be determined by titration, the zeta potential of the nanomaterials is determined by light-scattering electrophoresis or electro-acoustophoresis methods. It should be noted that the aqueous phase chemistry (e.g., electrolyte concentration, type of electrolytes and pH) significantly impacts the zeta potential and should be reported together with the zeta potential.
Liquid contact angle assessment elucidates additional information about surface chemistry, charge, and energy 47–51. Furthermore, the interfacial free energy at the site of cohesion (per unit area) is indicative of the inherent (thermodynamic) stability or aggregation propensity of a given material emerged in water 52, 53: The higher the free energy at the site of adhesion, the higher the chance of an interaction taking place to lower the surface energy. Alternatively, the free energy of adhesion (per unit area) of two different materials (e.g., a nanoparticle and a sand grain) immersed in water gives an indication of inherent (thermodynamic) stability or deposition propensity. Thus, the higher the free energy, the more hydrophilic, stable, and deposition resistant is the material 53.
(ii) Aggregation
ENMs posses a high surface area compared to micron size or bulk materials and could utilize this characteristic to interact with other substances in the suspending medium. This could lead to the formation of aggregates with a much larger hydrodynamic diameter than the parental material and can also interfere with analytes in the assay. In an extreme case, EMNs may precipitate e.g. onto the cells at the bottom of a plate leading to a local dose higher than the originally intended concentration. This aggregation is mainly due to some weak inter-particle interactions such as van der Waals forces and electrostatic attractions. Although ENM surface charge, pH and ionic strength influence the agglomeration potential, there are no hard and fast rules. An additional compounding factor is that aggregation is also a function of the ENM concentration.
ENM aggregation can be measured by dynamic light scattering (DLS) techniques. For the purpose of a quick screening procedure to select suitable assay buffer system in which to perform HTS, high throughput dynamic light scattering is an excellent choice. Dynamic light scattering measurement – as used in e.g. the Dynapro™ plate reader (Wyatt Technology) - relies on measuring the Brownian motion that is proportional to the hydrodynamic diameter of the particles. DLS can be used as a convenient and effective technique for determining nanoparticle size distribution in the same format as being used for HTS, e.g., by using 384 well plates.
As DLS measures only the size of the particle in solution, it is important to keep in mind that precipitated materials at the bottom of the wells are not being measured. As an alternative method, a sedimentation approach using UV-vis spectroscopy can be used as a rough measure for evaluating nanoparticle sedimentation. Without aggregation and stability measurements, accurate dosing is impossible and will result in questionable HTS results. Unfortunately, few studies have addressed the stability of the dispersions towards assay development, including looking at the effect of proteins or other chemical surfactants on nanoparticle stability in tissue culture media.
Conceptual approach to high throughout toxicology screening
In the planning stage of selecting a HTS approach for ENM safety screening it is important to be cognizant of the workflows approaches that are used for toxicological screening of small molecules. The available approaches can be broken down into (i) classical plate reader-based assays using time-honored readouts such as luminescence, fluorescence, absorption, time-resolved fluorescence and fluorescence polarization or (ii) high content screening (HCS) approaches. Time-resolved fluorescence energy resonance transfer (TR-FRET) or fluorescence polarization (FP) platforms are less often used because of high cost or sensitivity to interference from contaminants and temperature variations.
Because traditionally plate reader assays have been used for small molecule safety assessment, they have yielded multiple platforms and read-out modes that can be used for toxicological assessment. These platforms include the use of luminescence, fluorescence or absorption approaches to screen for mutagenicity or genotoxicity in bacteria, yeast or mammalian backgrounds. Examples include the bacterial-based VitoTox test54 that uses luciferase expression from the recN promoter in response to DNA damage. A similar-based approach in yeast is the GreenScreen assay55 in which the RAD54 promotor drives GFP expression that can be quantified by fluorescence. In the yeast-based RadarScreen56 the GFP is replaced by β-galactosidase, the activity of which is recorded by colorigenic or luminogenic substrates. Further up in the evolutionary chain, the GreenScreen HC assay makes use of a TK6 lyphoblastoma cell line that reports damage to a GADD45α promoter linked to a firefly luciferase reporter gene.
Cytotoxicity can be easily assessed by utilizing dyes such as propidium iodide (PI) and calcein-AM that can be assessed in a plate reader assay 57. While PI is impermeable to the intact surface membrane, calcein AM is a membrane permeable fluorescein-conjugated derivative that is cleaved by esterases and not capable of exiting the cell unless the membrane integrity is compromised. Cytotoxicity can also manifest itself as a depletion of cellular gluthathione content that can be assessed by monochlobimane fluorescence 58 or as increased production of radical oxygen species (ROS) that can be quantified by dichlorofluoresceine acetate 59. The redox potential of the cell can also be followed using dyes such as MTT or Alamar Blue60. Luminescence-based alternatives are CytoLite (measures NADH) 61, ATP-Lite or CellTiter Glo (measure ATP) 62. Another approach is to assess the generation of apoptosis, which is frequently induced by hazardous ENM, through a Z-DEVD-aminoluciferin 63 assay that assesses caspase 3 and 7 activation or the use of LETD-aminoluciferin that assesses caspase 8 and 9 activation. Cleavage of these peptides by the caspases in question releases aminoluciferin that can be converted by firefly luciferase.
Another high throughput approach to toxicity assessment is to screen for endocrine disruptors using easy to perform reporter gene assays 64. This has allowed the assessment of androgen, estrogen, progesterone and glucocorticoid receptors in CHO cells through the use of luciferase and reporter gene assays.
HCS based assays are relatively new toxicological screening tools and provide significant advantages over the classical plate reader assays in terms of specificity and sensitivity. O’Brien et al. published a landmark study66 in 2006 during which they compared conventional single readouts to a five-parameter HCS assay that utilize a combination of fluorescent compounds for toxicological assessment. The results were quite striking. While the single parameter readout had limited sensitivity (<25%) but high specificity (about 90%), the HCS approach showed a sensitivity of 93% and a specificity of 98%. Even more interestingly, although they used only a hepatocyte (HepG2) cell line, they were able to detect 92% of the toxic drugs, even those with non-hepatotoxic potential. Thus, HCS is potentially one of the best tools available for high throughput ENM screening. In fact, in 2008 Kotov recognized HCS as a universal tool for ENM cytotoxicity profiling and characterized cadmium telluride quantum dots and gold nanopartciles using this technique 115. However, while a variety of assays are available for toxicity screening of small organic molecules, it remains to be seen whether a wide range of ENM toxicology responses can be covered by HCS approaches. This will require discovery of ENM toxicological pathways in which the HCS approach can be used for hypothesis development about ENM properties capable of engaging those injury pathways. Last but not least novel assay platforms are also required for the assessment of the environmental impact of the ENM in organisms such as bacteria, yeast, zebra fish and oyster embryos. In order to elucidate the use of high throughput approaches for ENM screening, let us review the individual steps in such an effort.
Methodological approach to ENM toxicity testing
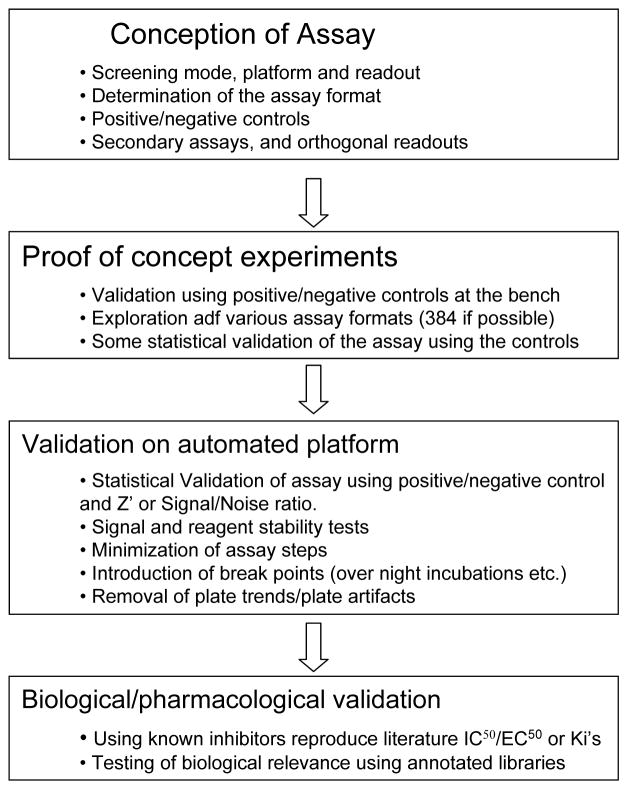
While the essential steps and workflow requirements are identical to small molecule screening (figure 2), it is important to elucidate special requirements for ENM toxicity screening, which overall poses more challenges that screening of small molecules:
Figure 2.
Assay Development Workflow. Adapted from: Molecular Screening R.Damoiseaux Handbook of Drug Discovery Wiley and Sons, forthcoming
(i) Conceptualization of assay development
This is the single most important step in workflow development. The first requirement is selection of a platform as well as a read-out for the assay. A list of possible readouts that can be used for toxicological screening appear in Table 3. For a target-based assay, it is important to identify and isolate the target, e.g., a cell-free biochemical assay that can be used as the sole target. Examples include testing for abiotic ROS generation by ENMs through the use the fluorescent dye, 2,7-dichlorofluoresceine 59. This assay can be further refined for high throughput testing of ENM that generate oxygen radicals through photoactivation, e.g., UV-induced electron-hole pair activation by TiO2 67. While a cell-free screening allows quantitative assessment under abiotic conditions, this is not necessarily indicative of how ENM react in cellular environments where, for instance, ROS generation can be dampened by a homeostatic antioxidant response. Thus, it is essential to develop cell-based approaches as a screening platform for toxicological impact on mammalian cells, bacteria, yeasts or lower-level organisms in the environment. In these cell-based systems one needs to select appropriate toxicological, cellular signaling or stress pathways for HCS. Among the injury responses that have been elucidated to date 70 oxygen radical generation, induction of cellular oxidative stress 71, organellar damage through partial or complete nanoparticle dissolution with the release of toxic metal ions 72, cationic surface charge 73, protein damage or triggering of the protein unfolding response 74, membrane leakage, generation of inflammation (including chronic granulomatous inflammation), induction of frustrated phagocytosis in phagocytic cells, fibrogenic responses, and activation of cell signaling cascades 75 have emerged as important mechanistic paradigms.
Table 3.
Examples of toxicity paradigms, possible analytes, readout modes and potential problems when using various readouts for ENM toxicity screening.
| Toxicity Type or paradigm | Analyte | Probes | Readout Mode | Utility | Potential problem |
|---|---|---|---|---|---|
| Cytotoxicity | Cell number/proliferation | Hoechst 33342/DAPI | Fluorimetry/High content assay | Cell quantification, nuclear content | Background signal from NPs with blue fluorescence |
| Membrane leakage | Propidium Iodide/Syto 9 | Fluorimetry/High content assay | Compromised cell membrane integrity | Background signal from NPs with red fluorescence (e.g. QDs) | |
| Membrane integrity | LDH assay | Absorbance at 490 nm | Cell viability | NPs may inhibit enzyme and/or absorb at 490 nm (CNTs, Ag) | |
| ATP | ATPlite™ | Luminescence | Mitochondrial activity and Viability status of cells | Not appropriate for NPs that may inhibit enzyme and/or absorb light eg. CNTs | |
| Mitochondrial membrane potential | JC1/TMRM/chloromethyl-X-rodhamine | Fluorimetry/High content assay | Loss of MMP | Background signal from NPs with red or green fluorescence | |
| Metabolic activity | MTT, WST-1, XTT. | Absorbance | Mitochondrial activity and Viability status of cells | NPs may inhibit enzyme and/or absorb light or substrates | |
| Intracellular calcium flux | Fluo-4/Fura 2-AM/Rho 2- AM | Fluorometry/High content assay | Increased Intra-cellular calcium level | Background signal from NPs with green fluorescence | |
| Apoptosis | Calcein-AM | Fluorometry/High content assay | Mitochondrial membrane permeability transition (MPT) | Background signal from NPs with green fluorescence | |
| Genotoxicity | DNA cleavage | Micro-nuclei assay (HCS), BrDU incorporation | High Content Screening | Chromosome damage | Background signal from NPs with blue fluorescence |
| Inflammation | IL-1, IL-8, TNF-α | Antibody based ELISA or TR-FRET | Luminescence/TR-FRET | Expression level of inflammatory markers | Not appropriate for NPs that interfere with TR-FRET (e.g. absorb protein) or luminescence reactions |
| NF-kB and AP-1 activation | Reporter genes | Luminescence | Activation of inflammatory pathways | Not appropriate for NPs that may inhibit enzyme and/or absorb light | |
| Oxidative stress | GSH, ROS | Absorbance/HCS using fluoresence probes | Fluorometry/High content assay | Free radical generation, Glutathione depletion | Not appropriate for NPs that may interfere with fluorescence output |
| Fibrogenesis | TGF-1b, Collagen 1&3, MMPs | Fluorescence probe coupled antibody | Fluorometry/High content assay | Induction of fibrogenesis | Not appropriate for NPs that may interfere with fluorescence output |
In addition to screening of cells, whole-organism screening has been used successfully for toxicity studies 68. Whole organism screening using platforms such as the zebrafish allows for the screening of several developmental stages. While this type of screening can make the range of targets that can be covered even more complete, these methods have not yet been developed into to high throughput approaches.
It is important for ENM toxicity screening to consider that cell-based and organism-based platforms differ in the temporal manifestation of their biological outcomes. Thus, while in vitro responses at cellular level often take place over the course of minutes to hours, the response in zebrafish embryos may require days and could therefore have an impact on the utility of the high throughput approach.
It is important to consular that the responsiveness of a particular cell type to potentially hazardous ENM properties could be target-specific and that cellular phenotype, lineage, transformed stage, and stage of differentiation are important. As an example, Weissleder showed that the predictive power of an in vitro ENM cytotoxicity assay is greatly improved by including multiple cell lines (4 different cell types) and using at least four different doses of the materials 69. Thus, the choice of cell type is essential to the success of HTS.
After the selection of the assay mode and toxicological paradigm for screening, one needs to decide on the appropriate assay read-out. This choice has to take into consideration the type of material because several ENM compositions restrict the toxicological endpoints that can be assessed (Table 3). In contrast to small molecules, several unique ENM properties such as high adsorption capacity, optical properties, catalytic activity etc could interfere in the assay, leading to false positive or negative results 76. And old aphorism in HTS is “You get what you screen for” definitely holds true for toxicity screening. While designing this screening assay, care should be taken to counter the potential artifacts by inclusion of adequate controls and complementary assays that have an orthogonal readout. Thus, if the first assay relies on fluorescence, the second should use a luminescence read-out. It is also important to consider that while some ENM cytotoxicity assays (e.g. MTT) use tetrazolium derivatives and optical density as readout, that this can lead to invalid results with carbon based nanomaterials (carbon black, multiwalled carbon nanotubes, C60) or QDs due to dye/dye interactions and/or NM adsorption of the dye products 77. In a similar fashion the assessment of membrane integrity by measuring lactate dehydrogenase (LDH) activity in the culture supernatants through the use of the tetrazolium salts is prone to interference by ENMs. In this instance, the ENM could inhibit the enzymatic conversion and/or ability of the nanomaterial to absorb at the reading wavelength 78, 79.
While a HTS assay for each type of toxicity is theoretically possible, the cellular response that is being tested should be clinically or pathologically relevant. This approach could be supplemented with a larger panel of assays that cover additional injury mechanisms. General toxicity metrics such as cell number and redox potential are very useful to assess toxicity even at sub-lethal level. It is obvious that in addition to mammalian screening assays, HTS should also be implemented in bacteria, yeast and zebrafish in order to gain a comprehensive understanding of ENM toxicity profiling in the environment.
(ii) Proof of principle experiments
This step implements small scale testing that is typically performed manually at the benchtop. The goal is to establish a basic assay that incorporates positive and negative controls, work out basic assay parameters such as reagents, concentrations and incubation times and assay formats. Positive and negative controls, e.g. toxic and non-toxic ENM reference materials, are included in order to explore the viability of the HTS approach. This involves the calculation of the assay quality assessment parameter known as the Z′, and is based on the data obtained from the evaluation of a small number of negative and positive control samples. A Z′>0.5 is required for implementation of a HTS approach 80. At this stage it is also necessary to check on interference in the assay read-out as a result of the ENM properties discussed above.
(iii) Validation on the automated patform
Not all assays can be automated since the workflow has to be very simple and the assay results highly reproducible to make it a candidate for automation. Because every step in an assay could introduce some variability it is imperative to simplify the assay as much as possible. For example, two reagents might be added together, thus saving an assay step. It is also necessary to know precisely where breakpoints are allowed, when the assay can be stopped for an extended period of time without loss of performance. It is also important to analyze dose-response relationships in assessing ENM toxicity in order to validate assay performance as well as ensuring that the assays is carried out in the linear range of the dose response curve. This requirement is similar to the qHTS approach of the NIH Chemical Genomics Center.81 However, while small organic molecules can be stored in dilution series, a ENM dilution series can not be stored but has to be made fresh for each experiment. This makes the screening process much more complicated.
(iv) Biological/pharmacological validation
After establishment of the automated assay, it is imperative to check if the selected assay has the biological relevance originally aimed for. This requires the comparison of positive and negative ENM controls as well as comparison to small molecule agonists/antagonists, signaling pathway inhibitors, antioxidants, charge neutralization, alteration of dissolution properties, surface coatings etc. For validation, a small library of ENMs with known properties or a set of small molecules with known pharmacological profile can be subjected to this assay and the results should indicate whether the assay generates a result profile that reflects the anticipated outcome.
Examples of successful implementation of HTS to assess ENM hazard potential
Although the use of HTS for ENM safety assessment is still in its infancy, some screening assays that have already been established have proven very useful and have allowed for prioritizing ENMS for in vivo testing based on the screening results. Shaw et al devised a panel of assays in HTS format to evaluate the adverse biological effects of a panel of paramagnetic iron oxide nanoparticles with potential medical application in molecular imaging 69. They measured cytotoxic events such as ATP content, reducing equivalents, caspase-mediated apoptosis and mitochondrial membrane potential in different cell lines. Their analysis enabled them to identify nanomaterials with similar biologic activities elucidating the structure-activity relationships between particle properties and biological outcome 69. Moreover, they demonstrated that the predictive power of in vitro assay increased as a function of the number of cell lines and cellular events tested 69.
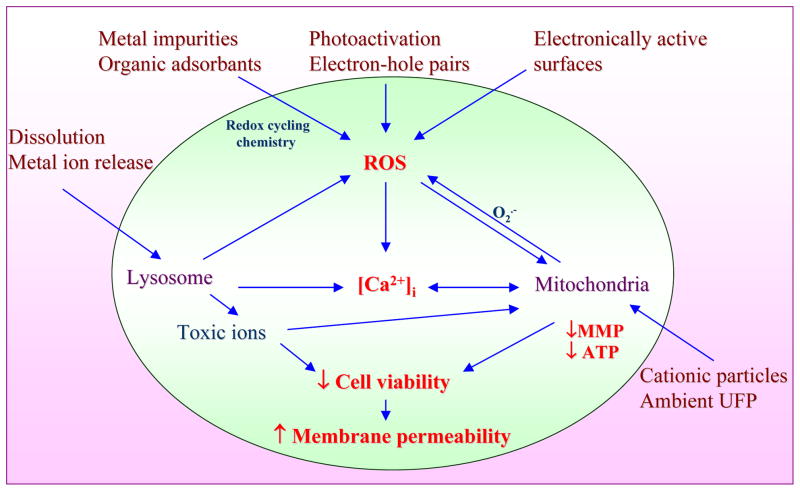
Upon considering toxicological paradigms, one of the best HTS examples that have emerged is the screening of ENM hazard potential according to the principle of cellular oxidative stress. In particular, we have demonstrated that ZnO nanoparticles are capable of engaging a toxicological pathway in a bronchial epithelial cell line that can be picked up by a multi-parametric oxidative stress assay that is also valid in the pro-inflammatory effects of the particles at cellular level. The in vitro screening, in turn, is analogous to the effect of welding fumes causing metal fume fever by exciting inflammation in human lung 82, 83. The origin of this assay is lies in prior demonstration that ambient particulate matter and a number of redox active ENMs are capable of inducing an incremental cellular oxidative stress according to ‘hierarchical oxidative stress paradigm’ (figure 3). Different levels of oxidant stress have been classified as antioxidant defense (Tier 1), pro-inflammatory (Tier 2), and cytotoxic (Tier 3) cellular responses, each of which are induced by specific sensing and signaling pathway. Thus, Tier 1 responses are initiated by the activation of the transcription factor, Nrf2, that transcriptionally activates the anti-oxidant response element (ARE) in the promoters of >200 phase II antioxidant defense, anti-inflammatory and cytoprotective genes. This pathway exerts anti-inflammatory effects and restores cellular redox equilibrium, including new GSH synthesis. This pathway may be engaged at relatively low levels of oxidative stress as well as electrophilic chemicals such as sulforaphane and resveratrol that are found in foods 84. Failure of this homeostatic response, leads excessive ROS production to activate pro-inflammatory pathways such as the mitogen-activated protein kinase (MAPK) and nuclear factor-kappa B (NF-κB) cascades. In the context of oxidative stress, these pathways and the accompanying cytokines and chemokines represent Tier 2 of the hierarchical oxidative stress response and are relevant to particulate matter related disease processes such as asthma and atherosclerosis. Examples of pro-inflammatory responses in macrophages and epithelial cells include TNF-α and interleukin 8 (IL-8) production, respectively. These are also the characteristic cytokines that are found in the lung and bronchoalveolar lavage fluid of welders that develop metal fume fever 72. The increased redox in-equilibrium at the highest level of oxidative stress (Tier 3), triggers intracellular Ca2+ flux, perturbs mitochondria to the extent that is triggering cellular apoptosis or apoptosis-necrosis. The cytotoxic response pathway can be triggered by a number of ENM types capable of ROS production as a result of the presence of transition metal impurities, ability to shed toxic metal ions, presence of electronically active surface (e.g., semiconductors), photo activation or ability to catalyze biotic ROS production 70, 71.
Figure 3. Hierarchical oxidative stress model explains cellular events occurring sequentially as the level of oxidative stress increases.
A mild oxidative stress activates the protective antioxidant machinery in an attempt to restore the redox equilibrium (Tier 1). Higher levels of oxidative stress activate MAPK and Nf-kB cascade of molecular events leading to pro-inflammatory responses (Tier 2). Oxidative stress beyond the tolerable limit of cells, triggers cell death pathways often manifested as increased cytosolic Ca2+ level, lowering of mitochondrial membrane potential, opening of mitochondrial permeability transition and cell membrane damage (Tier 3).
A variety of biological assays and biomarkers have been developed for screening Tier 1,2 and 3 cellular oxidative stress responses. Although, these endpoints were initially developed as individual assays that can be studied independently or in a batch, this is a labor intensive task that could theoretically be integrated in a multi-parametric assay that is useful for HTS (Table 3). After extensive experimentation we found compatible cellular dyes that could be used for contemporaneous screening of a number of Tier 3 responses by epifluorescence microscopy. This assay utilizes the DNA intercalating dye, Hoechst 33342, to assess cell number and nuclear size, 5,5′,6,6′-tetrachloro-1,1′,3,3′ tetraethylbenzimidazolylcarbocyanine iodide (JC 1) to assess perturbation of mitochondrial membrane potential (MMP), Fluo 4 to assess intracellular calcium flux and propidium iodide (PI) to report increased membrane permeability in dying cells. An example of the fluorescence changes that develop in cells being treated with ZnO nanoparticles is shown in figure 4. The data demonstrate that the drop in MMP (panel A) in a bronchial epithelial cell line (BEAS-2B) is accompanied by green cytoplasmic fluorescence distinct from the red fluorescence (and granular) staining of healthy mitochondria in untreated cells. Plasma membrane damage, leading to PI uptake and increased cellular [Ca2+] flux results in increased red and green fluorescence, respectively (panel B). The functional linke between cellular ROS production, [Ca2+]i flux, mitochondrial depolarization and plasma membrane damage is explained in figure 5 and explains the elements of a multiparametric assay that reflects sublethal and lethal oxidative stress responses.
Figure 4. High content screening for assaying cytotoxic events triggered by nanoparticle interaction.
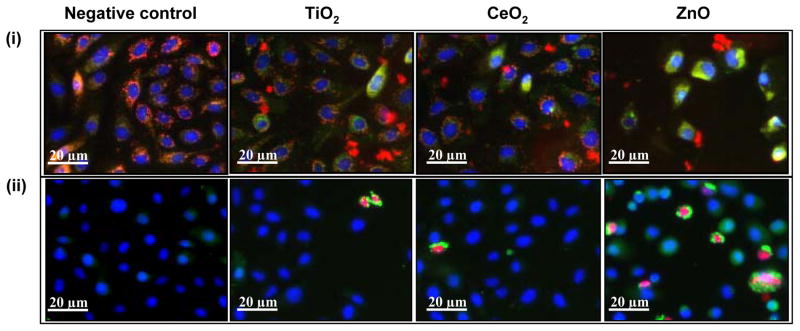
(i) BEAS-2B cells subjected to nanoparticles, stained with nucleic acid staining Hoechst 33342 and mitochondrial dye JC1. Healthy cells shows blue nuclei and red mitochondria (Negative control, TiO2 and CeO2) while the mitochondrial depolarization causes the cytoplasm to fluoresce green (green cells in ZnO). (ii) BEAS-2B cells treated with nanopaticles and stained with a dye cocktail of Hoechst 33342, fluo-4 (for intracellular Ca2+) and propidium iodide (for accessing membrane damage). Healthy cells shows blue nuclei, while damaged cells shows green cytoplasm and red nuclei (cells in ZnO group). Depending upon the color profile, the percentage of cells affected can be assayed and nanoparticles can be ranked for their cytotoxic potential.
Figure 5. Hazardous nanomaterial properties leading to ROS generation and induction of integrated events in cytotoxicity pathway.
Nanomaterials induce ROS production as a direct consequence of specific material properties or as a consequence of triggering cellular injury responses leading to oxidant radical generation. ROS production could trigger a range of oxidative stress effects as outlined in the hierarchical oxidative stress model. The induction of cellular toxicity at the highest level of oxidative stress involves a number of interrelated cellular responses that include intracellular Ca2+ release and mitochondrial perturbation leading to cell death with accompanying changes in cell membrane integrity and nuclear PI uptake.
It is possible to assess Tier 2 oxidative stress responses in high throughput mode, either by measuring the cytokine release in the cell culture supernatant or by monitoring the activation of signaling cascades linked to transcriptional activation of cytokine promoters. This can be accomplished by developing appropriate reporter gene assays. The commonly tested human and murine inflammatory markers are interleukin-8 (IL-8), TNF-α and IL-6. The most common approach is to measure the production of cytokines or chemokines by classical ELISAs that can be combined with HTS, e.g., harvesting cellular supernatants through robotic processing. Because this requires a washing step that is problematic, the assay could be modified by using TR-FRET technology to measure cytokines in the supernatant with the cells. The detection of signaling pathways involved in inflammation through the use of reporter gene assays is feasible but not very specific. A number of key cellular signaling cascades are involved in oxidative stress, inflammation and cytotoxicity. These include the Nrf2, Jun kinase (JNK), NF-κB, AP-1 and P38 pathways.
While screening for oxidative stress is considered a pathway-specific approach, ENM toxicity screening in a bacterial or yeast platforms can only be achieved by using phenotypic screening approaches. Among the phenotypic assays, growth assays utilizing optical density (OD) assessments offer the most straightforward approach to ENM toxicity screening. Frequently used OD metrics include doubling time and carrying capacity. However, these assays do not reflect the populations of bacteria that are dead or alive. A critical issue in bacterial ENM toxicity testing is the fact that frequently used growth media do not reflect the oligotrophic conditions that may be encountered in a real-life exposure environment. Assay buffers that reflect these oligotrophic conditions might be more helpful.
More information can be gained using assays that assess bacterial viability rather than measuring total biomass. These assessments can be performed by tailoring the assay buffer to reflect a real world exposure environment. One example is an assay that utilizes Alamar Blue to assess the redox potential of the live bacteria85. Another more universal assay is performance of ‘live/lead’ assays that distinguishes the live from dead bacteria utilizing a pair of nucleic acid dyes, namely green-fluorescent SYTO® 9 and red-fluorescent propidium iodide (PI). While SYTO® 9 penetrates cell membranes and labels live and dead bacteria the PI stain only permeates the membrane of non-viable bacteria. Thus, the dead bacteria exhibit significantly less green florescence while showing a predominant red signal. The green to red fluorescence ratio represents a quantitative index of bacterial viability that is obtainable within minutes after the addition of the dyes 86–92. The live/dead assay has been applied to a variety of bacteria cultures including Acidianus brierleyi, Bacillus cereus, B. subtilis, Clostridium, perfringens, Escherichia coli, Klebsiella pneumoniae, Micrococcus luteus, Mycobacterium phlei, Pseudomonas aeruginosa, P. syringae, Salmonella oranienburg, Serratia marcescens, Shigella sonnei, Staphylococcus aureus, Streptococcus pyogenes, and Sulfolobus acidocaldarius 89. The results obtained from the live/dead assay are normally in good agreement with standard plate counts methods. This approach has been used by a number of research groups to evaluate the toxicological in response to ENMs, including Ag, Au, ZnO, TiO2, QDs, etc 77, 93–97. Since the assay is ratio-metric it is more robust than standard single point assays. However, it needs to be mentioned that the assay requires establishment of a calibration curve which is different for each bacterial strain and the buffer the assay is being performed in. Not surprisingly, the composition of the assay buffer exerts a big influence on the ENM toxicity assessment.
Data analysis and modeling of toxicological properties of ENMs
The lack of systematic toxicological assessment of well-characterized ENMs has hindered the development of predictive ENM toxicological models. HTS is one of the most powerful methods to acquire such data but has to be supplemented with equally powerful data analysis tools to establish property-activity relationships. HCS or HTS can be used to assess the influence of deliberate experimental variation of nanoparticle properties such as composition, size, charge, shape, aspect ratio, crystallinity, state of aggregation or dispersal. The same approach can be used to probe the effect of a variation in environmental parameters such as pH, temperature, salinity, solution chemistry and presence of natural dispersing agents. However, due to the lack of a priori quantitative models of NP toxicity, the challenge is to determine if the generated data sets span an adequate range (magnitude) of selected NP characteristics and toxicological parameters. Here, the development of models based on experimental data (i.e., data-driven models) has proven to be useful in situations where there is no prior knowledge of the complete set of equations governing the modeled phenomenon.
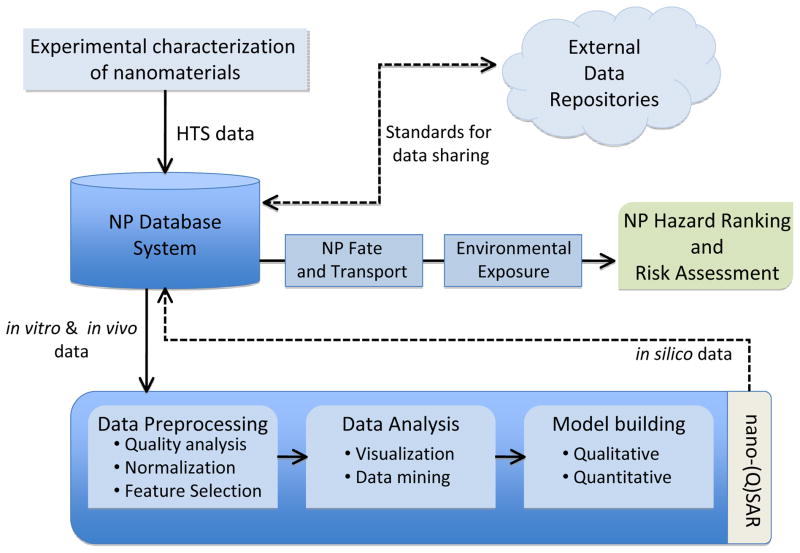
The development of a computational framework to assess the toxicological effects of nanomaterials (NM) requires a novel integrative approach that combines information technologies that allows data management through advanced algorithms and modeling approaches. The three main elements of the data analysis framework are illustrated in figure 6 and include: (a) an ENM database system, (b) modeling, and (c) hazard-ranking.
Figure 6.
Components of a computational Framework for Predictive nano-Toxicology.
Experimental data related to ENM toxicity are derived from multiple assay platforms. The heterogeneous data set is quite complex and highly dimensional as a result of ENM characteristics, screening approach, assay platform etc. It has been recognized that, unlike single chemical molecules, chemical and structural descriptors that specify ENMs is a challenging endeavor. To this end, a number of initiatives have emerged that focus on standardizing the diverse aspects of nanotechnology data sets. Central to this effort is the development of a common nomenclature for nanoparticles to facilitate linkage of different ENM toxicity screening efforts. Towards this goal, a systematic approach towards codifying ENMs has been put forth by Gentleman and Chan 14 while Tomalia 98 suggested a system for ENM classification that is premised on atom mimicry and the periodic table of elements. Other efforts in this area are directed at the adoption of an approach similar to that used with Life Science Identifier 99 in order to identify bioinformatics data objects. For example, the cancer nanotechnology laboratory portal is designed to facilitate data sharing activities by providing support for the annotation of ENM with physicochemical and in vitro characterizations, as well as the distribution of these characterizations and associated protocols in a secure fashion.
Although computational chemistry and predictive toxicology for nanoparticles are still in their infancy, theses fields are fully developed for chemical compounds. Initiatives such as the EPA ToxCast™ Program and the collaborative OpenTox Initiative have already identified the main issues (e.g., ensuring unified access to distributed toxicological resources including data, computer models, validation and reporting) and provide the basis for the development of a predictive toxicology framework for chemicals. Lessons learned from these initiatives are key towards establishing an integrated framework for predictive toxicity of ENM.
HTS data preprocessing
The application of data-driven techniques for data mining and model development requires high data quality to ensure correctness and reliability of the results. Errors such as liquid handling errors contribute to cell growth variability or fluctuations in nanoparticle concentration and may introduce significant levels of noise in HTS data 100. Such errors can be partially compensated for by using replicate measurements and procedural quality controls such as positive and negative controls to reduce assay variability. Indeed, the use of control wells (i.e., cells not exposed to ENMs) helps to characterize plate-to-plate variability in multi-plate assays and to establish the proper assay background levels. The normalization of raw data between plates using control wells removes interplate variability and leads to experimental measurements that are comparable across plates. A detailed discussion of statistical methods for pre-processing HTS data can be found elsewhere 101.
Since the readouts from HTS plates are in form of numbers, the categorization of the results requires distinguishing between significantly reactive and non-reactive ENMs. The common practice is to apply predefined activity thresholds to discriminate between active and non-active NP. However, this approach tends to be biased towards the detection of highly active NPs whereas lower activities close to the threshold have a higher probability of being masked due to measurement errors. Different methods have been proposed in the literature to address this critical issue, including the use of local thresholds based on similarity clustering 102, 103.
HTS Data Analysis
Since HTS data are high dimensional, it is a common practice to use dimension reduction (or feature extraction) methods to project the data onto lower dimensional feature spaces more suitable for interpretation and visualization. Related to dimension reduction, cluster analysis can be used in the context of HTS data to extract similar activity patterns and to perform ENM grouping based on similar biological effects and physicochemical properties.
Agglomeration algorithms for clustering begin with each element as a separate cluster and merge them into successively larger clusters. Divisive algorithms begin with the whole set and proceed to divide it into successively smaller clusters. In contrast, partition clustering methods such as K-means determine a single cluster partition.
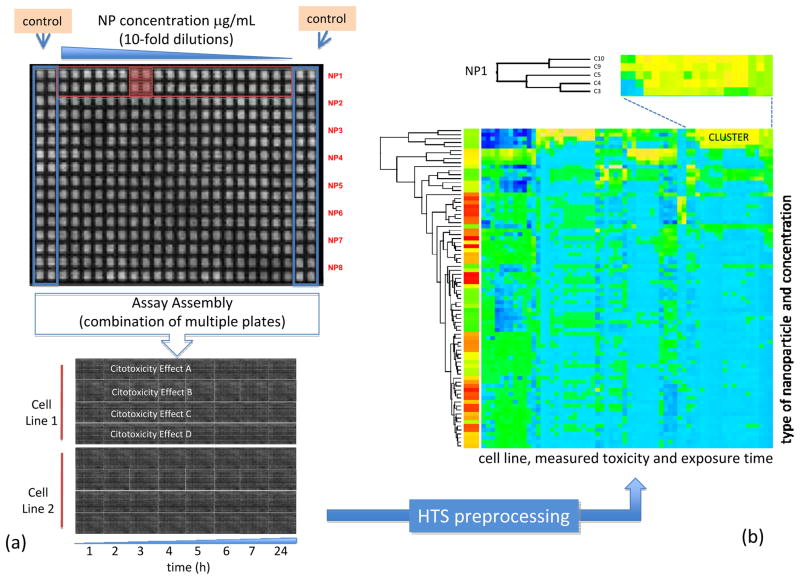
Heat-map clustering has played an important role in HTS data analysis 104. Heat-maps provide, through row and/or column clustering, ordered representations of data that facilitate the identification of similarity patterns of large datasets. The same principles that have been used for heat-map based analysis of gene expression data can be adapted to the analysis of HTS nanoparticle toxicity datasets. Once properly preprocessed, the different plates containing data for specific HTS assays can be arranged in a form that is suitable for heat-map analysis. Figure 7 illustrates the use of heat-maps to analyze a HTS data set obtained from the experimental setup described in figure 7a. Figure 7b depicts the cytotoxic activity patterns projected over the heat-map where rows and columns corresponding to nanoparticles at a specific concentration and to the measured cytotoxic effects (after some exposure time), respectively. In this representation blue colors indicate no harmful activity while yellow/orange indicates adverse effects with respect to the oxidative stress paradigm presented above, e.g., reactive oxygen species (ROS) generation, membrane damage, loss of mitochondrial membrane potential and intracellular Ca flux. Specific patterns of activity can be extracted from this representation.
Figure 7.
(a) Setup information for a HTS cytotoxicity assay showing plate arrangement. (b) Clustered heat-map corresponding to this data, blue colors indicate no activity, while yellow indicates toxic activity. Visual inspection identifies clusters of biologically active nanoparticles
A complementary approach to heat maps is the use of topology preservation methods which are capable of maintaining, in the projected space, the distances measured in the original feature space. The self-organizing map (SOM) algorithm105 is the most important of these algorithms. The SOM approach provides a valuable computational tool that can facilitate knowledge extraction from high dimensional HTS data. Compared with heat maps, SOM projections facilitate the discovery of hidden relationships between variables by providing topologically ordered representations of the input space. The SOM is based on an unsupervised competitive learning approach through which the SOM grid is generated. The initial generation of the SOM grid is entirely data-driven and once the initial generation of the SOM grid is finished, the grid can be used for clustering of the data. The clustering structure of the data can be visualized over the SOM grid by displaying the distances between cluster centers (distance matrices) or by projecting features onto the different component planes which allows for a more detailed evaluation of the parameters in these planes.
Summary
There is the expectation that nanotechnology will help solve some of mankind’s most pressing problems such as energy, environmental cleanup, preservation of water resources, improved diagnostics and therapeutics etc. It is already clear that ENMs have the potential to revolutionize every aspect of daily life with applications ranging from electronics, building materials, airplane bodies, clothes, cosmetic products etc. However, because of their unique properties, ENMs also has the potential to create hazardous interactions at the nano/bio interface. For example, some single walled CNTs have the capacity to induce granulomatous inflammation and fibrosis in rodent lung exposure models 14. The presence of CNTs in consumer products such as brake pads or tires may create inhalable wear particles and demonstrates the pressing need to establish screening procedures for ENM hazard assessment as well as for prioritizing animal experimentation. The use of animals as a primary screening platform does not have the capacity to keep up with the rate of expansion of nanotechnology. HTS has the necessary capacity as a primary screening tool and can be applied for ENM similar to its use in the the pharmaceutical industry for flagging potentially toxic small organic molecules. HCS in particular appears to be a very promising platform for ENM screening. High throughput methods have helped the pharmaceutical industry to save resources through early recognition of the potential toxicological properties of drug candidates. A similar application in the nanotechnology industry should allow us to identify materials with potential hazardous properties and prioritizing animal testing. The ideal would be to establish predictive toxicological paradigms where in vitro screening principles can be established that are representative of in vivo injury responses. Having established the relevance, observations can then be accomplished in vitro with only occasional in vivo relevance checks. How much of what we currently know about in vitro ENM toxicology is directly applicable to human disease is currently unknown. However, the screening for oxidative stress responses does represent a predictive platform for ambient air pollution particles where there is some correlation between in vitro oxidative stress responses and in vivo outcomes of pro-inflammatory disease processes such as asthma and atherosclerosis 109–113. It would appear that the in vitro injury responses that register in our multi-parametric assay also have some in vivo relevance, e.g., linkage of cellular oxidative stress by ZnO to metal fume fever in welders 112. There is also an emerging data that suggest that pro-inflammatory responses in bronchial epithelial cells may be predictive of some classes of ENM to generate neutrophilic inflammation in rodent lungs 113,114. Thus, it would appear as if the shallow dose-response characteristics of nuisance dusts such as carbon black and TiO2 in vitro as well as in animal lung differ from the steep dose-response characteristics of more toxic ENMs such as Co, Ni, Cu and crystalline silica 113,114. Moreover, these differences in pro-inflammatory effects were in some cases also reflected by higher levels of oxidative stress in response to the more potent lung toxicant.
We can expect to uncover more ENM toxicity paradigms and injury pathways through the implementation of high throughput methods, which could be used together with compositional and combinatorial ENM libraries to also establish qualitative structure-activity relationships that will reveal more of the secrets at the nano-bio interface. In addition, this knowledge will also be able to promote safe-by-design methods to produce ENMs with improved safety profiles. However, the lack of a systematic ENM nomenclature has stymied our efforts at establishing robust structure-activity relationship to date. As a result, there are a lot of gaps in our understanding of the specific ENM properties that are responsible for hazard generation under biological conditions. It is also becoming clear that chemisorption or the presence of impurities such as transition metals contribute to ENM toxicity. This is particularly relevant from the perspective that there are potentially a large number of commercially available nanomaterials that are not adequately characterized and may contain impurities that lead to toxicity.
While HTS methodologies to date have focused largely on mammalian cellular screening systems it is necessary to develop similar screening efforts for prokaryotic systems and lower environmental lifeforms to also ENM safety screening for protection of the environment. For example, we have instigated high throughput screening assays in E. Coli and C. Cervisiae using genome-wide knockout libraries to study the applicability of these platforms to ENM eco-toxicological screening. Similar efforts are on the way in the use of zebra fish and oyster embryos. Last but not least efforts are on the way for using reporter cell lines selected to represent the most important cell types, lineages and cellular responses to perform HTS ENM screening.
Conclusion
HTS is an extremely useful methodology that has enhanced the ability for knowledge generation about ENM hazard in our multi-disciplinary University of California Center for the Environmental Impact of Nanotechnology. The data generated to date show that a number of toxicological paradigms can be effectively incorporated into high throughput or high content efforts and that the resulting data can be used for initial hazard ranking and prioritizing of ENM screening in the wider health and safety context.
Biographies

André Nel is a Professor of Medicine and Chief of the Division of NanoMedicine at UCLA. He directs the UC Center for the Environmental Impact of Nanotechnology. Dr. Nel obtained his MD and Doctorate degrees in Cape Town, South Africa, and did Clinical Immunology and Allergy training at UCLA. He is the Director of the UCLA Asthma and Immunology Disease Center and a peer-selected member of the Best Doctors of America. His chief research interests are: (i) Nanomedicine, including drug and siRNA delivery systems; (ii) Nanobiology with interest in interfacial nanomaterial properties that can be used to improve biosafety; (iii) Nanotoxicology, with interest in predictive toxicological screening paradigms, nanomaterial safety testing, high throughput screening, and nanomaterial impact on the environment.

Dr. Damoiseaux is the Scientific Director of the Molecular Shared Screening Resources (MSSR) at the California NanoSystems Institute of UCLA. He obtained his Ph.D. degree while working on directed molecular evolution at the University of Lausanne (Switzerland) and joined the Novartis Institute for Functional Genomics (GNF) in La Jolla, CA in 2001 and resides at the University of California, Los Angeles since 2004. He oversees all drug discovery as well as the functional genomics projects at the MSSR and feels at home at the interface of chemistry, biology and engineering. His research diverse interests span biofilms, toxicological issues of nano-materials and high throughput materials discovery and high throughput screening.
References
- 1.Baughman RH, Zakhidov AA, de Heer WA. Carbon nanotubes - the route toward applications. Science. 2002;297(5582):787–792. doi: 10.1126/science.1060928. [DOI] [PubMed] [Google Scholar]
- 2.Kotov NA, Winter JO, Clements IP, Jan E, Timko BP, Campidelli S, Pathak S, Mazzatenta A, Lieber CM, Prato M, Bellamkonda RV, Silva GA, Kam NWS, Patolsky F, Ballerini L. Nanomaterials for Neural Interfaces. Advanced Materials. 2009;21(40):3970–4004. [Google Scholar]
- 3.Ariga K, Hu XL, Mandal S, Hill JP. By what means should nanoscaled materials be constructed: molecule, medium, or human? Nanoscale. 2010;2(2):198–214. doi: 10.1039/b9nr00105k. [DOI] [PubMed] [Google Scholar]
- 4.Patra CR, Bhattacharya R, Mukhopadhyay D, Mukherjee P. Fabrication of gold nanoparticles for targeted therapy in pancreatic cancer. Advanced Drug Delivery Reviews. 2010;62(3):346–361. doi: 10.1016/j.addr.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Cheng FY. Combination of Lightweight Elements and Nanostructured Materials for Batteries. Accounts of Chemical Research. 2009;42(6):713–723. doi: 10.1021/ar800229g. [DOI] [PubMed] [Google Scholar]
- 6.Dong A, Yu H, Wang F, Buhro WE. Colloidal GaAs quantum wires: Solution-liquid-solid synthesis and quantum-confinement studies. Journal of the American Chemical Society. 2008;130(18):5954–5961. doi: 10.1021/ja711408t. [DOI] [PubMed] [Google Scholar]
- 7.Fierro JLG. Metal Oxides: Chemistry and Applications. CRC Press; Boca Raton, FL: 2006. Metal Oxides: Chemistry and Applications. [Google Scholar]
- 8.Gao XL, Chen J, Chen JY, Wu BX, Chen HZ, Jiang XG. Quantum Dots Bearing Lectin-Functionalized Nanoparticles as a Platform for In vivo Brain Imaging. Bioconjugate Chemistry. 2008;19(11):2189–2195. doi: 10.1021/bc8002698. [DOI] [PubMed] [Google Scholar]
- 9.Goldberger J, Fan R, Yang PD. Inorganic nanotubes: A novel platform for nanofluidics. Accounts of Chemical Research. 2006;39(4):239–248. doi: 10.1021/ar040274h. [DOI] [PubMed] [Google Scholar]
- 10.Haddon RC. Carbon nanotubes. Accounts of Chemical Research. 2002;35(12):997–997. doi: 10.1021/ar020259h. [DOI] [PubMed] [Google Scholar]
- 11.Hochbaum AI, Yang PD. Semiconductor Nanowires for Energy Conversion. Chemical Reviews. 2010;110(1):527–546. doi: 10.1021/cr900075v. [DOI] [PubMed] [Google Scholar]
- 12.Liu JF, Miller GP. Field-assisted nanopatterning. Journal of Physical Chemistry C. 2007;111(29):10758–10760. [Google Scholar]
- 13.Richards CI, Hsiang JC, Senapati D, Patel S, Yu JH, Vosch T, Dickson RM. Optically Modulated Fluorophores for Selective Fluorescence Signal Recovery. Journal of the American Chemical Society. 2009;131(13):4619–4621. doi: 10.1021/ja809785s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao YL, Stoddart JF. Noncovalent Functionalization of Single-Walled Carbon Nanotubes. Accounts of Chemical Research. 2009;42(8):1161–1171. doi: 10.1021/ar900056z. [DOI] [PubMed] [Google Scholar]
- 15.Muller-Kuhrt L. Putting nature back into drug discovery. Nat Biotechnol. 2003;21(6):602. doi: 10.1038/nbt0603-602. [DOI] [PubMed] [Google Scholar]
- 16.Niederberger M. Nonaqueous sol-gel routes to metal oxide nanoparticles. Accounts of Chemical Research. 2007;40(9):793–800. doi: 10.1021/ar600035e. [DOI] [PubMed] [Google Scholar]
- 17.Patzke GR, Krumeich F, Nesper R. Oxidic nanotubes and nanorods - Anisotropic modules for a future nanotechnology. Angewandte Chemie-International Edition. 2002;41(14):2446–2461. doi: 10.1002/1521-3773(20020715)41:14<2446::AID-ANIE2446>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 18.Pokhrel S, Simion CE, Teodorescu VS, Barsan N, Weimar U. Synthesis, Mechanism, and Gas-Sensing Application of Surfactant Tailored Tungsten Oxide Nanostructures. Advanced Functional Materials. 2009;19(11):1767–1774. [Google Scholar]
- 19.Nel A, Xia T, Madler L, Li N. Toxic Potential of Materials at the Nanolevel. Science. 2006;311(5761):622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 20.Hench LL, West JK. The Sol-Gel Process. Chemical Reviews. 1990;90(1):33–72. [Google Scholar]
- 21.Sanders DP. Advances in Patterning Materials for 193 nm Immersion Lithography. Chemical Reviews. 2010;110(1):321–360. doi: 10.1021/cr900244n. [DOI] [PubMed] [Google Scholar]
- 22.Switzer JA, Gudavarthy RV, Kulp EA, Mu GJ, He Z, Wessel AJ. Resistance Switching in Electrodeposited Magnetite Superlattices. Journal of the American Chemical Society. 2010;132(4):1258. doi: 10.1021/ja909295y. [DOI] [PubMed] [Google Scholar]
- 23.Service R. Nanotechnology - Can high-speed tests sort out which nanomaterials are safe? Science. 2008;321(5892):1036–1037. doi: 10.1126/science.321.5892.1036. [DOI] [PubMed] [Google Scholar]
- 24.Service RF. American Chemical Society meeting: Nanomaterials show signs of toxicity. Science. 2003;300(5617):243–243. doi: 10.1126/science.300.5617.243a. [DOI] [PubMed] [Google Scholar]
- 25.Colvin VL. The potential environmental impact of engineered nanomaterials. Nature Biotechnology. 2003;21(10):1166–1170. doi: 10.1038/nbt875. [DOI] [PubMed] [Google Scholar]
- 26.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environmental Health Perspectives. 2005;113(7):823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pereira DA, Williams JA. Origin and evolution of high throughput screening. British journal of pharmacology. 2007;152(1):53–61. doi: 10.1038/sj.bjp.0707373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dimasi JA. Risks in new drug development: approval success rates for investigational drugs. Clin Pharmacol Ther. 2001;69(5):297–307. doi: 10.1067/mcp.2001.115446. [DOI] [PubMed] [Google Scholar]
- 29.Gibb S. Toxicity testing in the 21st century: a vision and a strategy. Reprod Toxicol. 2008;25(1):136–8. doi: 10.1016/j.reprotox.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Judson RS, Houck KA, Kavlock RJ, Knudsen TB, Martin MT, Mortensen HM, Reif DM, Rotroff DM, Shah I, Richard AM, Dix DJ. In vitro Screening of Environmental Chemicals for Targeted Testing Prioritization: The ToxCast Project. Environ Health Perspect. 118(4):485–92. doi: 10.1289/ehp.0901392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Auffan M, Rose J, Bottero JY, Lowry GV, Jolivet JP, Wiesner MR. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat Nanotechnol. 2009;4(10):634–641. doi: 10.1038/nnano.2009.242. [DOI] [PubMed] [Google Scholar]
- 32.Warheit DB. How meaningful are the results of nanotoxicity studies in the absence of adequate material characterization? Toxicol Sci. 2008;101(2):183–185. doi: 10.1093/toxsci/kfm279. [DOI] [PubMed] [Google Scholar]
- 33.Card JW, Magnuson BA. Proposed Minimum Characterization Parameters for Studies on Food and Food-Related Nanomaterials. J Food Sci. 2009;74(8):VI–VII. doi: 10.1111/j.1750-3841.2009.01366.x. [DOI] [PubMed] [Google Scholar]
- 34.Powers KW, Brown SC, Krishna VB, Wasdo SC, Moudgil BM, Roberts SM. Research strategies for safety evaluation of nanomaterials. Part VI. Characterization of nanoscale particles for toxicological evaluation. Toxicological Sciences. 2006;90(2):296–303. doi: 10.1093/toxsci/kfj099. [DOI] [PubMed] [Google Scholar]
- 35.Lowell S. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density. 2006. [Google Scholar]
- 36.Bootz A, Vogel V, Schubert D, Kreuter J. Comparison of scanning electron microscopy, dynamic light scattering and analytical ultracentrifugation for the sizing of poly(butyl cyanoacrylate) nanoparticles. European Journal of Pharmaceutics and Biopharmaceutics. 2004;57(2):369–375. doi: 10.1016/S0939-6411(03)00193-0. [DOI] [PubMed] [Google Scholar]
- 37.Jillavenkatesa A, Kelly JF. Nanopowder characterization: challenges and future directions. Journal of Nanoparticle Research. 2002;4(5):463–468. [Google Scholar]
- 38.Bootz A, Russ T, Gores F, Karas M, Kreuter J. Molecular weights of poly(butyl cyanoacrylate) nanoparticles determined by mass spectrometry and size exclusion chromatography. European Journal of Pharmaceutics and Biopharmaceutics. 2005;60(3):391–399. doi: 10.1016/j.ejpb.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Fritz H, Maier M, Bayer E. Cationic polystyrene nanoparticles: Preparation and characterization of a model drug carrier system for antisense oligonucleotides. Journal of Colloid and Interface Science. 1997;195(2):272–288. doi: 10.1006/jcis.1997.5172. [DOI] [PubMed] [Google Scholar]
- 40.Wiesner M. Environmental Nanotechnology: Applications and Impacts of Nanomaterials. McGraw-Hill Professional; 2007. [Google Scholar]
- 41.Pokhrel S, Birkenstock J, Schowalter M, Rosenauer A, Mädler L. Growth of Ultrafine Single Crystalline WO3 Nanoparticles Using Flame Spray Pyrolysis. Crystal Growth & Design. 2010;10(2):632–639. [Google Scholar]
- 42.Billinge SJL. Strain, nano-phase separation, multi-scale structures and function of advanced materials. In: Bishop AR, Shenoy SR, Sridhar S, editors. Intrinsic Multiscale Structure and Dynamics in Complex Electronic Oxides, Proceedings. World Scientific Publ Co Pte Ltd; Singapore: 2003. pp. 25–40. [Google Scholar]
- 43.Deb P, Biswas T, Sen D, Basumallick A, Mazumder S. Characteristics of Fe2O3 nanoparticles prepared by heat treatment of a nonaqueous powder precipitate. Journal of Nanoparticle Research. 2002;4(1–2):91–97. [Google Scholar]
- 44.Ohnuma M, Hono K, Onodera H, Ohnuma S, Fujimori H, Pedersen JS. Microstructures and magnetic properties of Co-Al-O granular thin films. Journal of Applied Physics. 2000;87(2):817–823. [Google Scholar]
- 45.Hubbard CR, Evans EH, Smith DK. The reference intensity ratio, I/Ic, for computer simulated powder patterns. Journal of Applied Crystallography. 1976;9(2):169–174. [Google Scholar]
- 46.Lo YL, Yu TC, Su LS, Huang YS. Modified total intensity ratio methods for measuring cell gap of twisted nematic liquid crystal cells. Optics Communications. 2008;281(18):4560–4565. [Google Scholar]
- 47.Jawor A, Jeong BH, Hoek EMV. Synthesis, characterization, and ion-exchange properties of colloidal zeolite nanocrystals. Journal of Nanoparticle Research. 2009;11(7):1795–1803. [Google Scholar]
- 48.van Oss CJ. Acid-base interfacial interactions in aqueous-media. Colloids Surf, A. 1993;78:1–49. [Google Scholar]
- 49.van Oss CJ. The Properties of Water and their Role in Colloidal and Biological Systems. Vol. 16 Academic Press; Amsterdam: 2008. [Google Scholar]
- 50.van Oss CJ, Giese RF. Influence of the pH of water on its electron-accepticity and donicity. Journal of Adhesion. 2005;81(3–4):237–244. [Google Scholar]
- 51.Huang XF, Bhattacharjee S, Hoek EMV. Is Surface Roughness a “Scapegoat” or a Primary Factor When Defining Particle-Substrate Interactions? Langmuir. 2010;26(4):2528–2537. doi: 10.1021/la9028113. [DOI] [PubMed] [Google Scholar]
- 52.Fowkes FM. Determination of Interfacial Tensions, Contact Angles, and Dispersion Forces in Surfaces by Assuming Additivity of Intermolecular Interactions in Surfaces. Journal of Physical Chemistry. 1962;66(2):382. [Google Scholar]
- 53.van Oss CJ. Interfacial Forces in Aqueous Media. Marcel Dekker, Inc; New York, NY: 1994. XIII: Surface tension components and parameters of liquids and solids; pp. 170–185. [Google Scholar]
- 54.Verschaeve L, Van Gompel J, Thilemans L, Regniers L, Vanparys P, van der Lelie D. VITOTOX bacterial genotoxicity and toxicity test for the rapid screening of chemicals. Environ Mol Mutagen. 1999;33(3):240–8. [PubMed] [Google Scholar]
- 55.Van Gompel J, Woestenborghs F, Beerens D, Mackie C, Cahill PA, Knight AW, Billinton N, Tweats DJ, Walmsley RM. An assessment of the utility of the yeast GreenScreen assay in pharmaceutical screening. Mutagenesis. 2005;20(6):449–54. doi: 10.1093/mutage/gei062. [DOI] [PubMed] [Google Scholar]
- 56.Westerink WM, Stevenson JC, Lauwers A, Griffioen G, Horbach GJ, Schoonen WG. Evaluation of the Vitotox and RadarScreen assays for the rapid assessment of genotoxicity in the early research phase of drug development. Mutat Res. 2009;676(1–2):113–30. doi: 10.1016/j.mrgentox.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 57.Marbeuf-Gueye C, Salerno M, Quidu P, Garnier-Suillerot A. Inhibition of the P-glycoprotein- and multidrug resistance protein-mediated efflux of anthracyclines and calceinacetoxymethyl ester by PAK-104P. Eur J Pharmacol. 2000;391(3):207–16. doi: 10.1016/s0014-2999(00)00047-9. [DOI] [PubMed] [Google Scholar]
- 58.Fernandez-Checa JC, Kaplowitz N. The use of monochlorobimane to determine hepatic GSH levels and synthesis. Anal Biochem. 1990;190(2):212–9. doi: 10.1016/0003-2697(90)90183-a. [DOI] [PubMed] [Google Scholar]
- 59.Gabriel C, Camins A, Sureda FX, Aquirre L, Escubedo E, Pallas M, Camarasa J. Determination of nitric oxide generation in mammalian neurons using dichlorofluorescin diacetate and flow cytometry. J Pharmacol Toxicol Methods. 1997;38(2):93–8. doi: 10.1016/s1056-8719(97)00066-x. [DOI] [PubMed] [Google Scholar]
- 60.Nakayama GR, Caton MC, Nova MP, Parandoosh Z. Assessment of the Alamar Blue assay for cellular growth and viability in vitro. J Immunol Methods. 1997;204(2):205–8. doi: 10.1016/s0022-1759(97)00043-4. [DOI] [PubMed] [Google Scholar]
- 61.Chan JH, Dua HS, Powell-Richards A, Jones DR, Harris IM. Effect of ABO blood group mismatching on corneal epithelial cells: an in vitro study. Br J Ophthalmol. 2001;85(9):1104–9. doi: 10.1136/bjo.85.9.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hannah RBM, Moravec R, Riss T. CellTiter-GloTM luminescent cell viability assay: A sensitive and rapid method for determining cell viability. Promega Cell Notes. 2001;(2):11–13. [Google Scholar]
- 63.O’Brien MA, Daily WJ, Hesselberth PE, Moravec RA, Scurria MA, Klaubert DH, Bulleit RF, Wood KV. Homogeneous, bioluminescent protease assays: caspase-3 as a model. J Biomol Screen. 2005;10(2):137–48. doi: 10.1177/1087057104271865. [DOI] [PubMed] [Google Scholar]
- 64.Sonneveld E, Riteco JA, Jansen HJ, Pieterse B, Brouwer A, Schoonen WG, van der Burg B. Comparison of in vitro and in vivo screening models for androgenic and estrogenic activities. Toxicol Sci. 2006;89(1):173–87. doi: 10.1093/toxsci/kfj009. [DOI] [PubMed] [Google Scholar]
- 65.Schoonen WG, de Ries RJ, Joosten JW, Mathijssen-Mommers GJ, Kloosterboer HJ. Development of a high-throughput in vitro bioassay to assess potencies of progestagenic compounds using chinese hamster ovary cells stably transfected with the human progesterone receptor and a luciferase reporter system. Anal Biochem. 1998;261(2):222–4. doi: 10.1006/abio.1998.2681. [DOI] [PubMed] [Google Scholar]
- 66.O’Brien PJ, Irwin W, Diaz D, Howard-Cofield E, Krejsa CM, Slaughter MR, Gao B, Kaludercic N, Angeline A, Bernardi P, Brain P, Hougham C. High concordance of drug-induced human hepatotoxicity with in vitro cytotoxicity measured in a novel cell-based model using high content screening. Arch Toxicol. 2006;80(9):580–604. doi: 10.1007/s00204-006-0091-3. [DOI] [PubMed] [Google Scholar]
- 67.Onoue S, Igarashi N, Yamada S, Tsuda Y. High-throughput reactive oxygen species (ROS) assay: an enabling technology for screening the phototoxic potential of pharmaceutical substances. J Pharm Biomed Anal. 2008;46(1):187–93. doi: 10.1016/j.jpba.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 68.Usenko CY, Harper SL, Tanguay RL. In vivo evaluation of carbon fullerene toxicity using embryonic zebrafish. Carbon N Y. 2007;45(9):1891–1898. doi: 10.1016/j.carbon.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shaw SY, Westly EC, Pittet MJ, Subramanian A, Schreiber SL, Weissleder R. Perturbational profiling of nanomaterial biologic activity. Proceedings of the National Academy of Sciences. 2008;105(21):7387–7392. doi: 10.1073/pnas.0802878105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meng H, Xia T, George S, Nel AE. A Predictive Toxicological Paradigm for the Safety Assessment of Nanomaterials. ACS Nano. 2009;3(7):1620–1627. doi: 10.1021/nn9005973. [DOI] [PubMed] [Google Scholar]
- 71.Xia T, Kovochich M, Brant J, Hotze M, Sempf J, Oberley T, Sioutas C, Yeh JI, Wiesner MR, Nel AE. Comparison of the Abilities of Ambient and Manufactured Nanoparticles To Induce Cellular Toxicity According to an Oxidative Stress Paradigm. Nano Letters. 2006;6(8):1794–1807. doi: 10.1021/nl061025k. [DOI] [PubMed] [Google Scholar]
- 72.Xia T, Kovochich M, Liong M, Mädler L, Gilbert B, Shi H, Yeh JI, Zink JI, Nel AE. Comparison of the Mechanism of Toxicity of Zinc Oxide and Cerium Oxide Nanoparticles Based on Dissolution and Oxidative Stress Properties. ACS Nano. 2008;2(10):2121–2134. doi: 10.1021/nn800511k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xia T, Kovochich M, Liong M, Zink JI, Nel AE. Cationic Polystyrene Nanosphere Toxicity Depends on Cell-Specific Endocytic and Mitochondrial Injury Pathways. ACS Nano. 2008;2(1):85–96. doi: 10.1021/nn700256c. [DOI] [PubMed] [Google Scholar]
- 74.Jung EJ, Avliyakulov NK, Boontheung P, Loo JA, Nel AE. Pro-oxidative DEP chemicals induce heat shock proteins and an unfolding protein response in a bronchial epithelial cell line as determined by DIGE analysis. Proteomics. 2007;7(21):3906–3918. doi: 10.1002/pmic.200700377. [DOI] [PubMed] [Google Scholar]
- 75.Xiao GG, Wang M, Li N, Loo JA, Nel AE. Use of Proteomics to Demonstrate a Hierarchical Oxidative Stress Response to Diesel Exhaust Particle Chemicals in a Macrophage Cell Line. J Biol Chem. 2003;278(50):50781–50790. doi: 10.1074/jbc.M306423200. [DOI] [PubMed] [Google Scholar]
- 76.Kroll A, Pillukat MH, Hahn D, Schnekenburger J. Current in vitro methods in nanoparticle risk assessment: Limitations and challenges. European Journal of Pharmaceutics and Biopharmaceutics. 2009;72(2):370–377. doi: 10.1016/j.ejpb.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 77.Monteiro-Riviere NA, Inman AO, Zhang LW. Limitations and relative utility of screening assays to assess engineered nanoparticle toxicity in a human cell line. Toxicology and Applied Pharmacology. 2009;234(2):222–235. doi: 10.1016/j.taap.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 78.Casey A, Herzog E, Davoren M, Lyng FM, Byrne HJ, Chambers G. Spectroscopic analysis confirms the interactions between single walled carbon nanotubes and various dyes commonly used to assess cytotoxicity. Carbon. 2007;45(7):1425–1432. [Google Scholar]
- 79.Worle-Knirsch JM, Pulskamp K, Krug HF. Oops They Did It Again! Carbon Nanotubes Hoax Scientists in Viability Assays. Nano Letters. 2006;6(6):1261–1268. doi: 10.1021/nl060177c. [DOI] [PubMed] [Google Scholar]
- 80.Zhang, Chung, Oldenburg A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. Journal of biomolecular screening: the official journal of the Society for Biomolecular Screening. 1999;4(2):67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 81.Inglese J, Auld DS, Jadhav A, Johnson RL, Simeonov A, Yasgar A, Zheng W, Austin CP. Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc Natl Acad Sci U S A. 2006;103(31):11473–8. doi: 10.1073/pnas.0604348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martin CJ, Le XC, Guidotti TL, Yalcin S, Chum E, Audette RJ, Liang CK, Yuan BS, Zhang X, Wu J. Zinc exposure in Chinese foundry workers. American Journal of Industrial Medicine. 1999;35(6):574–580. doi: 10.1002/(sici)1097-0274(199906)35:6<574::aid-ajim4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 83.Rohrs LC. Metal-Fume Fever from Inhaling Zinc Oxide. Archives of Internal Medicine. 1957;100(1):44–49. [PubMed] [Google Scholar]
- 84.Rubiolo JA, Mithieux G, Vega FV. Resveratrol protects primary rat hepatocytes against oxidative stress damage: activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur J Pharmacol. 2008;591(1–3):66–72. doi: 10.1016/j.ejphar.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 85.DeForge LE, Billeci KL, Kramer SM. Effect of IFN-gamma on the killing of S. aureus in human whole blood. Assessment of bacterial viability by CFU determination and by a new method using alamarBlue. J Immunol Methods. 2000;245(1–2):79–89. doi: 10.1016/s0022-1759(00)00279-9. [DOI] [PubMed] [Google Scholar]
- 86.Laflamme C, Lavigne S, Ho J, Duchaine C. Assessment of bacterial endospore viability with fluorescent dyes. Journal of Applied Microbiology. 2004;96(4):684–692. doi: 10.1111/j.1365-2672.2004.02184.x. [DOI] [PubMed] [Google Scholar]
- 87.Shimomura Y, Ohno R, Kawai F, Kimbara K. Method for assessment of viability and morphological changes of bacteria in the early stage of colony formation on a simulated natural environment. Applied and Environmental Microbiology. 2006;72(7):5037–5042. doi: 10.1128/AEM.00106-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boulos L, Prevost M, Barbeau B, Coallier J, Desjardins R. LIVE/DEAD (R) BacLight (TM): application of a new rapid staining method for direct enumeration of viable and total bacteria in drinking water. Journal of Microbiological Methods. 1999;37(1):77–86. doi: 10.1016/s0167-7012(99)00048-2. [DOI] [PubMed] [Google Scholar]
- 89.Leuko S, Legat A, Fendrihan S, Stan-Lotter H. Evaluation of the LIVE/DEAD BacLight kit for detection of extremophilic archaea and visualization of microorganisms in environmental hypersaline samples. Applied and Environmental Microbiology. 2004;70(11):6884–6886. doi: 10.1128/AEM.70.11.6884-6886.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sayes CM, Gobin AM, Ausman KD, Mendez J, West JL, Colvin VL. Nano-C-60 cytotoxicity is due to lipid peroxidation. Biomaterials. 2005;26(36):7587–7595. doi: 10.1016/j.biomaterials.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 91.Porter J, Deere D, Pickup R, Edwards C. Fluorescent probes and flow cytometry: New insights into environmental bacteriology. Cytometry. 1996;23(2):91–96. doi: 10.1002/(SICI)1097-0320(19960201)23:2<91::AID-CYTO1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 92.Kaprelyants AS, Kell DB. Rapid Assessment of Bacterial Viability and Vitality by Rhodamine 123 and Flow-Cytometry. Journal of Applied Bacteriology. 1992;72(5):410–422. [Google Scholar]
- 93.Choi O, Deng KK, Kim NJ, Ross L, Surampalli RY, Hu ZQ. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Research. 2008;42(12):3066–3074. doi: 10.1016/j.watres.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 94.Park JS, Park K, Moon HT, Woo DG, Yang HN, Park KH. Electrical Pulsed Stimulation of Surfaces Homogeneously Coated with Gold Nanoparticles to Induce Neurite Outgrowth of PC12 Cells. Langmuir. 2009;25(1):451–457. doi: 10.1021/la8025683. [DOI] [PubMed] [Google Scholar]
- 95.Chen J, Zhu JM, Cho HH, Cui KM, Li FH, Zhou XB, Rogers JT, Wong STC, Huang XD. Differential cytotoxicity of metal oxide nanoparticles. Journal of Experimental Nanoscience. 2008;3(4):321–328. [Google Scholar]
- 96.Jin X, Li M, Wang J, Marambio-Jones C, Huang X, Damoiseaux R, Hoek EMV. High-throughput Screening of Nano-silver Stability and Bacterial Toxicity in Simulated Fresh Water: Influence of Specific Ions. Environmental Science & Technology. 2010 doi: 10.1021/es100854g. in Preparation. [DOI] [PubMed] [Google Scholar]
- 97.Li M, Jin X, Marambio-Jones C, Huang X, Maedler L, Damoiseaux R, Hoek EMV. Tuning the Stability and Antibacterial Properties of ZnO Nanoparticles. Journal of Nanoparticle Research. 2010 in Preparation. [Google Scholar]
- 98.Tomalia DA. In quest of a systematic framework for unifying and defining nanoscale. Journal of Nanoparticle Research. 2009;11:61. doi: 10.1007/s11051-009-9632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Clark T, Martin S, Liefeld T. Globally distributed object identification for biological knowledgebases. Brief Bioinform. 2004;5(1):59–70. doi: 10.1093/bib/5.1.59. [DOI] [PubMed] [Google Scholar]
- 100.Wawer M, Bajorath J. Extraction of Structure-Activity Relationship Information from High-Throughput Screening Data. Curr Med Chem. 2009;16(31):4049–4057. doi: 10.2174/092986709789378189. [DOI] [PubMed] [Google Scholar]
- 101.Malo N, Hanley J, Cerquozzi S, Pelletier J, Nadon R. Statistical practice in high-throughput screening data analysis. Nature biotechnology. 2006;24(2):167–176. doi: 10.1038/nbt1186. [DOI] [PubMed] [Google Scholar]
- 102.Posner BA, Xi H, Mills JEJ. Enhanced HTS Hit Selection via a Local Hit Rate Analysis. J Chem Inf Model. 2009;49(10):2202–2210. doi: 10.1021/ci900113d. [DOI] [PubMed] [Google Scholar]
- 103.Sukuru SCK, Jenkins JL, Beckwith REJ, Scheiber J, Bender A, Mikhailov D, Davies JW, Glick M. Plate-Based Diversity Selection Based on Empirical HTS Data to Enhance the Number of Hits and Their Chemical Diversity. J Biomol Screen. 2009;14(6):690–699. doi: 10.1177/1087057109335678. [DOI] [PubMed] [Google Scholar]
- 104.Eisen M, Spellman P, Brown P, Botstein D. Cluster analysis and display of genome-wide expression patterns. P Natl Acad Sci Usa. 1998;95(25):14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.KOHONEN T. The self-organizing map. Proceedings of the IEEE. 1990;78(9):1464–1480. doi: 10.1109/5.58325. [DOI] [Google Scholar]
- 106.Sager TM, Porter DW, Robinson VA, Lindsley WG, Schwegler-Berry DE, Castranova V. Improved method to disperse nanoparticles for in vitro and in vivo investigation of toxicity. Nanotoxicology. 2007;1:118–129. [Google Scholar]
- 107.Ji Z, Jin X, George S, Xia T, Meng H, Wang X, Suarez E, Zhang H, Hoek EM, Godwin H, Nel AE, Zink JI. Dispersion and Stability Optimization of TiO(2) Nanoparticles in Cell Culture Media. Environ Sci Technol. 2010 doi: 10.1021/es100417s. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gao N, Keane MJ, Ong T, Ye J, Miller WE, Wallace WE. Effects of phospholipid surfactant on apoptosis induction by respirable quartz and kaolin in NR8383 rat pulmonary macrophages. Toxicol Appl Pharmacol. 2001;175(3):217–25. doi: 10.1006/taap.2001.9249. [DOI] [PubMed] [Google Scholar]
- 109.Li N, Wang M, Bramble LA, Schmitz DA, Schauer JJ, Sioutas C, Harkema JR, Nel AE. The adjuvant effect of ambient particulate matter is closely reflected by the particulate oxidant potential. Environ Health Perspect. 2009;117(7):1116–23. doi: 10.1289/ehp.0800319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li N, Harkema JR, Lewandowski RP, Wang M, Bramble LA, Gookin GR, Ning Z, Kleinman MT, Sioutas C, Nel AE. Ambient Ultrafine Particles Provide a Strong Adjuvant Effect in the Secondary Immune Response: Implication for Traffic-related Asthma Flares. Am J Physiol Lung Cell Mol Physiol. 2010 Jun 18; doi: 10.1152/ajplung.00115.2010. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ, Nel AE. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ Res. 2008;102(5):589–96. doi: 10.1161/CIRCRESAHA.107.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xia T, Li N, Nel AE. Potential health impact of nanoparticles. Annu Rev Public Health. 2009;30:137–50. doi: 10.1146/annurev.publhealth.031308.100155. [DOI] [PubMed] [Google Scholar]
- 113.Duffin R, Tran L, Brown D, Stone V. Proinflammogenic Effects of Low-Toxicity and Metal Nanoparticles In vivo and In vitro: Highlighting the Role of Particle Surface Area and Surface Reactivity. Inhalation Toxicology: International Forum for Respiratory Research. 2007:849–856. doi: 10.1080/08958370701479323. [DOI] [PubMed] [Google Scholar]
- 114.Rushton EK, Jiang J, Leonard SS, Eberly S, Castranova V, Biswas P, Elder A, Han X, Gelein R, Finkelstein J, Oberdorster G. Concept of assessing nanoparticle hazards considering nanoparticle dosemetric and chemical/biological response metrics. J Toxicol Environ Health. 2010;73(5):445–61. doi: 10.1080/15287390903489422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jan E, Byrne SJ, Cuddihy M, Davies AM, Volkov Y, Gun’ko YK, Kotov NA. High-content screening as a universal tool for fingerprinting of cytotoxicity of nanoparticles. ACS Nano. 2008;2(5):928–38. doi: 10.1021/nn7004393. [DOI] [PubMed] [Google Scholar]