Abstract
Reactive oxygen species (ROS), by-products of aerobic metabolism, cause oxidative damage to cells and tissue and not surprisingly many theories have arisen to link ROS-induced oxidative stress to aging and health. While studies clearly link ROS to a plethora of divergent diseases, their role in aging is still debatable. Genetic knock-down manipulations of antioxidants alter the levels of accrued oxidative damage, however, the resultant effect of increased oxidative stress on lifespan are equivocal. Similarly the impact of elevating antioxidant levels through transgenic manipulations yield inconsistent effects on longevity. Furthermore, comparative data from a wide range of endotherms with disparate longevity remain inconclusive. Many long-living species such as birds, bats and mole-rats exhibit high-levels of oxidative damage, evident already at young ages. Clearly, neither the amount of ROS per se nor the sensitivity in neutralizing ROS are as important as whether or not the accrued oxidative stress leads to oxidative-damage-linked age-associated diseases. In this review we examine the literature on ROS, its relation to disease and the lessons gleaned from a comparative approach based upon species with widely divergent responses. We specifically focus on the longest lived rodent, the naked mole-rat, which maintains good health and provides novel insights into the paradox of maintaining both an extended healthspan and lifespan despite high oxidative stress from a young age.
Keywords: Comparative biology of aging, mitochondria, naked mole-rat, oxidative stress, proteasome, autophagy, reactive oxygen species
1. INTRODUCTION
The oxidative stress theory of aging asserts that the decline in functionality that characterizes the aging process is due to progressively accumulating levels of oxidative damage. This intuitively logical theory has been around for more than a century and despite an equivocal array of data, this invincible theory has held steadfast as the key proximate mechanism that determines species maximum lifespan potential (MLSP) [1]. More than a century ago, Max Rubner first noted the inverse correlation between the mass specific rate of oxygen consumption and longevity in mammals. He further coupled MLSP with body size and metabolism and determined that the lifetime energy expenditure (LEE) was relatively constant [2, 3]. Twenty years later Raymond Pearl expanded upon this idea and proposed that differences in metabolic rate explained the lifespan extension of Drosophila melanogaster maintained at different temperatures and through this observation defined the “rate of living theory of aging” [4].
The toxic nature of oxygen was already a well-known phenomenon since the seminal work of Lavoisier in 1781 [5]. However, free radicals were first regarded as the cause of oxygen toxicity in 1954 [6] and soon afterward in 1956 Denham Harman proposed that physiological metals would cause reactive oxygen species (ROS) to form in cells potentially damaging nearby molecules, including DNA. These would cause mutations, and based on the belief at the time, such induction of mutations could cause both cancer and aging. Harman also proposed that administering compounds that could oxidize easily and absorb the ROS in the cell could slow down this mutation-induced aging [7]. Since that time, the free radical theory of aging has been repeatedly modified and renamed to the “oxidative stress theory of aging [8–11]. As such, more than 50 years later, it remains a key focal area for aging research. Research has focused on two broad categories to validate and expand upon the notion that oxidative stress is an integral component of aging: testing the levels of oxidatively damaged biomolecules in aging tissue and manipulating--either biochemically, genetically or behaviorally—various stressors to determine their effects on lifespan (Fig. 1; reviewed in [12] and [13]). The boldest version of the oxidative stress theory of aging makes the all-encompassing prediction that lifespan is determined by oxidative damage and, thus, that an increase in oxidative damage will contribute to a shorter lifespan. While a shortened lifespan may not be the product of accelerated aging, determining the lifespan of animal or plant species with compromised antioxidant pathways can be used further to explore this theory: it follows that if an organism has increased oxidative damage but exhibits no change in lifespan, the result falsifies the hypothesis.
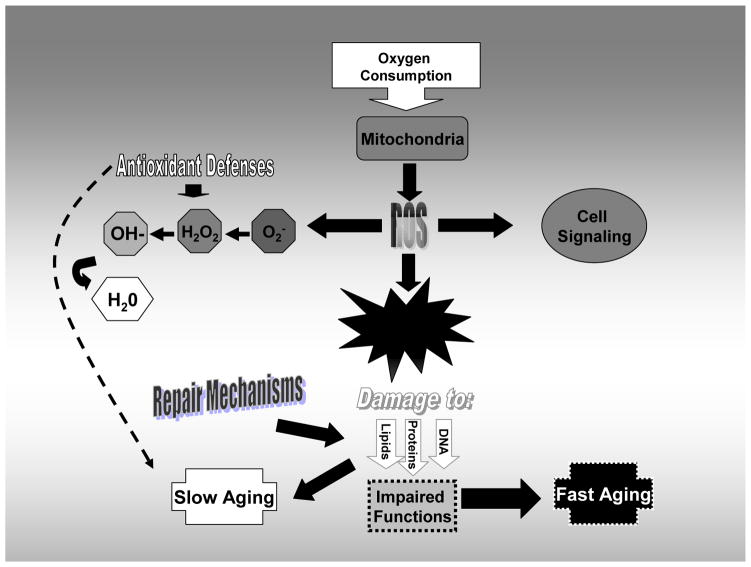
Fig. 1.
Schematic diagram outlining the components of the oxidative-stress theory of aging. The theory predicts that, as an inevitable byproduct of metabolic activity, reactive oxygen species (ROS) are produced. If these are not neutralized, oxidative damage to proteins, DNA and lipids may occur. However, as some damage may occur, repair mechanisms are in place to mitigate the damage. Without repair, accelerated aging occurs. With repair, we may have a slow-down in the aging process.
Here, we offer new insights into oxidative stress, longevity, and the role of oxidative stress in species longevity. We base our insights on research primarily using mammals and birds, and in particular, highlight research on the longest-lived rodent known, the naked mole-rat. This strictly subterranean, eusocial rodent, found in the northeast horn of Africa lives more than 30 years in captivity while maintaining cancer-free, good health well into its third decade of life [14]. The lack of spontaneous neoplasia is most unusual among captive wild-caught rodents (such as Mus musculus and Peromyscus species) as well as domesticated laboratory strains of mice and rats. Approximately 70% of domesticated laboratory rodent deaths are attributed to various types of cancers [15]. Understanding the mechanisms that facilitate cancer resistance in captive naked mole-rats may reveal important insights into cancer prevention. We hypothesized that this extraordinary longevity relative to its shorter-lived rodent cousins may be explained by the production of less ROS and/or extremely efficient mechanisms to protect this species against oxidative damage.
2. OXIDATIVE STRESS, DISEASE AND LONGEVITY
ROS are formed during oxidative phosphorylation in the mitochondria. During this process, electrons from NADH or FADH2 are transferred through the electron transport chain (ETC) to oxygen and the energy released in the process is used to power proton transport across the mitochondrial inner membrane at complexes I, III, and IV. The proton motive force generated is used to drive the ATP-synthesis reactions. During this process, however, electron leakage may occur predominantly in complex I and III, leading to the generation of superoxide [16, 17] and it is estimated that up to 3%, of oxygen consumed during respiration ends up as oxygen radicals [18]. This is likely to be an overestimate as other studies report less than 0.15% of the products of maximum oxygen consumption become ROS [16]. The rate of superoxide production is primarily dependent upon the local oxygen concentration and the presence of reducing equivalents, although the exact mechanisms that lead to superoxide formation are still not fully understood. At physiological concentrations, ROS may have important intracellular signaling functions affecting cell function, growth and development [19–21]. However, excessive mitochondrial ROS production is detrimental. Oxygen radicals may react with numerous other compounds to form a variety of other reactive species all of which are capable of indiscriminately damaging nearby macromolecules including phospholipids, proteins and DNA. Oxidized lipids are themselves potent ROS, autocatalyzing and self-propagating this unregulated process. Genotoxic intermediates of lipid peroxidation may also play a role in eliciting age associated DNA mutation [22]. ROS cause oxidative damage to proteins by showing that oxidative damage to a histidine in glutamate synthetase resulted in enzyme inactivation [23]. The damage also modified the residue to a carbonyl group. Additionally, carbonyl groups reportedly increase with age and are resistant to normal proteolytic degradation, making them the preferred marker of protein damage as a result of ROS [24]. The harmful effects of ROS have led to the evolution of numerous antioxidant and cytoprotective mechanisms that neutralize and detoxify the free radicals before they can induce substantial damage.
A complex suite of mechanisms has evolved to eliminate ROS and thereby offset their potential for accruing oxidative damage. The primary lines of defense include a) enzymatic antioxidants including superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (Gpx) b) hydrophilic scavengers such as reduced glutathione (GSH) c) lipophilic scavengers such as tocopherols, flavonoids and carotenoids as well as d) the proteins involved in reducing oxidized antioxidants and thereby recycling them for further use (e.g., GSH reductase, peroxiredoxins). As a second line of defense against free radicals, cells possess a number of detection and repair mechanisms for the different types of DNA damage (nucleotide excision repair (NER), base excision repair (BER), mismatch repair (MMR), double-strand break repair (DSBR)) and also rely on proteasome degradation and autophagy to remove damaged proteins and organelles [25]. Likewise lipid peroxidation can trigger autophagic response leading to a degradation of oxidized products by lysosomes [26, 27]. Despite the plethora of antioxidants mechanisms, some ROS always evade detection and subsequent neutralization, inducing damage that is seldom completely repaired. As a consequence, according to the oxidative stress theory, damage accrues and accumulates with age. Furthermore, ROS have been implicated in the causation of several age-related diseases from cancer to diabetes. Better processing of these free radicals resulting in less damage could ameliorate these diseases (reviewed in [28]).
Research has persistently tried to understand how antioxidant defenses work, either by overexpressing key genes or knocking them out in various species. Manipulation of antioxidant genes shows a myriad of phenotypes in different animal models from yeast to mice. The invertebrate phenotypes have been described extensively (reviewed in [12, 13]). Here we focus specifically on the investigations conducted in vertebrate (mainly mouse) models.
Knockout Models
Glutathione peroxidase 1 (Gpx1) is ubiquitous in almost all mammalian tissues and is thought to be the main cellular scavenger of hydrogen peroxide (H2O2) [29]. The most significant pathological finding to date for Gpx1 knockouts is that the null mice develop cataracts, potentially resulting from increased ROS damage in the eye, at a considerably younger age than wild-type mice [30, 31]. Also, Gpx1−/− mice are very sensitive to paraquat and diquat, both potentiators of ROS which cause fatal oxidative damage to tissues in these animals [32]. Unfortunately, glutathione peroxidase 4 (Gpx 4) knockout mice are embryonically lethal indicating a vital developmental function [33], but recent studies using a spermatocyte-specific knockout show that the lack of Gpx4 leads to infertility possibly due to an increase of ROS in the germ cells [34]. Survival curves of Gpx1 −/− and Gpx4 +/− mice have not shown a difference in lifespan when compared to wild-type mice [13, 35].
Cu/Zn superoxide dismutase (SOD1) is a highly conserved enzyme responsible for the scavenging of the superoxide radical (O2−) in the cytosol and in the mitochondrial inter-membrane space. Formerly called erythrocuprein, the enzymatic function was first described by McCord and Fridovich (1969). They reported that CuZnSOD1 catalyzes the reaction where the first superoxide molecule is oxidized and the second molecule is reduced, turning two molecules of superoxide into O2 and H2O2 [36]. In 1993, Rosen et al., determined that mutations in the SOD1 gene correlated with familial amyotrophic lateral sclerosis (ALS) [37]. Reportedly, SOD1 is ubiquitously expressed in all tissues with highest concentrations found in neural tissue [38]. Mutations in this gene are the most common known cause of ALS, comprising close to 10% of all cases [39]. Copper is rapidly incorporated into both newly translated and pre-formed SOD1. Mouse fibroblasts labeled with radioactive copper (64Cu) show that the concentration of apo-SOD1 has an inverse relationship with copper levels [40]. It has been shown that SOD1 activity is induced under conditions of oxidative stress [41, 42].
Female mice without CuZnSOD are prone to infertility due to a dysfunction in ovarian development, in addition to signs of early, age-related hearing-loss and early onset of cataracts [43–47]. Furthermore, mice lacking SOD1 have an increased risk of developing hepatocellular carcinoma [48] and this predisposition to cancer may be the main reason for the 30% reduction in maximum lifespan of these animals [48]. Congenic SOD1 knockout animals have high levels of oxidative damage to all macromolecules [48]. Sod1−/− mice also exhibit a pronounced loss of muscle mass as they age, such that by 20 months of age they have ~50% of the muscle mass of that of their wild-type counterparts and accrue a significant amount of oxidative damage in skeletal muscle compared to wild-type mice [49]. More recently, Sod1−/− mice were found to exhibit an increase in blood glucose and blocked glucose-stimulated insulin production [50]. These collective differences in Sod1−/− mice phenotype relative to wild type suggest that the lack of an accelerated enzymatic method to remove superoxide anions results in biological toxicity with a long-term pathogenesis of oxidative stress and damage to biomolecules.
Mn superoxide dismutase (SOD2) is the main scavenger of superoxide in the mitochondrial matrix. Mutations in this gene may cause idiopathic cardiomyopathy and diabetes and increase the risk of breast cancer [51–53]. Depending on the genetic background, removal of the SOD2 gene in mice results in neonatal lethality predominantly via neurodegeneration within the first 24 days after birth [54–56]. These mice also have a significant increase in DNA oxidative damage and are very sensitive to hypoxia [57, 58]. Heterozygous transgenic mice show that a non-lethal decrease in Sod2 nevertheless increases oxidative damage and the sensitivity to oxidative stressors. However, while the animals have a higher incidence of cancer, their lifespans were indistinguishable from those of wild-type mice [59–61].
The third, though least abundant SOD enzyme is extracellular SOD (ECSOD) which is encoded by the Sod3 gene. ECSOD is located in the extracellular fluids including plasma, lymph and synovial fluid. It also has a substantial presence in the lung and a strong affinity to heparin [62, 63]. Polymorphisms in the SOD3 gene in human populations lead to increased susceptibility to type II diabetes [64]. Sod3 knockout mice are viable, can reproduce normally and do not show a decrease in lifespan. However, they do show sensitivity to hyperoxia [65].
Genetic manipulations of methionine sulfoxide reductase (MsrA; a reducing enzyme found in the cytoplasm and mitochondria that selectively reduces the sulfoxide of methionine) reveal a possible impact on longevity [66]. One group found that deletion of MsrA decreased longevity by 40% and increased sensitivity to oxidative stressors such as paraquat [67]. These knockout animals have also shown compromised cardiomyocyte activity following oxidative stress [68]. A more recent study also observed sensitivity to oxidative stress, however, no decrease in either median or maximum lifespan was observed [69].
Knockout studies of five of the six known peroxiredoxins--antioxidants that scavenge H2O2, organic peroxides and peroxynitrite--in mice all show some signs of oxidative damage related pathology and increased cancer risk [70, 71]. For instance peroxiredoxin 1 (Prx1) knockout mice have increased oxidative damage to both DNA and proteins [72, 73] and suffer from hemolytic anemia, an increase in cancer incidence and a 15% reduction in lifespan [73], but aside from Prx1, no data on lifespan are yet available regarding other peroxiredoxin knockout animals.
Although many of these animal models are more susceptible to oxidative stress and may even show similar dysfunction to that observed in human disease (See Table 1) clearly, there is no clear link between genetically reduced antioxidant expression and a decrease in lifespan.
Table 1.
Diseases and Disorders Associated with Antioxidant Defense Genes and the Effect of Genetic Manipulations of These Genes.
| Antioxidant Enyzme | Antioxidant Function | Genetic Manipulation | Phenotype(s) | Disorder/Disease Association | Ref(s). |
|---|---|---|---|---|---|
| Glutathione Peroxidase 1 | Scavenges H202 | Gpx1−/− Gpx TG in PD model |
Increased myo-carditis after viral infection. Protection against insulin resistance under high fat diet Lifespan not reported Reduced neural death, improved locomotion |
Cardiovascular Disease, Diabetes, Neurodegeneration | [255] [256] [257] |
| Glutathione Peroxidase 4 | Scavenges LOOH | Gpx4−/− | Embryonic Lethal. Infertile in spermatocyte-specific knockout |
Unknown | [33] [34] |
| Cu/Zn Superoxide Dismutase | Scavenger of O2·− | Sod1−/− | Multiple pathologies, 30% shortened lifespan | Neurodegeneration (ALS), Diabetes, Cancer | [48] |
| Mn Superoxide Dismutase | Scavenger of O2·− | Sod2−/− Sod2 TG |
Neonatal lethal Protection from insulin resistance under high fat diet |
Cardiovascular Disease, Cancer, Diabetes | [54] [258] |
| Extra-Cellular Superoxide Dismutase | Scavenger of O2·− | Sod3−/− Sod3 TG |
“Normal” Protection against LPS-induced inflammation in lungs |
Inflammation, Diabetes | [62] [64] [259] |
| Catalase | Scavenges H202 | Cat−/− M-Cat TG |
“Normal” Increase lifespan, protection against insulin resistance |
Cardiovascular Disease, Diabetes | [260] [79] [261] |
| Peroxiredoxin 1 | Scavenges peroxidea | Prdx1−/− | Hemolytic anemia | Cancer | [73] |
| Peroxiredoxin 3 | Scavenges peroxidea | Prx3 TG | protects the heart against post-MI remodeling and failure in mice | Cardiovascular disease | [92] |
| Thioredoxin 1 | Reduction of proteins by cysteine thiol-disulfide exchange | Trx1−/− Trx1 TG |
Embryonic lethal 20% increased lifespan, increased resistance to cerebral ischemia |
Inflammation, Stroke | [262] [85] |
| Methionine Sulfoxide Reductase | Repairs oxidized methionine | MrsA−/− | 40% shortened lifespan | Cardiovascular disease | [67] |
| Metallothionein | Metal and OH· scavenger | Mt1−/− MT-TG |
Sensitivity to ROS 15% increase in lifespan |
Cardiovascular disease | [91] [88] |
Peroxiredoxins scavenge H2O2, short chain organic peroxides, fatty acid alkyl hydroperoxides, and in the case of Prdx6, also lipid hydroperoxides
Overexpression of Antioxidant Enzymes
An alternative approach to studying the impact of antioxidants on longevity is to genetically overexpress antioxidant genes with the intention of extending lifespan. In mice, only a handful of studies examining lifespan in response to overexpression of antioxidant enzymes have been published. A key question in these studies is whether or not an increase of activity in one antioxidant enzyme will in fact reduce endogenous oxidative stress, cause down-regulations of other antioxidants or even upset the balance of oxidant removal leading ultimately to a counterintuitive increase in oxidative damage. A large cohort study showed that overexpression of CuZnSOD did not increase lifespan [74]. High overexpression of both CuZnSOD and MnSOD leads to harmful phenotypes such as muscular dystrophy, neuronal degeneration and infertility, with at least the muscular dystrophy correlated ironically with higher levels of oxidative damage such as lipid peroxidation, [75–77]. Furthermore, although overexpression of CAT showed protection in a cell culture model, at the whole organism level it actually led to an increase in gamma irradiation sensitivity [78, 79]. Mice overexpressing CAT (2- to 4-fold) in the peroxisome did not show a significant extension of maximal lifespan, although median lifespan showed a modest 10% increase [79]. Transgenic mice that overexpress human CAT in mitochondria (MCAT) showed a statistically significant extension of both median and maximum lifespan of approximately 20% and equivocal changes in lifespan when expressed in other organelles [80]. No increases in lifespan was evident with overexpression of MnSOD [81]. Age-related changes in oxidative damage (8-Oxo-2′-deoxyguanosine and mitochondrial DNA deletions in skeletal muscle), H2O2 production (in heart-specific mitochondria) and H2O2-induced aconitase inactivation (in heart tissue) were attenuated in MCAT animals [80]. In a more recent study, mice cardiomyocyte-targeted overexpression of CAT or endothelial nitric oxide synthase (eNOS) showed a reduction in vascular H2O2 and an increased expression of EC-SOD potentially leading to improved cardiac aging [82, 83]. However, whether this finding can lead to life extension is not yet known. Thioredoxin (Trx) facilitates the reduction of other proteins by cysteine thiol-disulfide exchange [84]. Overexpression of human thioredoxin (Hs Trx1) in mice reportedly leads to a 20% increase in lifespan, as well as increased resistance to cerebral ischemia and to UV-induced oxidative stress [85, 86]. In these animals HsTrx1 was three to six times more prevalent in tissues than endogenous Trx1 [85]. Metallothionein (MT), a scavenger of metal ions OH− is also highly induced by oxidative stress [87]. Transgenic mice overexpressing metallothionein (MT-TG) showed a 15% increase in mean lifespan relative to control mice [88]. Furthermore, their cardiomyocytes have attenuated age-related increases in superoxide generation, cytochrome c release, p47phox (Neutrophil cytosol factor-1) expression and higher aconitase activity [89] In contrast, knockout mouse models of MT show enhanced toxicity from ROS especially in cardiomyocytes [90, 91]. However, a lifespan study of MT null mice has not been conducted. The Mastushima group reported that overexpression of Prx3 protects the heart against post-mycardial infarction remodeling and failure in mice. Prx3 overexpression reduced left ventricular dilatation and dysfunction and attenuated myocyte hypertrophy, interstitial fibrosis and apoptosis of the non-infarcted myocardium. These beneficial effects of the Prx3 gene overexpression were also associated with attenuation in oxidative stress and mtDNA decline and dysfunction [92].
Clearly, overexpression of the antioxidants discussed above enhance protective mechanisms that could contribute to extended healthspan and lifespan. While lifespan studies have not been conducted on every antioxidant gene that is overexpressed, despite the many signs of enhanced protection, the available data show that the impact on lifespan is modest.
Studies in Humans
In humans, natural variation, measurable by genetic polymorphisms is a useful modality to investigate the effects of antioxidant enzyme alterations on lifespan. An abundant amount of literature exists on whether or not certain polymorphisms may present risk factors for disease where a subset of those risk factors includes oxidative stress. Studies in aged human cells containing mutated mtDNA or defective mitochondria show that a compromised metabolic function creates a higher rate of ROS production [93–95]. In contrast, a study on Japanese centenarians reported that 20% of this population possessed a mitochondrial gene variant in a coding a subunit of NADH dehydrogenase, Mt5178A [96] associated with low ROS production. Not only did the people in this study survive to be over 100 years of age, but they were 50% less likely to be hospitalized for age-related disease [96, 97].
A polymorphism in the mitochondrial localization sequence of MnSOD (the Val/Ala polymorphism at position -9 from the mature protein or 16 from the full-length precursor; V16A) is a known risk factor in many oxidative-stress-related pathologies such as diabetic nephropathy, hypertension and certain, but not all cancers [98–101]. However while the Ala allele has been found by some studies to increase the risk of breast cancer [102, 103] others cannot find a correlation [104, 105].
The cumulative indices of oxidative markers and oxidative stress in healthy humans related to aging and gender have been measured in multiple biological fluids and in many instances confounding results have been reported [106–115]. In several of these investigations some of the parameters tested showed significant correlations between age and an increase of pro-oxidative capacity together with a decrease of antioxidants [109, 110, 112, 113]. This was demonstrated by the observations of increases of plasma malondialdehyde (MDA) and 4-hydroxynonenal (HNE), erythrocytic glutathione disulfide (GSSG), cysteinylcysteine (CysCys) and by a slight decrease of erythrocytic GSH with age [108–113]. However, not all markers behaved predictably and the conclusions varied among the studies. For example the plasma protein carbonyls reached the lowest value in aged individuals in the Gil et al., (2006) study. Indeed that study reported that metabolic and nutritional influences play a greater role than does the balance between anti-oxidants and oxidants. Age-related changes in antioxidants (SOD, GPx and CAT, carotenoids and tocopherols) in humans do not show a consistent pattern; generally erythrocytic activity of SOD and GPx increased while plasma values were unchanged [108, 110–112, 114, 115]. Similarly oxidative damage indices are also equivocal. Some studies report positive and linear correlations with increasing age were significant with regards to MDA, HNE, protein carbonyls and GSSG [108, 110, 113, 114]; levels of urinary isoprostanes reportedly are unchanged with age; and assessment of DNA damage by comet assay and the FLARE (Fragment Length Analysis using Repair Enzymes) method are contradictory with the former increasing while the latter remains unchanged [114]. Lastly, there does not appear to be any sexual dimorphism with regards to the selected measures of oxidative stress in age-matched men or women [112–114]. One of the limitations of these studies is the cross-sectional nature. The subjects cannot be followed to track whether more favorable markers of oxidative stress (low oxidative damage; high antioxidant capacity) lead to increased longevity and no study has examined ROS production. Direct measures of antioxidants, the results of their manipulations or correlations with disease and oxidant polymorphisms have not shown unequivocal support for the theory. More than likely, the successful aging seen in these populations in not only linked to their antioxidant/oxidative damage profile, but also how the body responds to the damage to maintain a “survivable” set point for homeostasis and thereby avoiding the common debilitating age-associated diseases that ultimately increase the chances of dying.
Oxidative Damage and Alzheimer’s Disease
Oxidative damage has also been implicated in Alzheimer’s disease (AD) (reviewed in [116]), the most common age-related neurodegenerative disease currently affecting ~30 million people (www.alz.org). Accumulation of neurotoxic amyloid beta (Aβ) is considered one of the pathological hallmarks of AD. The greatest risk factor for AD is age and to date there is no therapy for this devastating disease. Lipid peroxidation has been extensively studied because of the abundance of fatty acyl groups which account for ~10% of neural tissue. Moreover, the brain is an energetically expensive organ, with high oxygen consumption and having a high iron content along with ascorbates, which may further damage proteins by binding to them under pro-oxidant conditions. Finally, the limited antioxidant defenses yield the brain prone to oxidative stress and damage (reviewed in [117]).
Clinical assessments and postmortem studies have shown an inverse relationship between docosahexaenoic acid (DHA) and AD (reviewed in [118]) linking oxidative damaged lipids and AD. Furthermore, a clear association has been made between overproduction of free radicals arising from dysfunctional mitochondria and from Aβ with oxidative damage in brain regions with AD pathology (hippocampus and cortex). However, the cerebellum which remains clear of AD pathology, shows low oxidative stress and no neuronal loss [117]. An increase in antioxidant levels has also been reported in AD brains and dietary manipulations increasing antioxidant activity have been investigated as a therapeutic targets for AD (reviewed in [117]). A promising clinical trial recently showed that attenuating metal proteins was effective in improving cognition in patients with AD [119] further highlighting the role of oxidative damage in the pathologies observed in this disease.
3. THE COMPARATIVE BIOLOGY OF OXIDATIVE STRESS; A POTENTIAL DETERMINANT OF SPECIES LONGEVITY
As reiterated above, the oxidative stress theory of ageing asserts that as an inevitable by-product of aerobic metabolism, the electron transport chain in the mitochondria produce ROS and that those species capable of living long lives will accrue less cumulative damage as a result of more complete neutralization by antioxidants. Furthermore, one would predict that long-lived species may also have structural macromolecule characteristics and/or better repair processes that make them more resistant to the cumulative effects of oxidative damage.
Although there is considerable support for this proximate mechanism of aging in invertebrates, [120–122], there are several contradictory studies even when using the same species (e.g. Drosophila, [123, 124]. Furthermore, the high phenotypic diversity and reproductive modality among invertebrates, coupled with the metabolic dependence upon temperature and terrestrial or aquatic lifestyle differentially influence the interactions between ROS production, antioxidant defense and maximum species lifespan potential. Endothermic vertebrate classes (birds and mammals) exhibit markedly higher oxygen flux than aquatic and terrestrial invertebrates and the ectothermic vertebrates (fish, amphibians and reptiles). Given their high metabolic flux, endotherms ought to be more susceptible to the harmful effects of potentially higher levels of unchecked ROS. However, birds exhibit a 1.5-fold higher mass specific basal metabolic rate (BMR) than do similar-sized mammals and in contrast to the predicted detrimental effects on longevity caused by the inevitable ROS-induced damage due to oxygen consumption, some live approximately twice as long as do similar-sized mammals [125].
Most biologists are aware of the importance of scaling biological traits with body size [126] and the famous “mouse-to-elephant curve” produced by Benedict [127] which profoundly demonstrated mammalian metabolic intensity to be inversely related to body mass. A very similar allometric relationship exists for birds, from humming birds to ostriches [128]. The allometric relationships for metabolic rate between birds and mammals are indistinguishable, despite the fact that endothermy arose independently in these two evolutionarily distinct vertebrate classes. In both birds and mammals, doubling of body mass, leads to a 15–20% decline in mass specific metabolic rate [129, 130]. Lindstedt and Calder [131] showed that longevity in birds and mammals increased similarly with increasing body size, although birds generally live twice as long as similar-sized mammals [129]. BMR is affected by several variables including body mass and phylogeny. When these effects of size and phylogeny on BMR are statistically corrected, the relationship between BMR and MLSP no longer holds in either birds or mammals [132]. The combination of a relatively higher metabolic rate and a longer lifespan also means that birds, on average, will have three-fold greater lifetime energy expenditure (LEE) than mammals. This raises critical questions about how aerobic metabolism and susceptibility to oxidative damage might differ between birds and mammals and if exceptionally long-lived mammals exhibit a profile more in keeping with that of long-lived birds. Both these outcomes refute the explanatory power of the rate of living theory for MLSP. Furthermore, when a more comprehensive assessment of >240 mammals is used to determine LEE, calculated values are not constant, but rather MLSP is negatively correlated with LEE. Furthermore BMR, the integral component upon which LEE is determined, is specifically measured at rest in post absorptive, non-growing, non-breeding, healthy adults housed in their thermoneutral zone and is not an accurate indicator of total daily energy expenditure; it does not take into account the energy costs associated with daily activity, foraging, digestion, growth or reproduction. If average daily metabolic rates (ADMR) or field metabolic rates (FMR) are used instead of BMR, LEE among mammals declines with increasing body mass [129, 130]. There are also multiple exceptions to the presumed constant relationship between metabolic rate and lifespan posited by the rate of living theory. For example voluntary exercise and its associated increase in metabolic rate does not shorten lifespan of rats [133] or humans [134] and is generally thought to extend healthy lifespan. Dietary caloric restriction (CR) is well known to extend lifespan in a wide range of species [135], yet this process is not accompanied by attenuations in mass specific metabolic rate resulting in elevated LEE [136]. Also, there is no inverse relationship between lifespan and mass-specific metabolic rates of individual mice, dogs or flies [137–139]. Intra-specific data also provide compelling exceptions to this theory showing that within a species those individuals with the highest metabolic rates live longest and this is attributed to a decline in mitochondrial efficiency and metabolic uncoupling processes [139]. Similarly significant interspecific differences in MLSP within both groups of endotherms cannot be explained by metabolic rate differences. Rather, interspecifies differences in metabolic rate generally reflect climatic zones and geographical regions such as desert or mesic habitats [140–142], the season during the year when the measurements were taken [143] and/or whether animals live above or below ground [144, 145] Generally all subterranean rodents have low basal metabolic rates relative to that expected allometrically [144, 146]. However the low metabolic rates of African mole-rats species (Bathyergidae) with known MLSP data do not correlate with species longevity or other life history traits (Table 2) such as time to sexual maturity, gestation length or litter size, [147]). Furthermore, while BMR of naked mole-rats is 75% that of age-matched mice their greater longevity would result in the highest mass specific LEE of any known mammal [148].
Table 2.
Bathyergid Key Life History and Metabolic Traits.
| Species | Common Name | Avg Body Mass (g) | MLSP (years) | % MLSP Expected# | BMR (mL O2/g/h) | % BMR Expected* | Core Body Temp (°C) | Time to Sexual Maturity (days) | Gest. (Days) | Litter Size | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cryptomys hottentus | Common Mole Rat | 75 | 11 | 116 | 0.68 | 62 | 34 | 450 (f) | 81 | 3.5 | [263] |
| Fukomys damarensis | Damaraland mole-rat | 152 | 15.5 | 147 | 0.57 | 60 | 35 | 511(f) | 93 | 2.5 | [263, 264] |
| Fukomys mechowi | Giant mole-rat | 272 | 20 | 174 | 0.60 | 75 | 36 | unknown | 112 | 2.6 | [265] |
| Georychus capensis | Cape mole-rat | 197 | 11.2 | 100 | 0.60 | 68 | 36 | 304(f), 304(m) | 44 | 5 | [141] |
| Heterocephal us glaber | Naked mole-rat | 35 | 31 | 381 | 1.00 | 66 | 33 | 228 (f) | 77 | 16 | [195, 266] |
Information not specifically cited taken from the AnAge Database (http://genomics.senescence.info/species/)[147]
% predicted MLSP based upon the allometric equation of JP de Magalhaes [132]
% BMR expected based upon the allometric equations of BK McNab [144]
Membrane Composition
Membrane composition, and in particular mitochondrial membrane lipid composition, also varies in a systematic manner with body mass in both endothermic vertebrate classes (see [130] for review). Phospholipids in membranes of larger species contain proportionately less easily oxidized polyunsaturated fatty acids (PUFA), but rather have more monounsaturated fatty acids (MUFA) that are more resistant to oxidation. The products of lipid peroxidation are powerful ROS, and initiate a self-propagating autocatalytic process of oxidative damage to adjacent macromolecules. Interclass variability in membrane composition could explain the seven-fold difference in metabolism between reptiles and mammals as well as differences in metabolic rate attributed to intra-class variability in body size [130]. Differences in membrane composition in both mammals and birds also are correlated with their maximum lifespans such that shorter-lived smaller mammals have membranes rich in n-3 PUFAs (such as DHA) that are more susceptible to lipid peroxidation [149, 150]. These findings have given rise to the “membrane pacemaker theory of aging” (see [130] for review). Comparative studies assessing membrane composition of species with disparate longevity provide support for this theory. For instance long-lived naked mole-rats [151], echidnas [152] and humans [153] all have membranes with a lower peroxidation index than predicted by body size. Similarly, longer-lived strains within a species have membranes with less DHA [151] and while CR does not influence BMR, membrane fatty acid composition varies with the degree of restriction [154]. Membrane leakiness and concomitant mitochondrial membrane potential influences the proton gradient and thus the rate of ROS production. Membrane leakiness, like BMR is inversely related to body mass in both mammals and birds [155, 156]. The extent of membrane leakiness however is reportedly not directly due directly to membrane fatty acid composition [157].
ROS Production
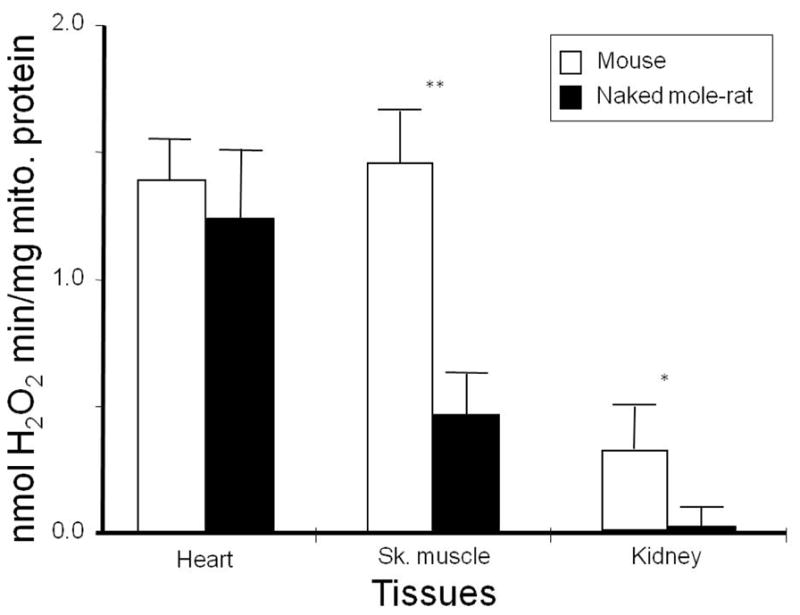
Both superoxide and H2O2 production per mg of mitochondrial protein scale inversely with body mass in a number of animals, from mice to horses [158, 159] further revealing that the potential for oxidative stress is inherently greater in smaller than in larger endotherms. Unless countered by other processes, oxidative stress could account for the relationship between body size and longevity. The rate of ROS production per unit of oxygen consumed was lower in pigeons than in rats, thus reconciling their ability to have a higher metabolic rate than rats and yet live far longer [160]. A similar inverse correlation between longevity and mitochondrial H2O2 production was found in a comparative study among three similar-sized rodents with widely divergent longevities [161] and in a larger comparative study using mitochondria from heart tissue of ten mammalian and two bird species that differed substantially in both body size and MLSP [162]. Significantly, even after statistically correcting for the influence of body size on longevity and ROS production, this relationship was upheld. Furthermore recent studies in reptiles also exhibit a similar relationship between MLSP and rate of mitochondrial H2O2 production [163]. Despite this generalized strong support, several inter-specific data sets do not back the oxidative stress theory of aging and these discrepancies lead us to be wary of fully supporting this august theory. For example, in a comparative study among long-lived little-brown bats (Myotis lucifugis, MLSP 34y); short-tailed shrews (Blarina brevicauda, MLSP 2 years) and white-footed mice (Peromyscus leucopus, MLSP 8 years), while ROS production in heart tissue was lowest in the long-lived species, ROS production in brain tissue was considerably higher than that of shorter-lived, white-footed mice and the species differences in ROS production did not correlate with the 17-fold and 4-fold difference in MLSP. Furthermore, in the Lambert study described above, in which rates of H2O2 production were measured under identical conditions, statistically indistinguishable levels of ROS production were evident among the two short- and long-lived rodent pairs, namely white-footed mice and wild-derived mice despite a two-fold difference in longevity; and among the naked mole-rats and the C57Bl/6 mice despite an eight-fold difference in MLSP. Similarly, Brazilian free-tailed bats (Tadarida brasiliensis, MLSP 12y) are approximately three times shorter-lived than the little brown bat (Myotis lucifugus, MLSP 34 y), yet have only a 10% lower ROS production rate. While ROS production in heart tissue was statistically similar among the long-lived naked mole-rats and C57Bl/6 mice ROS production in naked mole-rat skeletal muscle and kidneys showed significantly lower levels (Fig. 2) in the longer-lived species (unpublished data Lambert, Brand and Buffenstein). This tissue difference among the heart and other tissues may reflect the fact that the naked mole-rat heart is a smaller and slower pump than that of the mouse [139mg±0.6 for the naked mole-rat heart mass, or 0.36% of average body weight (n=15) and 143mg±0.3 or 0.56% of the average body weight (n = 10) for that of the mouse]; also, the naked mole-rat anesthetized heart operates between 150–220 beats per minute whereas that of mice under similar conditions beats at 450–500 beats per minute (Grimes, Lindsey, Lewis, and Buffenstein, unpublished data). Given the large difference in heart function, it is likely that the heart may not be the most appropriate organ for comparing mitochondrial function among species. This could be interpreted to mean that very small differences in rate of ROS formation have substantial effects on MLSP, but it also suggests that mitochondrial ROS production rates are tissue specific and are only one of many factors that may influence species longevity and that these are not the main determinants of longevity.
Fig. 2.
Hydrogen peroxide (H2O2) production by heart, skeletal muscle, and kidney mitochondria in mice and naked mole-rats. To assess ROS production by complex I, succinate was added to the assay and H2O2 generation rate was determined by monitoring the oxidation of either p-hydroxyphenylacetic acid or amplex red with the reaction being coupled to the enzymatic reduction of H2O2 by horseradish peroxidase as described [162]. While there was no significant difference in H2O2 production in heart tissue, naked mole-rats showed significantly less H2O2 in both skeletal muscle and kidney. (Lambert, Buffenstein and Brand, unpublished data).
It is worth mentioning that the detected inverse relationship between mitochondrial ROS and MLSP we observed is only evident when one substrate (succinate) is supplied in abundance [162]. This study suggests the reverse electron transfer through complex I may be an indicator of longevity. The substrate succinate is known to induce significant amounts of reverse electron transfer from the Q pool to complex I, which, in turn, is associated with high rates of ROS production when the membrane potential is also high [164]. There is considerable debate regarding whether or not this stalled electron transport occurs naturally and it is also not known why the correlation is not evident when multiple other substrates representative of forward electron transport through the various components of the ETC were employed. However, fatty acids and α-glycerophosphate also enter the electron transport chain through the Q pool and may reduce it to levels provoking reverse electron flow [165]. Complex I is considered the dominant site of in vivo ROS formation [166] and also the site responsible for the reductions in rates of mitochondrial ROS production following CR in mammals [167]. In this regard, it is significant that birds examined thus far (pigeons, budgerigars, and canaries) have proportionately less complex I in their mitochondria than mammals (rats, mice) [16, 168, 169]. Furthermore, the maximal rates of complex I ROS production per mg of heart mitochondrial protein in pigeons was about half that of rat mitochondria, reflecting exactly the proportionate difference in complex I content of their mitochondria [16]. If this were a general characteristic of avian mitochondria, birds ought to be predisposed to a reduced mitochondrial ROS production when compared to mammals.
In addition, it is important to determine whether this phenomenon would be observed in mitochondria from other vital tissues such as brain, liver, kidney or skeletal muscle. Mitochondria from different tissues of the same species have different phenotypes in physiological and pathological situations [170, 171], (Lambert, Brand and Buffenstein unpublished data). In addition, interspecies differences in mitochondrial organization may influence the efficiency of mitochondrial substrate utilization as well as proton leak. Furthermore, whether mitochondrial ROS would cause similar extent of damage in intact cells containing endogenous antioxidant systems is still an open question and probably more meaningful. Interestingly, targeted disruption of the mitochondrial gene SURF1, causes an increase in longevity in laboratory mice [172]. However, the cause of the phenotype may neither be dependent on membrane potential nor be related to ROS control, but instead depend on the handling of Ca2+ uptake [172].
Antioxidants
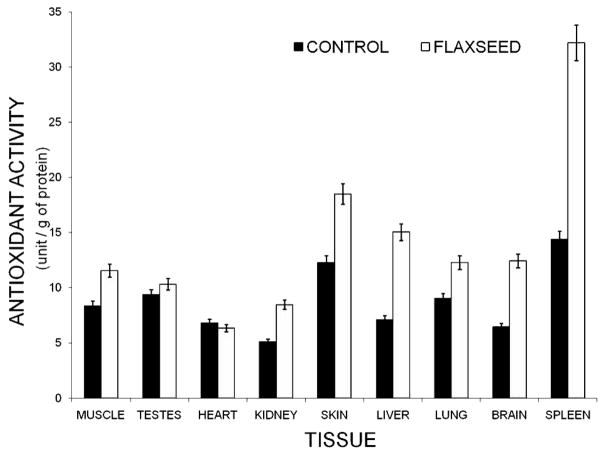
A complex suite of mechanisms have evolved to eliminate ROS and thereby offset their potential for accruing oxidative damage. Antioxidant activity as a determinant of MLS remains controversial with reports of a positive, negative and no correlation between anti-oxidant activity and MLSP [173–178]. CAT and GPx activities reportedly negatively correlated with MLSP [179] whereas Sohal et al., [158] found that SOD and CAT activities were positively correlated and indeed in the case of GPx, levels correlated positively with MLSP in brain samples but negatively in the liver and heart [176]. When birds, fish and amphibians were included for comparison for antioxidants activities and MLSP, strong negative correlations for some but not all antioxidants were obtained and results were tissue specific [160, 180, 181]. An often-touted explanation for this negative correlation is that antioxidant defenses are not the reason for extended longevity of long-living animals, but rather the lower antioxidant levels in long-living species may be indicative of lower levels of oxidative stress [173, 182–184] Similarly, we too have found that total antioxidant power varies among tissues and is responsive to dietary manipulation, as seen by the increase in total antioxidants following supplementation with peroxidation prone omega n-3 fatty acids (Fig. 3; Wywial and Buffenstein, unpublished data).
Fig. 3.
Total antioxidant power in naked mole-rats varied substantially among tissues. Furthermore, dietary supplementation for 6 weeks with flaxseed oil, an omega-3 fatty acid (7g PUFA per 100g ProNutro) [267] resulted in augmented total antioxidant activity (Wywial and Buffenstein, unpublished data). Total antioxidant power was measured quantitatively using a commercial colorimetric microplate assay for total antioxidants (Oxford Biomedical Research, Oxford, MI).
One difficulty in studying the role of antioxidants in longevity is that there is no consistent stoichiometric relation between pro-oxidation or antioxidant measures, making inferences between species from limited measures of only a few antioxidants essentially meaningless. Many members of the antioxidant defense system share redundant roles and are rapidly induced in response to elevated oxidative stress rather than maintained at high basal levels. In order to estimate the overall ability to defend against ROS, it is necessary to evaluate the activities of all antioxidants present in tissues and their interactions and not simply focus on a few key antioxidant enzymes.
No consistent differences in known processes that remove radicals and repair the damage have been found to correlate with MLSP [162, 175, 185, 186]. Many long-lived species, such as the humans, and naked mole-rat, show unremarkable antioxidant defenses [186, 187]. Clearly levels of antioxidant defense cannot account for lifespan difference across the animal kingdom and as such the anti-oxidant arsenal are not integral determinants of species longevity. These findings concur with studies showing that the effects of anti-oxidants on human health are equivocal [188] as are the efficacy of pharmacological mimetics of antioxidants [189]. Genetic overexpression of various antioxidants and their effects on lifespan in various animal models similarly yield conflicting results and knocking down antioxidant protection, while leading to more oxidative damage, does not impact upon longevity [35]. This observation correlates with the negative data from various dietary treatments with exogenous antioxidants that sometimes lead to extended median ages, but almost never extends MLSP [176, 183, 184]. If the oxidative stress theory holds true it would suggest that variation in rates of ROS importantly influences MLSP and that consequent oxidative damage is imperfectly countered by antioxidants.
Oxidative Damage
The oxidative stress theory of aging asserts that the decline in functionality that characterizes the aging process is due to progressively increasing oxidative damage. Most comparative studies compare data from young healthy adults providing a snap shot of oxidative damage at a given age of each species. The rationale behind this approach is that traits influencing rates of aging ought to be present throughout life and if these traits facilitate long life, even at an early age their impact should be evident. Accrued oxidative damage may not only influence aging but also may impact considerably on many life history characteristics dependent upon the apportioning of energy metabolism into fecundity, development, somatic maintenance and maximum species lifespan.
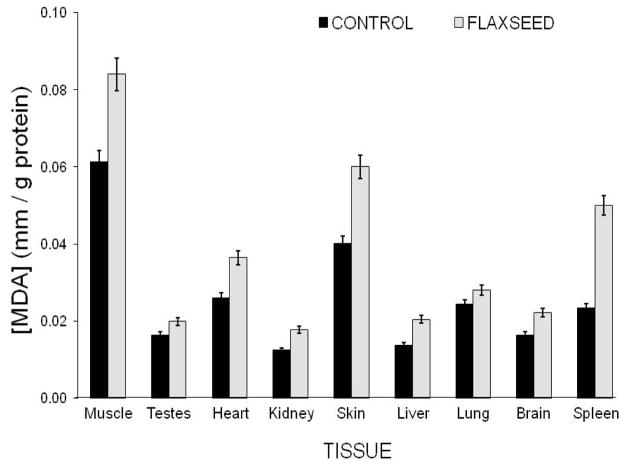
Several comparative studies show an inverse relationship between oxidative damage and longevity, already evident in young healthy adults [173, 190, 191]. However, many exceptions to this premise have been reported. For example within flies, the longer-lived Drosophila species has more protein carbonyls than the shorter-lived blow flies (Calliphora vicina) [192]. Similarly longer-lived birds have higher levels of oxidative damage to DNA than do shorter-lived mammals [193], and within mammals both long-lived vampire bats and naked mole-rats have higher levels of protein carbonyls than short-lived rats and mice measured under identical conditions [1, 175, 182, 194]. The level of oxidative damage, such as MDA differs substantially among tissues. Despite lower levels of ROS production in muscle than in heart tissues (Fig. 2), muscle tissue exhibits twice as much lipid peroxidation as observed in heart tissue (Fig. 4) and exhibits a similar level of antioxidant protection (Fig. 3). The degree of oxidative damage is depended upon dietary fatty acid content, as is evidenced by the significantly greater MDA levels following chronic dietary supplementation with n-3 rich flaxseed oil. High levels of oxidative damage in mole-rats and bats has been attributed to their high intracellular iron content wreaking havoc with redox [182, 195]. Such interspecific and ontogenetic differences in iron homeostasis are likely to have a huge impact on rates of aging and sudden increases in ROS-related damage leading to death.
Fig. 4.
Lipid peroxidation, as indicated by levels of malondehyde (MDA) in various tissues of naked mole rats. Note that MDA varies significantly within tissues (see Control profile), with skeletal muscle showing the highest levels. The degree of lipid peroxidation is influenced by dietary manipulations. After only six weeks of dietary supplementation with the omega-3 rich fatty acids present in ground flaxseeds, all tissues showed a significant increase in MDA. Wywial and Buffenstein, unpublished data). LPO was measured quantitatively using a commercial colorimetric assay (Oxford Biomedical Research, Oxford, MI).
Oxidative damage measurements made in tissue samples are the net result of several interacting processes associated with primary rates of ROS generation in mitochondria including autocatalytic secondary ROS production as a result of phospholipid peroxidation, neutralization of ROS by antioxidants as well as damage repair and the removal of damaged products by nucleases, proteases and autophagy that precede the sampling point. These net levels of oxidative damage as such may represent a “steady-state” level of damage tolerance rather than indicative of rates of oxidative stress. Steady-state levels of oxidative damage, based upon these premises reveal a lack of consistent patterns, with higher and lower levels of net damage reported in longer-lived birds, bats, and rodents [178, 186, 196, 197]. While it is possible that all the exceptions to the oxidative stress theory reflect unusual species that have been subjected to a different collage of evolutionary pressures and thus present with their own suite of “private mechanisms” to deal with aging, given the broad range of exceptions both within species, among species and among vertebrate classes, this seems unlikely [14]. Rather the lack of a consistent pattern in these measures of oxidative damage and lifespan challenge the validity of this theory.
This mode of comparative approach, however, is not without limitations and ignores the rates of age-related changes in oxidative stress and thereby fails to address the dynamics of aging and how this differs among species of disparate longevity, even though there is convincing evidence from numerous studies that aging is associated with increased oxidative stress and oxidative macromolecular damage in various tissues studied [193, 198]. Indeed age-dependent accumulation of oxidative damage is considered an intrinsic factor determining the rate of aging, with both damage to protein carbonyls, lipids and DNA increasing more markedly in the latter third of life [199–201]. For example oxidative damage to DNA gradually increases in a non-linear modality with age in rats, however levels appear to be tissue-specific such that DNA damage in the brain is greater than in liver and kidney [199]. The few studies that utilized both approaches are obviously more powerful for testing the oxidative stress theory of aging [186, 202–204]. These studies often yield contrary results to those simply using young adults. For example although naked mole-rats show high levels of oxidative damage, evident already at a young age, both cysteine oxidation and lipid peroxidation are maintained at those high steady state levels for at least two thirds of the extraordinary lifespan of naked mole-rats, providing some support for the oxidative stress theory of aging [205]. Clearly, this species has a higher threshold of damage tolerance and has mechanisms in place to facilitate structural resistance to the harmful effects of oxidative damage and still maintain cellular integrity and function.
Stress Resistance
Although antioxidant status and accrual of oxidative damage show mixed correlations with MLSP, stress and toxin resistance appears to play a significant role in the determination of lifespan in naturally long-lived species, as well as genetically modified models of longevity [206–209]. In worms and flies, manipulation of genes resulting in longer lifespan often result in increased resistance to exogenous stressors or toxins including heat shock, H2O2 and paraquat [210–212]. This toxin resistance is observed in rodent models of longevity as well. Dwarf mice, which can be drastically long-lived compared to wild-type littermates, show increased resistance in vitro and in vivo to an array of stressors [207, 213].
Most notably, fibroblasts cultured from naked mole-rats prove to be incredibly resistant to a variety of toxins, including oxidative stressors, when compared to fibroblasts from standard laboratory mice [205, 209]. However, while naked mole-rat fibroblasts showed resistance to toxicity from paraquat, consistent with the model of oxidative stress and longevity, the cells were acutely sensitive to H202 [209]. Interestingly, the toxicity of H202 differs from that of paraquat. Paraquat causes cellular toxicity through the production of O2− in the mitochodria disrupting NAPDH-dependent biochemistry and eventually leading to tissue damage and compromised organismal function [214]. H202, on the other hand, causes oxidative stress through the production of hydroxyl radicals that cause damage to membranes, lipids and nucleic acids [215]. Aside from the different mechanisms of radical formation, the standard culture conditions of 20% oxygen, or the use of serum-free conditions (affecting membranes and lipids as well) may have contributed to this seemingly paradoxical result pushing the naked mole-rat fibroblasts, already containing high levels of oxidative damage compared to age-typed laboratory mice beyond their ability to mitigate the damage [205, 209, 216, 217]. In any case, because the naked mole-rat appears to be resistant to a diverse range of toxins that have different modes of action, a pathway that may serve as a key regulator of cytoprotection may serve as the main mechanism for the profound resistance to toxins observed not only in the naked mole-rat, but other naturally and genetically-modified long-lived species. One such pathway is the nuclear factor erythroid-2 related factor-2 (Nrf2) signaling pathway, which regulates the transcription of 230 genes [218]. These genes include those involved in GSH synthesis and conjugation [219], free radical scavengers [220] as well as proteasomal subunits [221]. Nrf2 is a transcription factor expressed in all tissues of an organism and is conserved from worms to humans [210]. Under constitutive (non-stressful) conditions, Nrf2 is basally expressed at low levels and has a relatively short half-life (~15 minutes). Nrf2 is largely regulated by kelch-like ECH-associated protein 1 (Keap1), which targets Nrf2 for ubiquitination and degradation via the proteasome [222, 223]. Under stressful conditions (i.e. an increase in ROS or xenobiotic stressor), cysteines within Keap1 are modified, impeding the interaction with Nrf2. Levels of free Nrf2 increase resulting in an increase in half-life (~60 minutes) and increased nuclear levels of Nrf2. Nrf2 binds to the antioxidant response element to promote the transcription of cytoprotective molecules [224, 225].
In C. elegans, the Nrf2 homolog, Skn-1, was shown to be a mediator for the life-extending effects of CR [210]. Similar results were observed in Drosophila, in addition to a lifespan extension observed in flies that had decreased Keap1 expression [212]. In both of these invertebrate models, long-lived organisms show an increase in the signaling of Nrf2/Skn-1-regulated molecules, possibly contributing to their longevity. These trends are also observed in long-lived rodent models including the naked mole-rat [226]. Caloric and methionine-restricted mice, as well as dwarf mice, show signs of increased Nrf2-signalling activity further supported by increased toxin resistance [227–229].
Repair Processes
Repair processes play a vital role in maintaining cellular homeostasis and not surprisingly there are numerous cellular pathways to ensure quality control and the rapid removal of damaged molecules before these can impair functionality [230]. A gradual decline in some of these pathways occur during aging and the malfunction of these quality control mechanism form the basis of many age-associated diseases such as neurodegeneration, metabolic disorders and cancers [230]. Cells rely on various molecular chaperones to maintain the three dimensional structure of their proteins. Chaperones selectively binding to non-native protein conformations to form stable complexes and prevent the irreversible aggregation of non-native protein conformations [231]. Not surprisingly many of these chaperones are induced under stressful conditions to assist in the maintenance of protein quality. Expression of chaperones declines with age and manipulations that retard aging enhance chaperone expression, including the heat-shock response [230, 232, 233]. Previously we have shown that the proteome of naked mole-rats and bats is more resistant to both urea-induced and heat-induced unfolding than that of shorter-lived mice [205, 234]. This implies that long-lived species might have evolved enhanced chaperone-like activities to preserve protein structure and prevent misfolding/aggregation. If the damaged proteins cannot be repaired by these chaperones, the proteins are directed towards proteolytic degradation pathways either via the ubiquitin-mediated proteasomal system or via lysosomal mediated authophagy. Both of these systems are subject to age-related declines in functionality [235, 236].
Proteasome Activity
Amongst the many biological substrates of proteasome are oxidatively damaged proteins, the accumulation of which is one of the hallmarks of aging [237, 238]. Such proteins are suspected to inhibit the proteasome machinery, either by overwhelming the proteasome or by a noncompetitive inhibition [24]. In parallel, free radicals causing oxidative damage of substrates may modify the components of the proteasome system, effectively changing (most likely inhibiting) its actions [24]. Aging is also accompanied by the increase of protein carbonyl groups and aggregated cross-linked material which are resistant to proteolytic degradation and may act as inhibitors of the proteasome [24, 239–241]. Consequently, a model with reduced oxidative damage as observed in caloric restricted animals might reduce these potential inhibitors of the proteasome system [242, 243].
Data based upon the proteasome in fission yeast (Schizosac-charomyces pombe) reveal three basic principals of proteasome function [244]. 1) G0 cells require proteasome function during the maintenance of G0 quiescence. Temperature sensitive mutants of 26S proteasome subunits perturb this maintenance. 2) proteasome functions differ between G0 and vegetative proliferation phase (VEG). This conclusion comes from the examination of defective phenotypes of proteasome mutants in G0 phase, leading to a huge increase of oxygen stress responsive compounds and the massive decrease of mitochondria that did not occur in VEG. 3) proteasome dysfunction in G0 elicits defensive responses both by triggering the production of antioxidant components and inducing the degradation of mitochondria by autophagy. These defensive responses were not found in VEG cells, suggesting that proteasome functions in G0 are directly or indirectly involved in minimizing ROS [244]. Oxidative stress biomarkers, of which 20S proteasome was included, showed different responses depending on age in brown trout (Salmo trutta) exposed to paraquat. Fish aged five months showed no changes in carbonylation in liver tissue and 20S proteasome when exposed to the drug while the 12-month-old fish had both an increase in protein carbonylation in liver tissue and a decrease in 20S proteasome activity in brain tissue [245].
In mammalian studies, the influence of oxidative stress on the proteasome is strongly evident. Following injury to the brain or retina, the immunoproteasome activity is upregulated [246]. Furthermore, following immunoproteasome deficiency, the proteasome in the mouse retina could not respond to stress and as such became more susceptible to “oxidation-induced cell death” [247]. In rat brain and liver tissue preparations, exposure to oxidative damage-inducing agents, H2O2 and HNE, inhibited peptidase activity from the proteasome in a dose-dependent manner [248]. The effect was more pronounced in the brain. Interestingly, neither age of the animal or dietary restriction produced a significant effect on the oxidative-stress induced decline of proteasome activity observed in that study [248]. When proteasome activity is inhibited in neuronal cell crude lysates, an increase in both ubiquitinated and oxidized proteins is observed, as well as a selective increase in newly synthesized but insoluble proteins [249], again implicating the proteasome in oxidative-stress related protein homeostasis. An exception to the age-related decline of the proteasome is seen in the case of the naked mole-rat [205]. Perez et al., postulated that the robust maintenace of the proteasome in older mole rats (20yr) when compared to old mice (2yr), may contribute to a better preservation of the proteome and thus contribute to the increased longevity seen in the naked mole-rat [205]. Also, old naked mole-rats have significantly lower levels of ubiquitinated proteins than do old and young mice suggesting less accrual of oxidized or misfolded proteins. Chymotrypsin-like activity from the proteasome is higher in both young and old and naked mole-rats than that observed in their similarly aged mouse counterparts, supporting their hypothesis [205]. Naked mole-rat cytosolic and nuclear liver preparations also showed a more than two-fold increase in trypsin-like activity when compared to the activity from liver preparations of the same subcellular fractions obtained from livers of age-matched mice (Rodriguez KA and Buffenstein R, unpublished data) (Fig. 5).
Fig. 5.
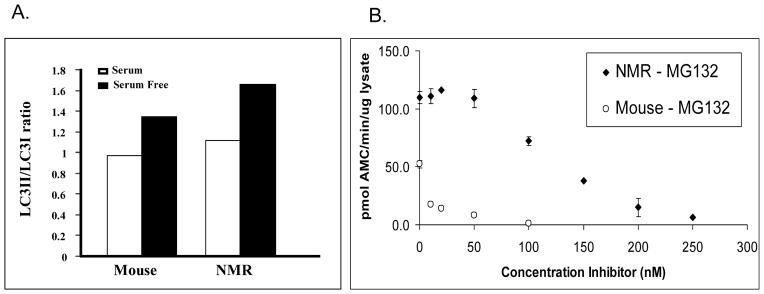
Both autophagy and proteasome activity show an increase in repair capacity in naked mole-rats when compared to mice. A) In serum-free media, the ratio of LC3II/LC3I increases significantly in naked mole-rats suggesting greater sensitivity in activating autophagy processes (Perez and Buffenstein, unpublished data). Data were collected using a Western blot technique and the ratios were calculated using Storm Image Quant software package (Molecular Dynamics, Sunnyvale, CA). B) Species differences in chymotrypsin-like (ChT-L) activity in liver cytosol lysates of young mice (4 m) and naked mole-rats (2y) in response to varying doses of proteasome inhibitor (MG132, N-(benzyl-oxycarbonyl) leucinyl-leucinal) (Rodriguez and Buffenstein, unpublished data). Data (presented as mean ± standard deviation) are based on three different experiments, run in triplicate, using a modification of the methods described by Rogers and Dean using the release of AMC from 100μM of the fluorogenic peptide Succ-LLVY-AMC (Boston Biochem, Boston MA) to measure proteasome ChT-L activity [268, 269]. Naked mole-rats not only show greater levels of proteasome activity compared to mice but also appear to be markedly resistant to a known inhibitor of proteasome activity.
Oxidative stress also links the proteasome to human disease. In a mouse model of ALS (SOD1G93A), a decrease in both the mRNA and protein expression in catalytic subunits β1 and β5 is observed during the progression of the disease in spinal cord tissue. A simultaneous increase is also noted in Lmp7 (β5i), an immunoproteasome subunit [250]. When these mice were crossed with ALS-mice possessing a fluorescently-tagged UPP substrate, revealed both and accumulation of the substrate, and ubiquitin suggesting an impairment of motor neuron UPP in the mutant SOD1-linked ALS mouse [250]. Furthermore, Sod1−/− samples show a relative decline of proteasome activity with both age and oxidative stress especially in the cytosolic and microsomal fractions of mouse liver tissue and a significant reduction in the contribution of activity from 26S in favor of 20S activity in tissue from young oxidatively stressed animals and from old animals (Rodriguez KA, Osmulski PA, and Gaczynska M, unpublished data). If indeed naked mole-rats have a higher steady-state level of oxidative stress as suggested by the studies referenced above [1, 175, 182, 194, 205], they might also have a higher contribution of activity from their 20S proteasomes and better maintenance of that activity with age.
Autophagy
Autophagy is an evolutionarily conserved catabolic pathway, present in all eukaryotic cells. This lysosomal system involves degradation of intact proteins and protein aggregates [251]. There are three pathways by which proteins and cellular organelles are delivered to the lysosome: microautophagy, macroautophagy, and chaperone-mediated autophagy. Surprisingly little research compares species differences regarding autophagy. Studies conducted on senescent avian and mammalian fibroblasts reveal that cells from longer-lived birds better maintain autophagasomal and lysosomal enzymes whereas these activities are down regulated with age in mammalian cell lines [252, 253]. Furthermore, our unpublished data reveal that this may indeed play an important role in the maintenance of protein quality in the naked mole-rat (Perez VI and Buffenstein R, unpublished data) as macroautophagy (degradation sensitive to 3-methyladenine, 3-MA) is substantially higher in fibroblasts maintained under serum starvation from a naked mole-rat compared to those from shorter-lived mice. Similarly when autophagy is assessed by monitoring markers of vacuole development (i.e., the conversion of LC3-I to LC3-II), the LC3-II/LC3-I ratio induced by serum deprivation was ~ 2-fold greater in naked mole-rat cells (Perez VI and Buffenstein R, unpublished data) (Fig. 5). This suggests perhaps that this exceptionally long-lived rodent, under basal conditions maintains higher levels of autophagy, thereby removing potentially toxic proteins and possibly its high ferritin load, before these can negatively impact upon organ functionality. Nevertheless exceptionally old (>29 yr) naked mole-rats accrue the age-associated yellowish-brown pigment lipofuscin which accumulates in various organs including skin, heart and liver, as a result of incomplete lysosomal digestion of cell products [254].
4. CONCLUSION
The plethora of contradictory results together with the lack of a general consensus among multiple species across the animal kingdom as well as among genetically manipulated mice in which oxidative stress is altered strongly suggest that species MLSP is unrelated to any of the oxidative stress parameters currently being measured. Certainly current data from genetically modified mice that exhibit high levels of oxidative damage in response to experimentally impaired antioxidant defenses show no concomitant changes in age-related mortality. Similarly high levels of oxidative damage to lipids, proteins and DNA in the naturally long-lived naked mole-rat coupled with low levels of Gpx that are not compensated for by other enzymatic antioxidants, do not negatively impact on their exceptional longevity. Instead they seem to have a higher set point of equilibrium for oxidative damage. In fact there may be great differences in tolerance and structural resistance to oxidative damage that may underlie the insignificant differences in ROS production and antioxidant protection, despite the large differences in species longevity. This may limit the role of oxidative stress in leading to diseases including cancer, myopathy, vascular disease and neurodegeneration known to result from unchecked ROS. Through the mitigation of damage naked mole-rats may have arrived at an adaptive state of flux. Finally, we hope that the studies reviewed here can act as a springboard to gain insights into the mechanisms on how certain species, like the naked mole-rat, are able to tolerate high levels of oxidative stress without an endpoint of cancer and neurodegenerative disease. Such information in the future can translate to a better understanding of oxidative stress and its relation to a healthy lifespan free of age-related disease in our own species.
Acknowledgments
This work was supported by an Ellison Senior Scholar award, a Breakthroughs in Gerontology award from the American Federation for Aging research and by the NIA/NIH to Rochelle Buffenstein [AG022891-01 and NIGMS S06-GM08168] and by grants from National Institutes of Health [grant numbers P01 AG025901, PL1 AG032118, P30 AG025708 and R01 AG033542], the W.M. Keck Foundation and the Ellison Medical Foundation [grant number AG-SS-2288-09] (to M.D.B.). Both Karl Rodriguez and Kaitlyn Lewis were supported by NIH Aging training grant [T32 AG021890-08].
References
- 1.Buffenstein R, Edrey YH, Yang TT, Mele J. The oxidative stress theory of aging: embattled or invincible? Insights from non-traditional model organisms. AGE. 2008;30:99–109. doi: 10.1007/s11357-008-9058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubner M. Uber den Einfluss der Korpergrosse auf stoff- und kraft-wechsel. Z Biol. 1883;19:535–62. [Google Scholar]
- 3.Rubner M. Das Problem der Lebensdauer. Munich: Oldenburg; 1908. [Google Scholar]
- 4.Pearl R. The Rate of Living. New York: Knopf; 1928. [Google Scholar]
- 5.Lavosier AL. Considérations générales sur la nature des acides, et sur les principes dont ils sont composés Mémoires de l’Académie Royale des Sciences de Paris. 1781. [Google Scholar]
- 6.Gershman R, Gilbert DL, Nye SW, Dwyer P, Fenn WO. Oxygen poisoning and X-irradiation: a mechanism in common. Science. 1954;119:623–6. doi: 10.1126/science.119.3097.623. [DOI] [PubMed] [Google Scholar]
- 7.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 8.Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–81. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 9.Harman D. The biological clock: the mitochondria? J Am Geriatr Soc. 1972;20:145–7. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 10.Hulbert AJ. On the importance of fatty acid composition of membranes for aging. J Theor Biol. 2005;234:277–288. doi: 10.1016/j.jtbi.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 11.Ishii N. Role of oxidative stress from mitochondria on aging and cancer. Cornea. 2007;9:S3–9. doi: 10.1097/ICO.0b013e31812f6745. [DOI] [PubMed] [Google Scholar]
- 12.Bokov A, Chaudhuri A, Richardson A. The role of oxidative damage and stress in aging. Mech Ageing Dev. 2004;125:811–26. doi: 10.1016/j.mad.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Muller FL, Lustgarten MS, Jang Y, Richardson A, VanRemmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 14.Buffenstein R. Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species. J Comp Physiol B. 2008;178:439–45. doi: 10.1007/s00360-007-0237-5. [DOI] [PubMed] [Google Scholar]
- 15.Ikeno Y, Hubbard GB, Lee S, et al. Housing density does not influence the longevity effect of calorie restriction. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 2005;60:1510–7. doi: 10.1093/gerona/60.12.1510. [DOI] [PubMed] [Google Scholar]
- 16.St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277:44784–90. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 17.Cadenas E, Davies K. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222–30. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 18.Speakman JR, Selman C. The free-radical damage theory: Accumulating evidence against a simple link of oxidative stress to ageing and lifespan. Bioessays. 2011;33:255–9. doi: 10.1002/bies.201000132. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Oberley LW. Redox regulation of transcriptional activators. Free Radic Biol Med. 1996;21:335–48. doi: 10.1016/0891-5849(96)00109-8. [DOI] [PubMed] [Google Scholar]
- 20.Chung HY, Sung B, Jung KJ, Zou Y, Yu BP. The molecular inflammatory process in aging. Antioxid Redox Signal. 2006;8:572–81. doi: 10.1089/ars.2006.8.572. [DOI] [PubMed] [Google Scholar]
- 21.Linnane AW, Kios M, Vitetta L. Healthy aging: regulation of the metabolome by cellular redox modulation and prooxidant signaling systems: the essential roles of superoxide anion and hydrogen peroxide. Biogerontology. 2007;8:445–67. doi: 10.1007/s10522-007-9096-4. [DOI] [PubMed] [Google Scholar]
- 22.Golden TR, Melov S. Mitochondrial DNA mutations, oxidative stress, and aging. Mech Ageing Dev. 2001;122:1577–89. doi: 10.1016/s0047-6374(01)00288-3. [DOI] [PubMed] [Google Scholar]
- 23.Levine RL. Oxidative modification of glutamine synthetase. I. Inactivation is due to loss of one histidine residue. J Biol Chem. 1983;258:11823–7. [PubMed] [Google Scholar]
- 24.Szweda PA, Friguet B, Szweda LI. Proteolysis, free radicals, and aging. Free Radic Biol Med. 2002;33:29–36. doi: 10.1016/s0891-5849(02)00837-7. [DOI] [PubMed] [Google Scholar]
- 25.Boesch P, Weber-Lotfi F, Ibrahim N, et al. DNA repair in organelles: Pathways, organization, regulation, relevance in disease and aging. Biochim Biophys Acta. 2011;1813:186–200. doi: 10.1016/j.bbamcr.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Hill BG, Haberzettl P, Ahmed Y, Srivastava S, Bhatnagar A. Unsaturated lipid peroxidation-derived aldehydes activate autophagy in vascular smooth-muscle cells. Biochem J. 2008;410(3):525–34. doi: 10.1042/BJ20071063. [DOI] [PubMed] [Google Scholar]
- 27.Guéraud F, Atalay M, Bresgen N, et al. Chemistry and biochemistry of lipid peroxidation products. Free Radic Biol Med. 2010;44:1098–124. doi: 10.3109/10715762.2010.498477. [DOI] [PubMed] [Google Scholar]
- 28.Madani A, Nehal M, Haque SS, Khan A. Perspective of oxidative stress in a biological system and prevention by naturally occurring antioxidant. Proc Natl Acad Sci USA. 2010;80:287–95. [Google Scholar]
- 29.Lawrence RA, Burk RF. Species, tissue and subcellular distribution of non Se-dependent glutathione peroxidase activity. J Nutr. 1978;108:211–5. doi: 10.1093/jn/108.2.211. [DOI] [PubMed] [Google Scholar]
- 30.Reddy VN, Giblin FJ, Lin LR, et al. Glutathione peroxidase-1 deficiency leads to increased nuclear light scattering, membrane damage, and cataract formation in gene-knockout mice. Invest Ophthalmol Vis Sci. 2001;42:3247–55. [PubMed] [Google Scholar]
- 31.Wolf N, Penn P, Pendergrass W, et al. Age-related cataract progression in five mouse models for anti-oxidant protection or hormonal influence. Exp Eye Res. 2005;81:276–85. doi: 10.1016/j.exer.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 32.Fu Y, Cheng WH, Porres JM, Ross DA, Lei XG. Knockout of cellular glutathione peroxidase gene renders mice susceptible to diquatinduced oxidative stress. Free Radic Biol Med. 1999;27:605–11. doi: 10.1016/s0891-5849(99)00104-5. [DOI] [PubMed] [Google Scholar]
- 33.Yant LJ, Ran Q, Rao L, et al. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic Biol Med. 2003;34:496–502. doi: 10.1016/s0891-5849(02)01360-6. [DOI] [PubMed] [Google Scholar]
- 34.Imai H, Hakkaku N, Iwamoto R, et al. Depletion of selenoprotein GPx4 in spermatocytes causes male infertility in mice. J Biol Chem. 2009;284:32522–32. doi: 10.1074/jbc.M109.016139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pérez VI, Bokov A, VanRemmen H, et al. Is the oxidative stress theory of aging dead? Biochem Biophys Acta. 2009;1790:1005–14. doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–55. [PubMed] [Google Scholar]
- 37.Rosen D, Siddique T, Patterson D, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Xu G, Borchelt DR. High molecular weight complexes of mutant superoxide dismutase 1: age-dependent and tissue-specific accumulation. Neurobiol Dis. 2002;9:139–48. doi: 10.1006/nbdi.2001.0471. [DOI] [PubMed] [Google Scholar]
- 39.Majoor-Krakauer D, Willems PJ, Hofman A. Genetic epidemiology of amyotrophic lateral sclerosis. Clin Genet. 2003;63:83–101. doi: 10.1046/j.0009-9163.2002.00001.x. [DOI] [PubMed] [Google Scholar]
- 40.Bartnikas TB, Gitlin JD. Mechanisms of biosynthesis of mammalian copper/zinc superoxide dismutase. J Biol Chem. 2003;278:33602–8. doi: 10.1074/jbc.M305435200. [DOI] [PubMed] [Google Scholar]
- 41.Brown NM, Torres AS, Doan PE, O’Halloran TV. Oxygen and the copper chaperone CCS regulate posttranslational activation of Cu, Zn superoxide dismutase. Proc Natl Acad Sci USA. 2004;101:5518–23. doi: 10.1073/pnas.0401175101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cuarano-Yzermens AL, Bartnikas TB, Gitlin JD. Mechanisms of the copper-dependent turnover of the copper chaperone for superoxide dismutase. J Biol Chem. 2006;281:13581–7. doi: 10.1074/jbc.M601580200. [DOI] [PubMed] [Google Scholar]
- 43.Matzuk MM, Dionne L, Guo Q, Kumar TR, Lebovitz RM. Ovarian function in superoxide dismutase 1 and 2 knockout mice. Endocrinology. 1998;139:4008–11. doi: 10.1210/endo.139.9.6289. [DOI] [PubMed] [Google Scholar]
- 44.Ho YS, Gargano M, Cao J, Bronson RT, Heimler I, Hutz RJ. Reduced fertility in female mice lacking copper-zinc superoxide dismutase. J Biol Chem. 1998;273:7765–9. doi: 10.1074/jbc.273.13.7765. [DOI] [PubMed] [Google Scholar]
- 45.McFadden SL, Ding D, Burkard RF, et al. Cu/Zn SOD deficiency potentiates hearing loss and cochlear pathology in aged 129, CD-1 mice. J Comp Neurol. 1999a;413:101–12. [PubMed] [Google Scholar]
- 46.McFadden SL, Ding D, Reaume AG, Flood DG, Salvi RJ. Age related cochlear hair cell loss is enhanced in mice lacking copper/zinc superoxide dismutase. Neurobiol Aging. 1999b;20:1–8. doi: 10.1016/s0197-4580(99)00018-4. [DOI] [PubMed] [Google Scholar]
- 47.Reddy VN, Kasahara E, Hiraoka M, Lin LR, Ho YS. Effects of variation in superoxide dismutases (SOD) on oxidative stress and apoptosis in lens epithelium. Exp Eye Res. 2004;79:859–68. doi: 10.1016/j.exer.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Elchuri S, Oberley TD, Qi W, et al. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcino-genesis later in life. Oncogene. 2005;24:367–80. doi: 10.1038/sj.onc.1208207. [DOI] [PubMed] [Google Scholar]
- 49.Muller FL, Song W, Liu Y, et al. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic Biol Med. 2006;40:1993–2004. doi: 10.1016/j.freeradbiomed.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 50.Wang X, Vatamaniuk MZ, Roneker CA, et al. Knockouts of SOD1 and GPX1 exert different impacts on murine islet function and pancreatic integrity. Antioxid Redox Signal. 2011;14:391–401. doi: 10.1089/ars.2010.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hiroi S, Harada H, Nishi H, Satoh M, Nagai R, Kimura A. Polymorphisms in the SOD2 and HLA-DRB1 genes are associated with nonfamilial idiopathic dilated cardiomyopathy in Japanese. Biochem Biophys Res Commun. 1999;261:332–9. doi: 10.1006/bbrc.1999.1036. [DOI] [PubMed] [Google Scholar]
- 52.Nakanishi S, Yamane K, Ohishi W, et al. Manganese superoxide dismutase Ala16Val polymorphism is associated with the development of type 2 diabetes in Japanese-Americans. Diabetes Res Clin Pract. 2008;81:381–5. doi: 10.1016/j.diabres.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 53.Cai Q, Shu XO, Wen W, Cheng JR, Dai Q, Gao YT, Zheng W. Genetic polymorphism in the manganese superoxide dismutase gene, antioxidant intake, and breast cancer risk: results from the Shanghai Breast Cancer Study. Breast Cancer Res. 2004;6:R647–55. doi: 10.1186/bcr929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Y, Huang TT, Carlson EJ, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376–81. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 55.Lebovitz RM, Zhang H, Vogel H, et al. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci USA. 1996;93:9782–7. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang T, Carlson E, Kozy H, Mantha S, Goodman S, Ursell P, Epstein C. Genetic modification of prenatal lethality and dilated cardiomyopathy in Mn superoxide dismutase mutant mice. Free Radic Biol Med. 2001;31:1101–10. doi: 10.1016/s0891-5849(01)00694-3. [DOI] [PubMed] [Google Scholar]
- 57.Melov S, Coskun P, Patel M, et al. Mitochondrial disease in superoxide dismutase 2 mutant mice. Proc Natl Acad Sci USA. 1999;96:846–851. doi: 10.1073/pnas.96.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Asikainen TM, Huang TT, Taskinen E, et al. Increased sensitivity of homozygous Sod2 mutant mice to oxygen toxicity. Free Radic Biol Med. 2002:175–86. doi: 10.1016/s0891-5849(01)00776-6. [DOI] [PubMed] [Google Scholar]
- 59.VanRemmen H, Salvador C, Yang H, Huang TT, Epstein CJ, Richardson A. Characterization of the antioxidant status of the heterozygous manganese superoxide dismutase knockout mouse. Arch Biochem Biophys. 1999;363:91–7. doi: 10.1006/abbi.1998.1060. [DOI] [PubMed] [Google Scholar]
- 60.VanRemmen H, Williams MD, Guo Z, et al. Knockout mice heterozygous for Sod2 show alterations in cardiac mitochondrial function and apoptosis. Am J Physiol Heart Circ Physiol. 2001;281:H1422–H32. doi: 10.1152/ajpheart.2001.281.3.H1422. [DOI] [PubMed] [Google Scholar]
- 61.VanRemmen H, Ikeno Y, Hamilton M, et al. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics. 2003;16:29–37. doi: 10.1152/physiolgenomics.00122.2003. [DOI] [PubMed] [Google Scholar]
- 62.Marklund SL. Extracellular superoxide dismutase. Methods Enzymol. 2002;349:74–80. doi: 10.1016/s0076-6879(02)49322-6. [DOI] [PubMed] [Google Scholar]
- 63.Marklund SL. Human copper-containing superoxide dismutase of high molecular weight. Proc Natl Acad Sci USA. 1982;79:7634–8. doi: 10.1073/pnas.79.24.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tamai M, Furuta H, Kawashima H, et al. Extracellular superoxide dismutase gene polymorphism is associated with insulin resistance and the susceptibility to type 2 diabetes. Diabetes Res Clin Pract. 2006;71:140–5. doi: 10.1016/j.diabres.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 65.Carlsson LM, Jonsson J, Edlund T, Marklund SL. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc Natl Acad Sci USA. 1995;92:6264–8. doi: 10.1073/pnas.92.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hansel A, Kuschel L, Hehl S, et al. Mitochondrial targeting of the human peptide methionine sulfoxide reductase (MSRA), an enzyme involved in the repair of oxidized proteins. FASEB J. 2002;16:911–3. doi: 10.1096/fj.01-0737fje. [DOI] [PubMed] [Google Scholar]
- 67.Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, Stadtman ER. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci USA. 2001;98:12920–5. doi: 10.1073/pnas.231472998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nan C, Li Y, Jean-Charles PY, et al. Deficiency of methionine sulfoxide reductase A causes cellular dysfunction and mitochondrial damage in cardiac myocytes under physical and oxidative stresses. Biochem Biophys Res Commun. 2011;402:608–13. doi: 10.1016/j.bbrc.2010.10.064. [DOI] [PubMed] [Google Scholar]
- 69.Salmon AB, Pérez VI, Bokov A, et al. Lack of methionine sulfoxide reductase A in mice increases sensitivity to oxidative stress but does not diminish life span. FASEB J. 2009b;23:3601–8. doi: 10.1096/fj.08-127415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wood ZA, Schroder E, Harris JR, Poole LB. Structure, mechanism, and regulation of peroxiredoxins. Trends Biochem Sci. 2003;28:32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- 71.Dubuisson M, Stricht DV, Clippe A, Etienne F, Nauser T, Kissner R, Koppenol WH, Rees JF, Knoops B. Human peroxiredoxin 5 is a peroxynitrite reductase. FEBS Lett. 2004;571:161–5. doi: 10.1016/j.febslet.2004.06.080. [DOI] [PubMed] [Google Scholar]
- 72.Egler RA, Fernandes E, Rothermund K, et al. Regulation of reactive oxygen species, DNA damage, and c-Myc function by peroxiredoxin 1. Oncogene. 2005;24:8038–50. doi: 10.1038/sj.onc.1208821. [DOI] [PubMed] [Google Scholar]
- 73.Neumann CA, Krause DS, Carman CV, et al. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature. 2003;424:561–5. doi: 10.1038/nature01819. [DOI] [PubMed] [Google Scholar]
- 74.Huang TT, Carlson EJ, Gillespie AM, Shi Y, Epstein CJ. Ubiquitous overexpression of Cu,Zn superoxide dismutase does not extend life span in mice. J Gerontol A Biol Sci Med Sci. 2000;55:B5–B9. doi: 10.1093/gerona/55.1.b5. [DOI] [PubMed] [Google Scholar]
- 75.Rando TA, Crowley RS, Carlson EJ, Epstein CJ, Mohapatra PK. Overexpression of copper/zinc superoxide dismutase: a novel cause of murine muscular dystrophy. Ann Neurol. 1998;44:381–6. doi: 10.1002/ana.410440315. [DOI] [PubMed] [Google Scholar]
- 76.Jaarsma D, Haasdijk ED, Grashorn JA, et al. Human Cu/Zn superoxide dismutase (SOD1) overexpression in mice causes mitochondrial vacuolization, axonal degeneration, and premature motoneuron death and accelerates motoneuron disease in mice expressing a familial amyotrophic lateral sclerosis mutant SOD1. Neurobiol Dis. 2000;7:623–43. doi: 10.1006/nbdi.2000.0299. [DOI] [PubMed] [Google Scholar]
- 77.Raineri I, Carlson EJ, Gacayan R, Carra S, Oberley TD, Huang TT, Epstein CJ. Strain-dependent high-level expression of a transgene for manganese superoxide dismutase is associated with growth retardation and decreased fertility. Free Radic Biol Med. 2001;31:1018–30. doi: 10.1016/s0891-5849(01)00686-4. [DOI] [PubMed] [Google Scholar]
- 78.Mele J, VanRemmen H, Vijg J, Richardson A. Characterization of transgenic mice that overexpress both copper zinc superoxide dismutase and catalase. Antioxid Redox Signal. 2006;8:628–38. doi: 10.1089/ars.2006.8.628. [DOI] [PubMed] [Google Scholar]
- 79.Chen X, Liang H, VanRemmen H, Vijg J, Richardson A. Catalase transgenic mice: characterization and sensitivity to oxidative stress. Arch Biochem Biophys. 2004;422:197–210. doi: 10.1016/j.abb.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 80.Schriner SE, Linford NJ, Martin GM, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–11. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 81.Pérez VI, VanRemmen H, Bokov A, Epstein CJ, Vijg J, Richardson A. The overexpression of major antioxidant enzymes does not extend lifespan of mice. Aging Cell. 2008;8:73–75. doi: 10.1111/j.1474-9726.2008.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oppermann M, Balz V, Adams V, et al. Pharmacological induction of vascular extracellular superoxide dismutase expression in vivo. J Cell Mol Med. 2009;13:1271–8. doi: 10.1111/j.1582-4934.2008.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Suvorava T, Kojda G. Reactive oxygen species as cardiovascular mediators: lessons from endothelial-specific protein overexpression mouse models. Biochem Biophys Acta. 2009;1787:802–10. doi: 10.1016/j.bbabio.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 84.Nordberg J, Arnér E. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Rad Bio Med. 2001;31:1287–312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 85.Takagi Y, Mitsui A, Nishiyama A, et al. Overexpression of thioredoxin in transgenic mice attenuates focal ischemic brain damage. Proc Natl Acad Sci USA. 1999;96:4131–6. doi: 10.1073/pnas.96.7.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mitsui A, Hamuro J, Nakamura H, et al. Overexpression of human thioredoxin in transgenic mice controls oxidative stress and life span. Antioxid Redox Signal. 2002;4:693–6. doi: 10.1089/15230860260220201. [DOI] [PubMed] [Google Scholar]
- 87.Bauman JW, Liu J, Liu YP, Klaassen CD. Increase in metallothionein produced by chemicals that induce oxidative stress. Toxicol Appl Pharmacol. 1991;110:347–54. doi: 10.1016/s0041-008x(05)80017-1. [DOI] [PubMed] [Google Scholar]
- 88.Yang X, Doser TA, Fang CX, et al. Metallothionein prolongs survival and antagonizes senescence-associated cardiomyocyte diastolic dysfunction: role of oxidative stress. FASEB J. 2006;20:1024–6. doi: 10.1096/fj.05-5288fje. [DOI] [PubMed] [Google Scholar]
- 89.Michalska AE, Choo KH. Targeting and germ-line transmission of a null mutation at the metallothionein I and II loci in mouse. Proc Natl Acad Sci USA. 1993;90:8088–92. doi: 10.1073/pnas.90.17.8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fu Z, Guo J, Jing L, Li R, Zhang T, Peng S. Enhanced toxicity and ROS generation by doxorubicin in primary cultures of cardiomyocytes from neonatal metallothionein-I/II null mice. Toxicol in Vitro. 2010;24:1584–91. doi: 10.1016/j.tiv.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 91.Yang H-Y, Wang Y-M, Peng S-Q. Metallothionein-I/II null cardiomyocytes are sensitive to Fusarium mycotoxin butenolide-induced cytotoxicity and oxidative DNA damage. Toxicon. 2010;55:1291–6. doi: 10.1016/j.toxicon.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 92.Matsushima S, Ide T, Yamato M, et al. Remodeling and failure after myocardial infarction in mice overexpression of mitochondrial peroxiredoxin-3 prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation. 2006;113:1779–1786. doi: 10.1161/CIRCULATIONAHA.105.582239. [DOI] [PubMed] [Google Scholar]
- 93.Xu JX. Radical metabolism is partner to energy metabolism in mitochondria. Ann NY Acad Sci. 2004;1011:57–60. doi: 10.1007/978-3-662-41088-2_6. [DOI] [PubMed] [Google Scholar]
- 94.Genova ML, Pich MM, Bernacchia A, Bianchi C, Biondi A, Bovina C. The mitochondrial production of reactive oxygen species in relation to aging and pathology. Ann NY Acad Sci. 2004;1011:86–100. doi: 10.1007/978-3-662-41088-2_10. [DOI] [PubMed] [Google Scholar]
- 95.Block G, Dietrich M, Norkus EP, Packer L. In: Oxidative stress in human’s populations. Culter R, Rodriguez H, editors. Singapore: World Scientific Publishing; 2003. [Google Scholar]
- 96.Tanaka M, Gong JS, Zhang J, Yaneda M, Yagi K. Mitochondrial genotype associated with longevity. Lancet. 1998;351:185–6. doi: 10.1016/S0140-6736(05)78211-8. [DOI] [PubMed] [Google Scholar]
- 97.Camougrand N, Rigoulet M. Aging and oxidative stress: studies of some genes involved both in aging and in response to oxidative stress. Respir Physiol. 2001;128:393–401. doi: 10.1016/s0034-5687(01)00314-0. [DOI] [PubMed] [Google Scholar]
- 98.Nomiyama T, Tanaka Y, Piao L, et al. The polymorphism of manganese superoxide dismutase is associated with diabetic nephropathy in Japanese type 2 diabetic patients. J Hum Genet. 2003;48:138–41. doi: 10.1007/s100380300021. [DOI] [PubMed] [Google Scholar]
- 99.Lee SJ, Choi MG, Kim DS, Kim TW. Manganese superoxide dismutase gene polymorphism (V16A) is associated with stages of albuminuria in Korean type 2 diabetic patients. Metabolism. 2006;55:1–7. doi: 10.1016/j.metabol.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 100.Mollsten A, Marklund SL, Wessman M, et al. A functional polymorphism in the manganese superoxide dismutase gene and diabetic nephropathy. Diabetes. 2007;56:265–9. doi: 10.2337/db06-0698. [DOI] [PubMed] [Google Scholar]
- 101.Crawford A, Fassett RG, Coombes JS, et al. Glutathione peroxidase, superoxide dismutase and catalase genotypes and activities and the progression of chronic kidney disease. Nephrol Dial Transplant. 2011;16 doi: 10.1093/ndt/gfq828. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 102.Cai Q, Shu X, Wen W, et al. Genetic polymorphism in the manganese superoxide dismutase gene, antioxidant intake, and breast cancer risk: results from the Shanghai Breast Cancer Study. Breast Cancer Res. 2004;6:R647–R55. doi: 10.1186/bcr929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mitrunen K, Sillanpaa P, Kataja V, et al. Association between manganese superoxide dismutase (MnSOD) gene polymorphism and breast cancer risk. Carcinogenesis. 2001;22:827–9. doi: 10.1093/carcin/22.5.827. [DOI] [PubMed] [Google Scholar]
- 104.Tamimi RM, Hankinson SE, Spiegelman D, Colditz GA, Hunter DJ. Manganese superoxide dismutase polymorphism, plasma anti-oxidants, cigarette smoking, and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:989–96. [PubMed] [Google Scholar]
- 105.Millikan RC, Player J, Cotret ARd, et al. Manganese superoxide dismutase Ala-9Val polymorphism and risk of breast cancer in a population-based case-control study of African Americans and whites. Breast Cancer Res. 2004;6:R264–R74. doi: 10.1186/bcr786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Aejemelaeus RT, Holm P, Kaukinen U, et al. Age-related changes in the peroxyl radical scavenging capacityof human plasma. Free Radic Biol Med. 1997;23:69–75. doi: 10.1016/s0891-5849(96)00591-6. [DOI] [PubMed] [Google Scholar]
- 107.Kim JW, No JK, Yu BP, Chung HY, editors. Analysis of redox status in serum during aging. New York: New York Academy of Science; 2001. [Google Scholar]
- 108.Meccoci P, Polidori CM, Troiano L, Cherubini A, Cechetti R, Pini G. Plasma antioxidants and longevity: a study on healthy centenarians. Free Radic Biol Med. 2000;28:1243–8. doi: 10.1016/s0891-5849(00)00246-x. [DOI] [PubMed] [Google Scholar]
- 109.Jones DP, Mody VC, Carlson JL, Lynn MJ, Sternberg P. Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic Biol Med. 2002;33:1290–300. doi: 10.1016/s0891-5849(02)01040-7. [DOI] [PubMed] [Google Scholar]
- 110.Erden-Inal M, Sunal E, Kanbak G. Age-related changes in the glutathione redox system. Cell Biochem Funct. 2002;20:61–6. doi: 10.1002/cbf.937. [DOI] [PubMed] [Google Scholar]
- 111.Ozbay B, Dulger H. Lipid peroxidation and anti-oxidant enzymes in Turkish population: relation to age, gender, exercise, and smoking. Tohoku J Exp Med. 2002;197:119–24. doi: 10.1620/tjem.197.119. [DOI] [PubMed] [Google Scholar]
- 112.Junqueira VB, Barros SB, Chan SS, Rodriguez L, Giavarotti L, Abud RL. Aging and oxidative stress. Mol Aspects Med. 2004;25:5–16. doi: 10.1016/j.mam.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 113.Gil L, Siems W, Mazurek B, Gross J, Schroeder P, Voss P. Age-associated analysis of oxidative stress parameters in human plasma and erythrocytes. Free Radic Res. 2006;40:495–505. doi: 10.1080/10715760600592962. [DOI] [PubMed] [Google Scholar]
- 114.Frisard MI, Broussand A, Davies SS, Roberts LJ, Rood J, Jonge LE. Aging, resting metabolic rate,and oxidative stress damage: results from Louisiana healthy aging study. J Ger Med Sc. 2007;62:752–9. doi: 10.1093/gerona/62.7.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mendoza VM, Ruiz M, Sanchez MA, Retana R, Muñoz JL. Aging-related oxidative stress in healthy humans. Tohoku J Exp Med. 2007;213:261–268. doi: 10.1620/tjem.213.261. [DOI] [PubMed] [Google Scholar]
- 116.Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–44. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 117.Candore G, Bulati M, Caruso C, et al. Inflammation, cytokines, immune response, apolipoprotein E, cholesterol, and oxidative stress in Alzheimer disease: therapeutic implications. Rejuvenation Res. 2010;13:301–13. doi: 10.1089/rej.2009.0993. [DOI] [PubMed] [Google Scholar]
- 118.Calon F, Cole G. Neuroprotective action of omega-3 polyunsaturated fatty acids against neurodegenerative diseases: evidence from animal studies. Prostaglandins Leukot Essent Fatty Acids. 2007;77:287–93. doi: 10.1016/j.plefa.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 119.Faux NG, Ritchie CW, Gunn A, et al. PBT2 rapidly improves cognition in Alzheimer’s Disease: additional phase II analyses. J Alzheimers Dis. 2010;20:509–16. doi: 10.3233/JAD-2010-1390. [DOI] [PubMed] [Google Scholar]
- 120.Abele D, Strahl J, Brey T, Philipp EE. Imperceptible senescence: ageing in the ocean quahog Arctica islandica. Free Radic Biol Med. 2008;42:474–80. doi: 10.1080/10715760802108849. [DOI] [PubMed] [Google Scholar]
- 121.Abele D, Brey T, Philipp E. Bivalve models of aging and the determination of molluscan lifespans. Exp Gerontol. 2009;44:307–15. doi: 10.1016/j.exger.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 122.Philipp E, Brey T, Pörtner HO, Abele D. Chronological and physiological ageing in a polar and a temperate mud clam. Mech Ageing Dev. 2005;126:598–609. doi: 10.1016/j.mad.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 123.Miwa S, Riyahi K, Partridge L, Brand MD. Lack of correlation between mitochondrial reactive oxygen species production and life span in Drosophila, strategies for engineered negligible senescence: why genuine control of aging may be foreseeable. Ann NY Acad Sci. 2004;1019:388–91. doi: 10.1196/annals.1297.069. [DOI] [PubMed] [Google Scholar]
- 124.Ross RE. Age-specific decrease in aerobic efficiency associated with increase in oxygen free radical production in Drosophila melanogaster. J Insect Physiol. 2000;46:1477–80. doi: 10.1016/s0022-1910(00)00072-x. [DOI] [PubMed] [Google Scholar]
- 125.Buttemer WA, Battam H, Hulbert AJ. Fowl play and the price of petrel: long-living Procellariiformes have peroxidation resistant membrane composition compared with short-living Galliformes. Biol Lett. 2008;4:351–4. doi: 10.1098/rsbl.2008.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schmidt-Nielsen K. Scaling in biology: the consequences of size. J Exp Zool. 1975;194:287–307. doi: 10.1002/jez.1401940120. [DOI] [PubMed] [Google Scholar]
- 127.Benedict FG. Vital Energetics: a study in comparative basal metabolism. Washington D.C: Carnegie Institution of Washington; 1938. [Google Scholar]
- 128.McKechnie AE, Wolf BO. The allometry of avian basal metabolic rate: good predictions need good data. Physiol Biochem Zool. 2004;77:502–21. doi: 10.1086/383511. [DOI] [PubMed] [Google Scholar]
- 129.Speakman JR. Body size, energy metabolism and lifespan. J Exp Biol. 2005;208:1717–30. doi: 10.1242/jeb.01556. [DOI] [PubMed] [Google Scholar]
- 130.Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA. Life and death: Metabolic rate, membrane composition, and life span of animals. Physiol Rev. 2007;87:175–213. doi: 10.1152/physrev.00047.2006. [DOI] [PubMed] [Google Scholar]
- 131.Lindstedt SL, Calder WA. Body size and longevity in birds. Condor. 1976;78:91–4. [Google Scholar]
- 132.deMagalhães JP, Costa J, Church GM. An analysis of the relationship between metabolism, developmental schedules, and longevity using phylogenetic independent contrasts. J Gerontol A Biol Sci Med Sci. 2007;62:149–60. doi: 10.1093/gerona/62.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Holloszy JO, Smith EK, Vining M, Adams S. Effect of voluntary exercise on longevity of rats. J Appl Physiol. 1985;59:826–31. doi: 10.1152/jappl.1985.59.3.826. [DOI] [PubMed] [Google Scholar]
- 134.Lee IM, Hsieh CC, RSP Exercise intensity and longevity in men. The Harvard Alumni Health Study. J Am Med Assoc. 1995;279:1179–84. [PubMed] [Google Scholar]
- 135.Masoro EJ. Caloric restriction and aging: controversial issues. J Gerontol. 2006;61:14–9. doi: 10.1093/gerona/61.1.14. [DOI] [PubMed] [Google Scholar]
- 136.McCarter RJ, Palmer J. Energy metabolism and aging: a lifelong study of Fischer 344 rats. Am J Physiol. 1992;263:E448–E52. doi: 10.1152/ajpendo.1992.263.3.E448. [DOI] [PubMed] [Google Scholar]
- 137.Hulbert AJ, Usher MJ, Wallman JF. Food consumption and individual lifespan of adults of the blowfly, Calliphora stygia: a test of the “rate of living” theory of aging. Exp Gerontol. 2004;39:1485–90. doi: 10.1016/j.exger.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 138.Speakman JR, vanAcker A, Harper EJ. Age-related changes in metabolism and body composition of three dog breeds and their relationship to life expectancy. Aging Cell. 2003;2:265–75. doi: 10.1046/j.1474-9728.2003.00061.x. [DOI] [PubMed] [Google Scholar]
- 139.Speakman JR, Talbot DA, Selman C, et al. Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell. 2004;3:87–95. doi: 10.1111/j.1474-9728.2004.00097.x. [DOI] [PubMed] [Google Scholar]
- 140.Lovegrove BG. The metabolism of social subterranean rodents -- adaptation to aridity oecologia. 1986;69:551–5. doi: 10.1007/BF00410361. [DOI] [PubMed] [Google Scholar]
- 141.Lovegrove BG. Thermoregulation in the subterranean rodent Georychus capensis (Rodentia, Bathyergidae) Physiol Zool. 1987;60:174–80. [Google Scholar]
- 142.Lovegrove BG. The zoogeography of mammalian basal metabolic rate. American Naturalist. 2000;156:201–19. doi: 10.1086/303383. [DOI] [PubMed] [Google Scholar]
- 143.Li QF, Sun RY, Huang CX, et al. Cold adaptive thermogenesis in small mammals from different geographical zones of China. Comp Biochem Physiol A Mol Int Physiol. 2001;129:949–61. doi: 10.1016/s1095-6433(01)00357-9. [DOI] [PubMed] [Google Scholar]
- 144.McNab BK. Climatic adaptation in the energetics of heteromyid rodents. Comp Bioche Physiol A Physiol. 1979;62:813–20. [Google Scholar]
- 145.Bennett NC, Aguilar GH, Jarvis JUM, Faulkes CG. Thermoregulation in 3 species of Afrotropical subterranean mole-rats (Rodentia, Batherygidae) from Zambia and Angola and scaling with the genus Crytpomys. Oecologia. 1994;97:222–7. doi: 10.1007/BF00323153. [DOI] [PubMed] [Google Scholar]
- 146.Buffenstein R. Ecophysiological responses to a subterranean habitat; A Bathyergid perspective. Mammalia. 1996;60:591–605. [Google Scholar]
- 147.deMagalhães JP, Costa J. A database of vertebrate longevity records and their relation to other life-history traits. J Evol Bio. 2009;22:1770–4. doi: 10.1111/j.1420-9101.2009.01783.x. [DOI] [PubMed] [Google Scholar]
- 148.O’Connor TP, Lee A, Jarvis JU, Buffenstein R. Prolonged longevity in naked mole-rats: age-related changes in metabolism, body composition and gastrointestinal function. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:835–42. doi: 10.1016/s1095-6433(02)00198-8. [DOI] [PubMed] [Google Scholar]
- 149.Pamplona R, Portero-Otin M, Riba D, et al. Low fatty acid unsaturation: a mechanism for lowered lipoperoxidative modification of tissue proteins in mammalian species with long life spans. J Gerontol. 2000;55:B286–B91. doi: 10.1093/gerona/55.6.b286. [DOI] [PubMed] [Google Scholar]
- 150.Pamplona R, Portero-Otin M, Riba D, Ruiz C, Prat J, Bellmunt MJ, Barja G. Mitochondrial membrane peroxidizability index is inversely related to maximum lifespan in mammals. J Lipid Res. 1998;39:1989–94. [PubMed] [Google Scholar]
- 151.Hulbert AJ, Faulks SC, Buffenstein R. Peroxidation-resisant membranes can explain longevity of longest-living rodent and similarly-sized mice. J Gerontol A Biol Sci Med Sci. 2006;61:1009–18. doi: 10.1093/gerona/61.10.1009. [DOI] [PubMed] [Google Scholar]
- 152.Hulbert AJ, Beard LA, Grigg GC. The exceptional longevity of an egg-laying mammal, the short-beaked echidna (Tachyglossus aculeatus) is associated with peroxidation-resistant membrane composition. Exp Gerontol. 2008;43:729–33. doi: 10.1016/j.exger.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 153.Rabini RA, Moretti N, Staffolani R, et al. Reduced susceptibility to peroxidation of erythrocyte plasma membranes from centenarians. Exp Gerontol. 2002;37:657–63. doi: 10.1016/s0531-5565(02)00006-2. [DOI] [PubMed] [Google Scholar]
- 154.Faulks SC, Turner N, Else PL, Hulbert AJ. Calorie restriction in mice: effects on body composition, daily activity, metabolic rate, mitochondrial ROS production and membrane fatty acid composition. J Gerontol. 2006;61:781–94. doi: 10.1093/gerona/61.8.781. [DOI] [PubMed] [Google Scholar]
- 155.Porter RK, Hulbert AJ, Brand MD. Allometry of mitochondrial proton leak: influence of membrane surface area and fatty acid composition. Am J Physiol Regul Integr Comp Physiol. 1996;271:R1550–R60. doi: 10.1152/ajpregu.1996.271.6.R1550. [DOI] [PubMed] [Google Scholar]
- 156.Brand MD, Turner N, Ocloo A, Else PL, Hulbert AJ. Proton conductance and fatty acyl composition of liver mitochondria correlates with body mass in birds. Biochem J. 2003;376:741–8. doi: 10.1042/BJ20030984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Brookes PS, Hulbert AJ, Brand MD. The proton permeability of liposomes made from mitochondrial inner membrane phospholipids: no effect of fatty acid composition. Biochem Biophys Acta. 1997;1330:157–64. doi: 10.1016/s0005-2736(97)00160-0. [DOI] [PubMed] [Google Scholar]
- 158.Sohal RS, Svensson I, Brunk UT. Hydrogen peroxide production by liver mitochondria in different species. Mech Ageing Dev. 1990a;53:209–15. doi: 10.1016/0047-6374(90)90039-i. [DOI] [PubMed] [Google Scholar]
- 159.Sohal RS, Svensson I, Sohal BH, Brunk UT. Superoxide anion radical production in different animal species. Mech Ageing Dev. 1989;49:139–45. doi: 10.1016/0047-6374(89)90096-1. [DOI] [PubMed] [Google Scholar]
- 160.Barja G, Cadenas S, Rojas C, Lopez-Torres M, Perez-Campo R. A decrease of free-radical production near-critical targets as a cause of maximum longevity in animals. Comp Biochem Physiol Biochem Mol Biol. 1994;108:501–12. doi: 10.1016/0305-0491(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 161.Shi Y, Buffenstein R, Pulliam DA, VanRemmen H. Comparative studies of oxidative stress and mitochondrial function in aging. Integ Comp Biol. 2010;50:869–79. doi: 10.1093/icb/icq079. [DOI] [PubMed] [Google Scholar]
- 162.Lambert AJ, Boysen HM, Buckingham JA, et al. Low rates of hydrogen peroxide production by isolated heart mitochondria associate with long maximum lifespan in vertebrate homeotherms. Aging Cell. 2007;6:607–18. doi: 10.1111/j.1474-9726.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- 163.Robert KA, Brunet-Rossinni A, Bronikowski AM. Testing the ‘free radical theory of aging’ hypothesis: physiological differences in long-lived and short-lived colubrid snakes. Aging Cell. 2007;6:395–404. doi: 10.1111/j.1474-9726.2007.00287.x. [DOI] [PubMed] [Google Scholar]
- 164.Hansford RG, Hogue BA, Mildaziene V. Dependence of H2O2 formation by rat heart mitochondria on substrate availability and donor age. J Bioenerg Biomembr. 1997;29:89–95. doi: 10.1023/a:1022420007908. [DOI] [PubMed] [Google Scholar]
- 165.Schönfeld P, Wojtczak L. Fatty acids as modulators of the cellular production of reactive oxygen species. Free Radic Biol Med. 2008;45:231–41. doi: 10.1016/j.freeradbiomed.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 166.Miwa S, Brand MD. Mitochondrial matrix reactive oxygen species production is very sensitive to mild uncoupling. Biochem Soc Trans. 2003;31:1300–1. doi: 10.1042/bst0311300. [DOI] [PubMed] [Google Scholar]
- 167.Lopez-Torres M, Barja G. Lowered methionine ingestion as responsible for the decrease in rodent mitochondrial oxidative stress in protein and dietary restriction Possible implications for humans. Biochem Biophys Acta. 2008;1780:1337–47. doi: 10.1016/j.bbagen.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 168.Pamplona R, Portero-Otin M, Sanz A, Ayala V, Vasileva E, Barja G. Protein and lipid oxidative damage and complex I content are lower in the brain of budgerigar and canaries than in mice. Relation to aging rate. AGE. 2005;27:267–80. doi: 10.1007/s11357-005-4562-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lambert AJ, Buckingham JA, Boysen HM, Brand MD. Low complex I content explains the low hydrogen peroxide production rate of heart mitochondria from the long-lived pigeon, Columba livia. Aging Cell. 2010;9:78–91. doi: 10.1111/j.1474-9726.2009.00538.x. [DOI] [PubMed] [Google Scholar]
- 170.Forman HJ, Kennedy J. Dihydroorotate-dependent superoxide production in rat brain and liver. A function of the primary dehydrogenase. Arch Biochem Biophys. 1976;173:214–24. doi: 10.1016/0003-9861(76)90252-6. [DOI] [PubMed] [Google Scholar]
- 171.Panov A, Dikalov S, Hemendinger NSR, Rosenfeld JTGJ. Species- and tissue-specific relationships between mitochondrial permeability transition and generation of ROS in brain and liver mitochondria of rats and mice. Am J Physiol Cell Physiol. 2007;292:C708–C18. doi: 10.1152/ajpcell.00202.2006. [DOI] [PubMed] [Google Scholar]
- 172.Dell’Agnello C, Leo S, Agostino A, et al. Increased longevity and refractoriness to Ca21-dependent neurodegeneration in Surf1 knockout mice. Hum Mol Genet. 2007;16:431–44. doi: 10.1093/hmg/ddl477. [DOI] [PubMed] [Google Scholar]
- 173.Barja G. Rate of generation of oxidative stress-related damage and animal longevity. Free Radic Biol Med. 2002;33:1167–1172. doi: 10.1016/s0891-5849(02)00910-3. [DOI] [PubMed] [Google Scholar]
- 174.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Andziak B, O’Connor TP, Buffenstein R. Antioxidants do not explain the disparate longevity between mice and the longest-living rodent, the naked mole-rat. Mech Ageing Dev. 2005;126:1206–12. doi: 10.1016/j.mad.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 176.Perez-Campo R, Lopez-Torres M, Cadenas S, Rojas C, Barja G. The rate of free radical production as a determinant of the rate of aging: evidence from the comparative approach. J Comp Physiol B. 1998;168:149–58. doi: 10.1007/s003600050131. [DOI] [PubMed] [Google Scholar]
- 177.Sohal RS, Agarwal S, Dubey A, Orr WC. Protein oxidative damage is associated with life expectancy of houseflies. Proc Natl Acad Sci USA. 1993;90:7255–9. doi: 10.1073/pnas.90.15.7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wilhelm-Filho D, Althoff SL, Dafre AL, Boveris A. Antioxidant defenses, longevity and ecophysiology of South American bats. Comp Biochem Physiol C Toxicol Pharmacol. 2007;146:214–20. doi: 10.1016/j.cbpc.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 179.Cutler RG. Peroxide-producing potential of tissues: inverse correlation with longevity of mammalian species. Proc Natl Acad Sci USA. 1985;82:4798–802. doi: 10.1073/pnas.82.14.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lopez-Torres M, Perez-Campo R, Rojas C, Cadenas S, Barja G. Maximum life span in vertebrates: relationship with liver antioxidant enzymes, glutathione system, ascorbate, urate, sensitivity to peroxidation, true malondialdehyde, in vivo H2O2, basal and maximum aerobic capacity. Mech Ageing Dev. 1993;70:177–99. doi: 10.1016/0047-6374(93)90047-u. [DOI] [PubMed] [Google Scholar]
- 181.Perez-Campo R, Lopez-Torres M, Rojas C, Cadenas S, Barja G. Longevity and antioxidant enzymes, non-enzymatic antioxidants and oxidative stress in the vertebrate lung: a comparative study. J Comp Physiol B Biochem Syst Environ Physiol. 1994;163:682–9. doi: 10.1007/BF00369520. [DOI] [PubMed] [Google Scholar]
- 182.Ferreira-Cravo M, Welker AF, Andrade RG, Drew K, Hermes-Lima M. Physiological oxidative stress in the animal world. Comp Biochem Physiol A Mol Integr Physiol. 2007;148:S63–S4. [Google Scholar]
- 183.Sanz A, Pamplona R, Barja G. Is the mitochondrial free radical theory of aging intact? Antioxid Redox Signal. 2006;8:582–99. doi: 10.1089/ars.2006.8.582. [DOI] [PubMed] [Google Scholar]
- 184.Pamplona R, Barja G. Highly resistant macromolecular components and low rate of generation of endogenous damage: two key traits of longevity. Ageing Res Rev. 2007;6:189–210. doi: 10.1016/j.arr.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 185.Brunet-Rossinni AK. Reduced free-radical production and extreme longevity in the little brown bat (Myotis lucifugus) versus two non-flying mammals. Mech Ageing Dev. 2004;125:11–20. doi: 10.1016/j.mad.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 186.Andziak B, O’Connor TP, Qi WB, et al. High oxidative damage levels in the longest-living rodent, the naked mole-rat. Aging Cell. 2006;5:463–71. doi: 10.1111/j.1474-9726.2006.00237.x. [DOI] [PubMed] [Google Scholar]
- 187.Voituron Y, deFraipont M, Issartel J, Guillaume O, Clobert J. Extreme lifespan of the human fish (Proteus anguinus): a challenge for ageing mechanisms. Biol Lett. 2011;7:105–7. doi: 10.1098/rsbl.2010.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Meydani M, Lipman RD, Han SN, et al. The effect of long-term dietary supplementation with antioxidants. Ann NY Acad Sci. 1998;854:352–60. doi: 10.1111/j.1749-6632.1998.tb09915.x. [DOI] [PubMed] [Google Scholar]
- 189.Melov S, Ravenscroft J, Malik S, et al. Extension of life-span with superoxide dismutase/catalase mimetics. Science. 2000;289:1567–9. doi: 10.1126/science.289.5484.1567. [DOI] [PubMed] [Google Scholar]
- 190.Pamplona R, Portero-Otin M, Riba D, Ledo F, Gredilla R, Herrero A, Barja G. Heart fatty acid unsaturation and lipid peroxidation, and aging rate, are lower in the canary and the parakeet than in the mouse. Aging Clin Exp Res. 1999;11:44–9. [PubMed] [Google Scholar]
- 191.Pamplona R. Mitochondrial DNA damage and animal longevity: insights from comparative studies. J Aging Res. 2011;2011:807108. doi: 10.4061/2011/807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sohal RS, Sohal BH, Orr WC. Mitochondrial superoxide and hydrogen-peroxide generation, protein oxidative damage, and longevity in different species of flies. Free Radic Biol Med. 1995;19:499–504. doi: 10.1016/0891-5849(95)00037-x. [DOI] [PubMed] [Google Scholar]
- 193.Hamilton ML, VanRemmen H, Drake J, et al. Does oxidative damage to DNA increase with age? Proc Natl Acad Sci USA. 2001;98:10469–74. doi: 10.1073/pnas.171202698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hermes-Lima M, Welker AF, Ferreira-Cravo M, Campos EG. Physiological oxidative stress and low oxygen. Comp Bioche Physiol B Mol Integr Physiol. 2007;148:S50–S60. [Google Scholar]
- 195.Buffenstein R. The naked mole-rat; a new long-living model for human aging research. J Gerontol Biol Sci. 2005;60:1369–77. doi: 10.1093/gerona/60.11.1369. [DOI] [PubMed] [Google Scholar]
- 196.Portero-Otin M, Requena JR, Bellmunt MJ, Ayala V, Pamplona R. Protein nonenzymatic modifications and proteasome activity in skeletal muscle from the short-lived rat and the long-lived pigeon. Exp Gerontol. 2004;39:1527–35. doi: 10.1016/j.exger.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 197.Agarwal S, Sohal RS. Relationship between suceptibility to protein oxidation, aging, and maximum lifespan potential of different species. Exp Gerontol. 1996;31:365–72. doi: 10.1016/0531-5565(95)02039-x. [DOI] [PubMed] [Google Scholar]
- 198.VanRemmen H, Richardson A. Oxidative damage to mitochondria and aging. Exp Gerontol. 2001;36:957–68. doi: 10.1016/s0531-5565(01)00093-6. [DOI] [PubMed] [Google Scholar]
- 199.Kaneko T, Tahara S, Matsuo M. Non-linear accumulation of 8-hydroxy-2′-deoxyguanosine, a marker of oxidized DNA damage, during aging. Mut Res DNA Aging Gene Instab Aging. 1996;316(5–6):277–85. doi: 10.1016/s0921-8734(96)90010-7. [DOI] [PubMed] [Google Scholar]
- 200.Levine RL. Carbonyl modified proteins in cellular regulation, aging, and disease. Free Radic Biol Med. 2002;32:790–6. doi: 10.1016/s0891-5849(02)00765-7. [DOI] [PubMed] [Google Scholar]
- 201.Gil P, Farinas F, Casado A, Lopez-Fernandez E. Malondialdehyde: A possible marker of ageing. Gerontology. 2002;48:209–14. doi: 10.1159/000058352. [DOI] [PubMed] [Google Scholar]
- 202.Andziak B, Buffenstein R. Disparate patterns of agerelated changes in lipid peroxidation in long-lived naked mole-rats and shorter-lived mice. Aging Cell. 2006;5:525–32. doi: 10.1111/j.1474-9726.2006.00246.x. [DOI] [PubMed] [Google Scholar]
- 203.Pérez VI, Lewa CM, Cortez LA, et al. Thioredoxin 2 haploinsufficiency in mice results in impaired mitochondrial function and increased oxidative stress. Free Radic Biol Med. 2008;44:882–92. doi: 10.1016/j.freeradbiomed.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 204.Sasaki M, Ikeda H, Sato Y, Nakanuma Y. Proinflammatory cytokine-induced cellular senescence of biliary epithelial cells is mediated via oxidative stress and activation of ATM pathway: A culture study. Free Radic Res. 2008;42:625–32. doi: 10.1080/10715760802244768. [DOI] [PubMed] [Google Scholar]
- 205.Pérez VI, Buffenstein R, Masamsetti V, et al. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proc Natl Acad Sci USA. 2009;106:3059–64. doi: 10.1073/pnas.0809620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kapahi P, Boulton ME, Kirkwood TBL. Positive correlation between mammalian life span and cellular resistance to stress. Free Radical Bio Med. 1999;26:495–500. doi: 10.1016/s0891-5849(98)00323-2. [DOI] [PubMed] [Google Scholar]
- 207.Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K, Miller RA. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am J Physiol-Endoc M. 2005;289:E23–E9. doi: 10.1152/ajpendo.00575.2004. [DOI] [PubMed] [Google Scholar]
- 208.Harper JM, Salmon AB, Leiser SF, Galecki AT, Miller RA. Skin-derived fibroblasts from long-lived species are resistant to some, but not all, lethal stresses and to the mitochondrial inhibitor rote-none. Aging Cell. 2007;6:1–13. doi: 10.1111/j.1474-9726.2006.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Salmon AB, Akha AAS, Buffenstein R, Miller RA. Fibroblasts from naked mole-rats are resistant to multiple forms of cell injury, but sensitive to peroxide, ultraviolet light, and endoplasmic reticulum stress. J Ger Biol Sci Med Sci. 2008;63:232–41. doi: 10.1093/gerona/63.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Jasper H. SKNy worms and long life. Cell. 2008;132:915–6. doi: 10.1016/j.cell.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 211.McElwee JJ, Schuster E, Blanc E, Thomas JH, Gems D. Shared transcriptional signature in Caenorhabditis elegans dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. J Biol Chem. 2004;279:44533–43. doi: 10.1074/jbc.M406207200. [DOI] [PubMed] [Google Scholar]
- 212.Sykiotis GP, Bohmann D. Keapl/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell. 2008;14:76–85. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sun LY, Bokov AF, Richardson A, Miller RA. Hepatic response to oxidative injury in long-lived Ames dwarf mice. FASEB J. 2011;25:398–408. doi: 10.1096/fj.10-164376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bus JS, Cagen SZ, Olgaard M, Gibson JE. Mechanism of paraquat toxicity in mice and rats. Toxicology and Applied Pharmacology. 1976;35:501–13. doi: 10.1016/0041-008x(76)90073-9. [DOI] [PubMed] [Google Scholar]
- 215.Puppo A, Halliwell B. Formation of hydroxyl radicals in biological-systems - does myoglobin stimulate hydroxyl radical formation from hydrogen-peroxide. Free Radical Research Communications. 1988;4:415–22. doi: 10.3109/10715768809066910. [DOI] [PubMed] [Google Scholar]
- 216.Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nature Cell Biology. 2003;5:741–7. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Busuttil RA, Rubio M, Dolle MET, Campisi J, Vijg J. Oxygen accelerates the accumulation of mutations during the senescence and immortalization of murine cells in culture. Aging Cell. 2003;2:287–94. doi: 10.1046/j.1474-9728.2003.00066.x. [DOI] [PubMed] [Google Scholar]
- 218.Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–57. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 219.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 220.Nioi P, Hayes JD. Contribution of NAD(P)H: quinone oxidoreductase 1 to protection against carcinogenesis, and regulation of its gene by the Nrf2 basic-region leucine zipper and the arylhydrocarbon receptor basic helix-loop-helix transcription factors. Mutat Res-Fund Mol M. 2004;555:149–71. doi: 10.1016/j.mrfmmm.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 221.Kwak MK, Wakabayashi N, Greenlaw JL, Yamamoto M, Kensler TW. Antioxidants enhance mammalian proteasome expression through the Keap1-Nrf2 signaling pathway. Mol Cell Biol. 2003;23:8786–94. doi: 10.1128/MCB.23.23.8786-8794.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tong KI, Kobayashi A, Katsuoka F, Yamamoto M. Two-site substrate recognition model for the Keap1-Nrf2 system: a hinge and latch mechanism. Biol Chem. 2006;387:1311–20. doi: 10.1515/BC.2006.164. [DOI] [PubMed] [Google Scholar]
- 223.McMahon M, Itoh K, Yamamoto M, et al. The cap ‘n’ collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 2001;61:3299–307. [PubMed] [Google Scholar]
- 224.Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamato M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–203. [PubMed] [Google Scholar]
- 225.Kwak MK, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, Kensler TW. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway - Identification of novel gene clusters for cell survival. J Biol Chem. 2003;278:8135–45. doi: 10.1074/jbc.M211898200. [DOI] [PubMed] [Google Scholar]
- 226.Lewis KN, Mele J, Hayes JD, Buffenstein R. Nrf2, a guardian of healthspan and gatekeeper of species longevity. Integr Comp Biol. 2010;50:829–43. doi: 10.1093/icb/icq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Brown-Borg HM, Rakoczy SG. Calorie restriction affects brain glutathione metabolism in normal and long-living mice. Exp Gerontol. 2007;42:140. [Google Scholar]
- 228.Brown-Borg HM, Rakoczy SG, Uthus EO. Growth hormone alters methionine and glutathione metabolism in Ames dwarf mice. Mech Ageing Dev. 2005;126:389–98. doi: 10.1016/j.mad.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 229.Amador-Noguez D, Dean A, Huang WD, Setchell K, Moore D, Darlington G. Alterations in xenobiotic metabolism in the long-lived Little mice. Aging Cell. 2007;6:453–70. doi: 10.1111/j.1474-9726.2007.00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Morimoto RI, Cuervo AM. Protein homeostasis and aging: taking care of proteins from the cradle to the grave. J Ger Biol Sci Med Sci. 2009;64:167–70. doi: 10.1093/gerona/gln071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Fink AL. Chaperone-mediated protein folding. Physiol Rev. 1999;79:425–49. doi: 10.1152/physrev.1999.79.2.425. [DOI] [PubMed] [Google Scholar]
- 232.Swanlund JM, Kregel KC, Oberly TD. Autophagy following heat stress - The role of aging and protein nitration. Autophagy. 2008;4:936–9. doi: 10.4161/auto.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Swindell WR, Masternak MM, Kopchick JJ, Conover CA, Bartke A, Miller RA. Endocrine regulation of heat shock protein mRNA levels in long-lived dwarf mice. Mech Ageing Dev. 2009;130:393–400. doi: 10.1016/j.mad.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Salmon AB, Leonard S, Masamsetti V. The long lifespan of two bat species is correlated with resistance to protein oxidation and enhanced protein homeostasis. FASEB J. 2009a;23:2317–26. doi: 10.1096/fj.08-122523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ward WF. The relentless effects of the aging process on protein turnover. Biogerontology. 2000;1:195–9. doi: 10.1023/a:1010076818119. [DOI] [PubMed] [Google Scholar]
- 236.Martinez-Vicente M, Sovak G, Cuervo AM. Protein degradation and aging. Exp Gerontol. 2005;40:622–33. doi: 10.1016/j.exger.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 237.Zwickl P, Seemuller E, Kapelari B, Baumeister W. The proteasome: a supramolecular assembly designed for controlled proteolysis. Adv Protein Chem. 2001;59:187–222. doi: 10.1016/s0065-3233(01)59006-3. [DOI] [PubMed] [Google Scholar]
- 238.Bochtler M, Ditzel L, Groll M, Hartmann C, Huber R. The proteasome. Annu Rev Biophys Biomol Struct. 1999;28:295–317. doi: 10.1146/annurev.biophys.28.1.295. [DOI] [PubMed] [Google Scholar]
- 239.Friguet B, Stadtman ER, Szweda LI. Modification of glucose-6-phosphate dehydrogenase by 4-hydroxy-2-noneal. Formation of cross-linked protein that inhibits the multicatalytic protease. J Biol Chem. 1994;269:21639–43. [PubMed] [Google Scholar]
- 240.Bulteau AL, Petropoulos I, Friguet B. Age-related alterations of proteasome structure and function in aging epidermis. Exp Gerontol. 2000;35:767–77. doi: 10.1016/s0531-5565(00)00136-4. [DOI] [PubMed] [Google Scholar]
- 241.Reinheckel T, Ulrich O, Sitte N, Grune T. Differential impairment of 20S and 26S proteasome activities in human hematopoietic K562 cells during oxidative stress. Arch Biochem Biophys. 2000;377:65–8. doi: 10.1006/abbi.2000.1717. [DOI] [PubMed] [Google Scholar]
- 242.Altun M, Besche HC, Overkleeft HS, et al. Muscle wasting in aged, sarcopenic rats, is associated with enhanced activity in the ubiquitin proteasome pathway. J Biol Chem. 2010;285:39597–608. doi: 10.1074/jbc.M110.129718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zainal TA, Oberley TD, Allison DB, Sweda LI, Weindruch R. Caloric restriction of rhesus monkey lowers oxidative damage in skeletal muscle. FASEB J. 2000;14:1825–36. doi: 10.1096/fj.99-0881com. [DOI] [PubMed] [Google Scholar]
- 244.Takeda K, Yoshida T, Kikuchi S, et al. Synergistic roles of the proteasome and autophagy for mitochondrial maintenance and chronological lifespan in fission yeast. Proc Natl Acad Sci USA. 2010;107:3540–5. doi: 10.1073/pnas.0911055107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Carney-Almroth B, Johansson AA, Förlin L, Sturve J. Early-age changes in oxidative stress in brown trout, Salmo trutta. Comp Biochem Phys Part B. 2010;155:442–8. doi: 10.1016/j.cbpb.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 246.Ferrington DA, Hussong SA, Roehrich H, et al. Immunoproteasome responds to injury in the retina and brain. J Neurochem. 2008;106:158–69. doi: 10.1111/j.1471-4159.2008.05345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hussong SA, Kapphahn RJ, Phillips SL, Maldonado M, Ferrington DA. Immunoproteasome deficiency alters retinal proteasome’s response to stress. J Neurochem. 2010;113:1481–90. doi: 10.1111/j.1471-4159.2010.06688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Dasuri K, Nguyen A, Zhang L, et al. Comparison of liver and brain proteasomes for oxidative stress induced inactivation: influence of aging and dietary restriction. Free Rad Res. 2009;43:28–36. doi: 10.1080/10715760802534812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Dasuri K, Ebenezer PJ, Zhang Z, et al. Selective vulnerability of neurons to acute toxicity after proteasome inhibitor treatment: Implications for oxidative stress and insolubility of newly synthesized proteins. Free Rad Bio Med. 2010;49:1290–7. doi: 10.1016/j.freeradbiomed.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Cheroni C, Marino M, Tortarolo M, et al. Functional alterations of the ubiquitin-proteasome system in motor neurons of a mouse model of familial amyotrophic lateral sclerosis. Hum Mol Genet. 2009;18:82–96. doi: 10.1093/hmg/ddn319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ventruti A, Cuervo AM. Autophagy and neurodegeneration. Current Neurology Neurosci. 2008;7:443–51. doi: 10.1007/s11910-007-0068-5. [DOI] [PubMed] [Google Scholar]
- 252.Massey AC, Kiffin R, Cuervo AM. Autophagic defects in aging - Looking for an “emergency exit”? Cell Cycle. 2006;5:1292–6. doi: 10.4161/cc.5.12.2865. [DOI] [PubMed] [Google Scholar]
- 253.Strecker V, Mai S, Muster B, Beneke S, Burkle A, Bereiter-Hahn J, Jendrach M. Aging of different avian cultured cells: Lack of ROS-induced damage and quality control mechanisms. Mech Ageing Dev. 2010;131:48–59. doi: 10.1016/j.mad.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 254.Buffenstein R, Edrey YH, Hanes M, Pinto M, Mele J. Successful aging and sustained good health in the naked mole rat: a long-lived Mammalian model for biogerontology and biomedical research. ILAR J. 2011;52:41–53. doi: 10.1093/ilar.52.1.41. [DOI] [PubMed] [Google Scholar]
- 255.Ho YS, Magnenat JL, Bronson RT, Cao J, Gargano M, Sugawara M, Funk CD. Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia. J Bio Chem. 1997;272:16644–51. doi: 10.1074/jbc.272.26.16644. [DOI] [PubMed] [Google Scholar]
- 256.Loh K, Deng H, Fukushima A, et al. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009;10:260–72. doi: 10.1016/j.cmet.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Thiruchelvam M, Prokopenko O, Cory-Slechta DA, Richfield EK, Buckley B, Mirochnitchenko O. Overexpression of superoxide dismutase or glutathione peroxidase protects against the paraquat+maneb-induced Parkinson disease phenotype. J Biol Chem. 2005;280:22530–9. doi: 10.1074/jbc.M500417200. [DOI] [PubMed] [Google Scholar]
- 258.Hoehn KL, Salmon AB, Hohnen-Behrens C, et al. Insulin resistance is a cellular antioxidant defense mechanism. Proc Natl Acad Sci USA. 2009;106:17787–92. doi: 10.1073/pnas.0902380106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Bowler RP, Nicks M, Tran K, et al. Extracellular superoxide dismutase attenuates lipopolysaccharide-induced neutrophilic inflammation. Am J Respir Cell Mol Biol. 2004;31:431–9. doi: 10.1165/rcmb.2004-0057OC. [DOI] [PubMed] [Google Scholar]
- 260.Ho YS, Xiong Y, Ma W, Spector A, Ho DS. Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury. J Biol Chem. 2004;279:32804–12. doi: 10.1074/jbc.M404800200. [DOI] [PubMed] [Google Scholar]
- 261.Anderson EJ, Lustig ME, Boyle KE, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119:573–81. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Matsui M, Oshima M, Oshima H, Takaku K, Maruyama T, Yodoi J, Taketo MM. Early embryonic lethality caused by targeted disruption of the mouse thioredoxin gene. Dev Biol. 1996;178:179–85. doi: 10.1006/dbio.1996.0208. [DOI] [PubMed] [Google Scholar]
- 263.Bennett NC, Cotterill FPD, Spinks AC. Thermoregulation in two populations of Matabeleland mole-rat (Cryptomys hottentotus nimrodi) and remarks on the general thermoregulatory trends within the genus Cyrptomys (Rodentia: Bathyergidae) Jounal of Zoology. 1996;239:17–27. [Google Scholar]
- 264.Holmes MM, Goldman BD, Goldman SL, Seney ML, Forger NG. Neuroendocrinology and sexual differentiation in eusocial mammals. Front Neuroendocrinol. 2009;30:519–33. doi: 10.1016/j.yfrne.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Scharff A, Locker-Grütjena O, Kawalikab M, Burda H. Natural History of the giant mole-rat, Crytomys mechowi (Rodentia: Bathyergidae) from Zambia. J Mammalogy. 2001;82:1003–15. [Google Scholar]
- 266.Buffenstein R, Yahav S. Is the naked mole-rat Heterocephalus glaber an endothermic yet poikilothermic mammal? J Thermal Biol. 1991;16:227–32. [Google Scholar]
- 267.Abayasekara DRE, Wathes DC. Effects of altering dietary fatty acid composition on prostaglandin synthesis and fertility. Prostaglandins Leukotrienes and Essential Fatty Acids. 1999;61:275–87. doi: 10.1054/plef.1999.0101. [DOI] [PubMed] [Google Scholar]
- 268.Rodgers KJ, Dean RT. Assessment of proteasome activity in cell lysates and tissue homogenates using peptide substrates. Int J Biochem Cell Biol. 2003;35:716–27. doi: 10.1016/s1357-2725(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 269.Rodriguez KA, Gaczynska M, Osmulski PA. Molecular mechanisms of proteasome plasticity in aging. Mech Ageing Dev. 2010;131:144–155. doi: 10.1016/j.mad.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]