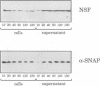
Abstract
Digitonin-permeabilized chromaffin cells secrete catecholamines by exocytosis in response to micromolar Ca2+ concentrations, but lose the ability to secrete in response to Ca2+ as the cells lose soluble proteins through the plasma membrane pores. Such secretory run-down can be retarded by cytosolic fractions, thus providing an assay for proteins potentially involved in the exocytotic process. We have used this assay to investigate the role of N-ethylmaleimide-sensitive fusion protein (NSF) and soluble NSF attachment proteins (SNAPs) in regulated exocytosis. Recombinant alpha- and gamma-SNAP stimulated Ca(2+)-dependent exocytosis, although recombinant NSF was ineffective, despite the fact that NSF and alpha-SNAP leak from the permeabilized cells with similar time courses. However, around one third of cellular NSF was found to be present in a non-cytosolic form and so it is possible that this is sufficient for exocytosis and that exogenous SNAPs stimulate the exocytotic mechanism by acting on the leakage-insensitive NSF. The stimulatory effect of alpha-SNAP displayed a biphasic dose-response curve and was maximal at 20 micrograms/ml. The effect of alpha-SNAP was Ca(2+)- and MgATP-dependent and was inhibited by N-ethylmaleimide and botulinum A neurotoxin, indicating a bona fide action on the exocytotic mechanism. Furthermore, Ca2+ concentrations which trigger catecholamine secretion acted to prevent the leakage of NSF and alpha-SNAP from permeabilized cells. These findings provide functional evidence for a role of SNAPs in regulated exocytosis in chromaffin cells.
Full text
PDF
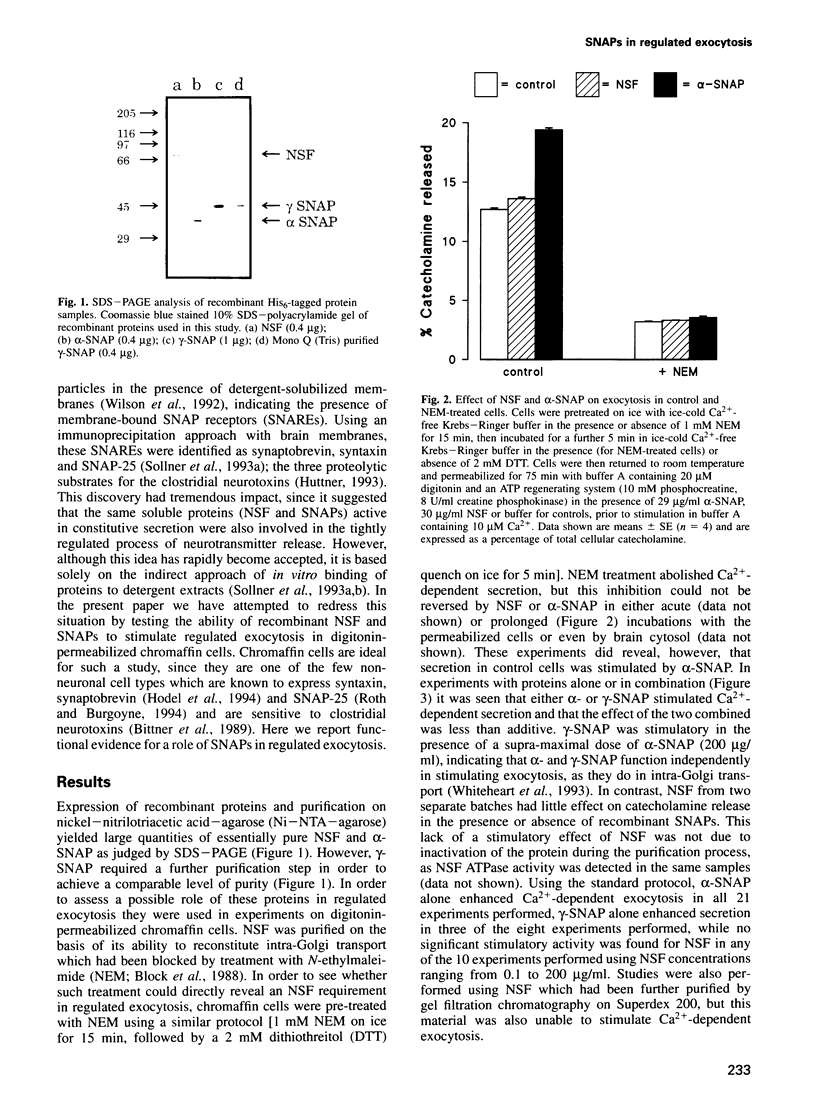
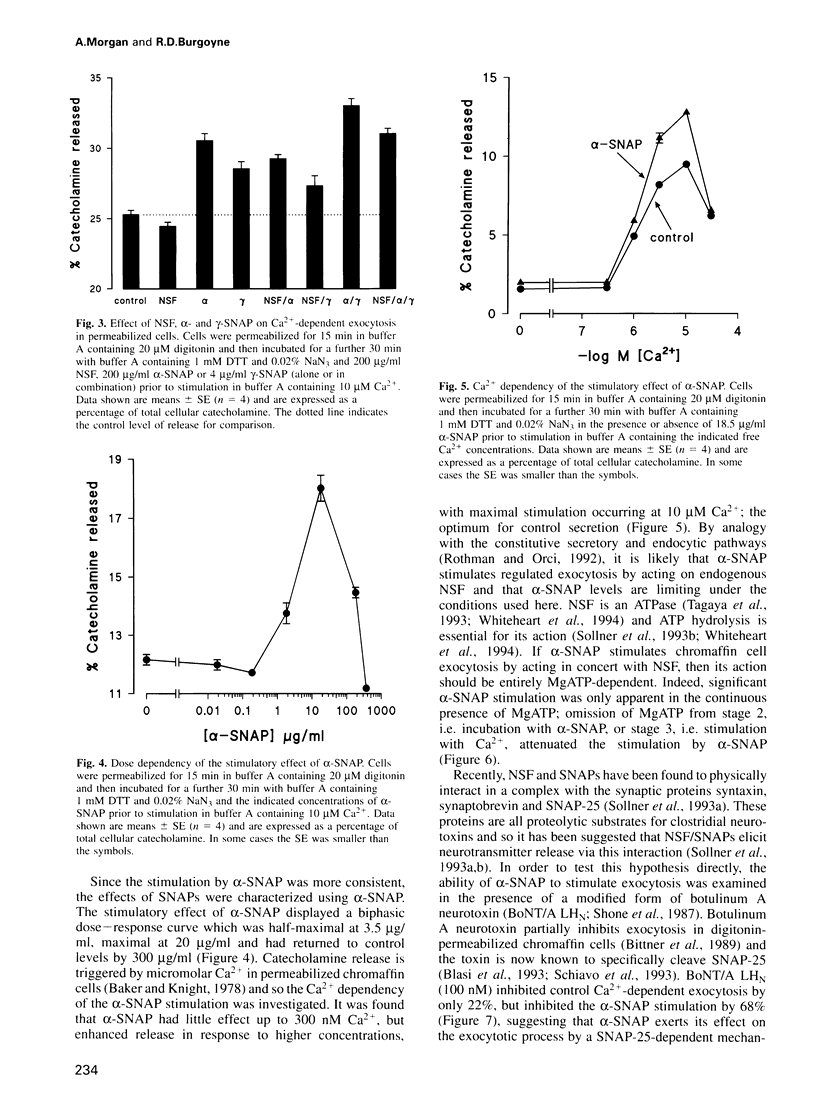
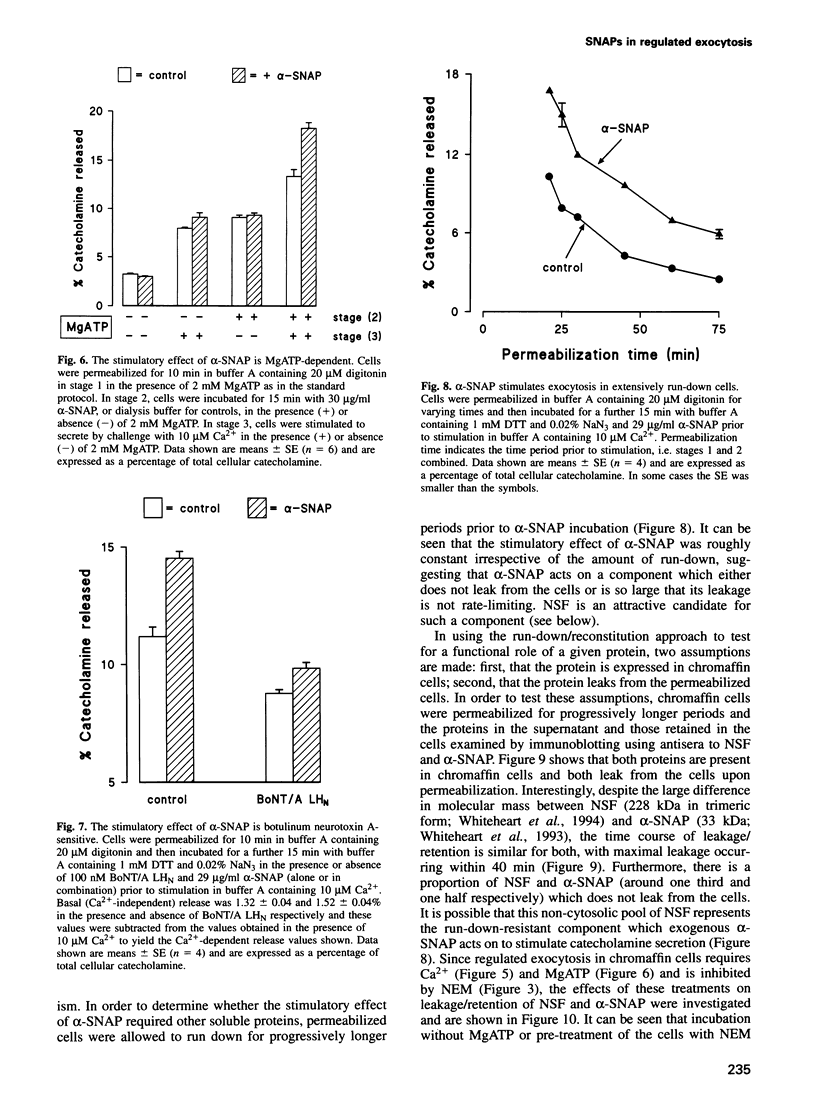




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S. M., Burgoyne R. D. The stimulatory effect of calpactin (annexin II) on calcium-dependent exocytosis in chromaffin cells: requirement for both the N-terminal and core domains of p36 and ATP. Cell Signal. 1990;2(3):265–276. doi: 10.1016/0898-6568(90)90054-e. [DOI] [PubMed] [Google Scholar]
- Ali S. M., Geisow M. J., Burgoyne R. D. A role for calpactin in calcium-dependent exocytosis in adrenal chromaffin cells. Nature. 1989 Jul 27;340(6231):313–315. doi: 10.1038/340313a0. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Knight D. E. Calcium-dependent exocytosis in bovine adrenal medullary cells with leaky plasma membranes. Nature. 1978 Dec 7;276(5688):620–622. doi: 10.1038/276620a0. [DOI] [PubMed] [Google Scholar]
- Beckers C. J., Block M. R., Glick B. S., Rothman J. E., Balch W. E. Vesicular transport between the endoplasmic reticulum and the Golgi stack requires the NEM-sensitive fusion protein. Nature. 1989 Jun 1;339(6223):397–398. doi: 10.1038/339397a0. [DOI] [PubMed] [Google Scholar]
- Bittner M. A., DasGupta B. R., Holz R. W. Isolated light chains of botulinum neurotoxins inhibit exocytosis. Studies in digitonin-permeabilized chromaffin cells. J Biol Chem. 1989 Jun 25;264(18):10354–10360. [PubMed] [Google Scholar]
- Blasi J., Chapman E. R., Link E., Binz T., Yamasaki S., De Camilli P., Südhof T. C., Niemann H., Jahn R. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993 Sep 9;365(6442):160–163. doi: 10.1038/365160a0. [DOI] [PubMed] [Google Scholar]
- Block M. R., Glick B. S., Wilcox C. A., Wieland F. T., Rothman J. E. Purification of an N-ethylmaleimide-sensitive protein catalyzing vesicular transport. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7852–7856. doi: 10.1073/pnas.85.21.7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Clague M. J. Annexins in the endocytic pathway. Trends Biochem Sci. 1994 Jun;19(6):231–232. doi: 10.1016/0968-0004(94)90143-0. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D. Control of exocytosis in adrenal chromaffin cells. Biochim Biophys Acta. 1991 Jul 22;1071(2):174–202. doi: 10.1016/0304-4157(91)90024-q. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A. Evidence for a role of calpactin in calcium-dependent exocytosis. Biochem Soc Trans. 1990 Dec;18(6):1101–1104. doi: 10.1042/bst0181101. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A., O'Sullivan A. J. A major role for protein kinase C in calcium-activated exocytosis in permeabilised adrenal chromaffin cells. FEBS Lett. 1988 Sep 26;238(1):151–155. doi: 10.1016/0014-5793(88)80246-1. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A. Regulated exocytosis. Biochem J. 1993 Jul 15;293(Pt 2):305–316. doi: 10.1042/bj2930305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clary D. O., Griff I. C., Rothman J. E. SNAPs, a family of NSF attachment proteins involved in intracellular membrane fusion in animals and yeast. Cell. 1990 May 18;61(4):709–721. doi: 10.1016/0092-8674(90)90482-t. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Rothman J. E. Purification of three related peripheral membrane proteins needed for vesicular transport. J Biol Chem. 1990 Jun 15;265(17):10109–10117. [PubMed] [Google Scholar]
- Diaz R., Mayorga L. S., Weidman P. J., Rothman J. E., Stahl P. D. Vesicle fusion following receptor-mediated endocytosis requires a protein active in Golgi transport. Nature. 1989 Jun 1;339(6223):398–400. doi: 10.1038/339398a0. [DOI] [PubMed] [Google Scholar]
- Greenberg A., Zinder O. Alpha- and beta-receptor control of catecholamine secretion from isolated adrenal medulla cells. Cell Tissue Res. 1982;226(3):655–665. doi: 10.1007/BF00214792. [DOI] [PubMed] [Google Scholar]
- Griff I. C., Schekman R., Rothman J. E., Kaiser C. A. The yeast SEC17 gene product is functionally equivalent to mammalian alpha-SNAP protein. J Biol Chem. 1992 Jun 15;267(17):12106–12115. [PubMed] [Google Scholar]
- Hay J. C., Martin T. F. Phosphatidylinositol transfer protein required for ATP-dependent priming of Ca(2+)-activated secretion. Nature. 1993 Dec 9;366(6455):572–575. doi: 10.1038/366572a0. [DOI] [PubMed] [Google Scholar]
- Hodel A., Schäfer T., Gerosa D., Burger M. M. In chromaffin cells, the mammalian Sec1p homologue is a syntaxin 1A-binding protein associated with chromaffin granules. J Biol Chem. 1994 Mar 25;269(12):8623–8626. [PubMed] [Google Scholar]
- Holz R. W., Bittner M. A., Peppers S. C., Senter R. A., Eberhard D. A. MgATP-independent and MgATP-dependent exocytosis. Evidence that MgATP primes adrenal chromaffin cells to undergo exocytosis. J Biol Chem. 1989 Apr 5;264(10):5412–5419. [PubMed] [Google Scholar]
- Hong R. M., Mori H., Fukui T., Moriyama Y., Futai M., Yamamoto A., Tashiro Y., Tagaya M. Association of N-ethylmaleimide-sensitive factor with synaptic vesicles. FEBS Lett. 1994 Aug 22;350(2-3):253–257. doi: 10.1016/0014-5793(94)00778-0. [DOI] [PubMed] [Google Scholar]
- Huttner W. B. Cell biology. Snappy exocytoxins. Nature. 1993 Sep 9;365(6442):104–105. doi: 10.1038/365104a0. [DOI] [PubMed] [Google Scholar]
- Kish P. E., Ueda T. Calcium-dependent release of accumulated glutamate from synaptic vesicles within permeabilized nerve terminals. Neurosci Lett. 1991 Jan 28;122(2):179–182. doi: 10.1016/0304-3940(91)90852-k. [DOI] [PubMed] [Google Scholar]
- Koffer A., Gomperts B. D. Soluble proteins as modulators of the exocytotic reaction of permeabilised rat mast cells. J Cell Sci. 1989 Nov;94(Pt 3):585–591. doi: 10.1242/jcs.94.3.585. [DOI] [PubMed] [Google Scholar]
- Lomneth R., Martin T. F., DasGupta B. R. Botulinum neurotoxin light chain inhibits norepinephrine secretion in PC12 cells at an intracellular membranous or cytoskeletal site. J Neurochem. 1991 Oct;57(4):1413–1421. doi: 10.1111/j.1471-4159.1991.tb08308.x. [DOI] [PubMed] [Google Scholar]
- Martin T. F., Walent J. H. A new method for cell permeabilization reveals a cytosolic protein requirement for Ca2+ -activated secretion in GH3 pituitary cells. J Biol Chem. 1989 Jun 15;264(17):10299–10308. [PubMed] [Google Scholar]
- Morgan A., Burgoyne R. D. Exo1 and Exo2 proteins stimulate calcium-dependent exocytosis in permeabilized adrenal chromaffin cells. Nature. 1992 Feb 27;355(6363):833–836. doi: 10.1038/355833a0. [DOI] [PubMed] [Google Scholar]
- Morgan A., Burgoyne R. D. Interaction between protein kinase C and Exo1 (14-3-3 protein) and its relevance to exocytosis in permeabilized adrenal chromaffin cells. Biochem J. 1992 Sep 15;286(Pt 3):807–811. doi: 10.1042/bj2860807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A., Wilkinson M., Burgoyne R. D. Identification of Exo2 as the catalytic subunit of protein kinase A reveals a role for cyclic AMP in Ca(2+)-dependent exocytosis in chromaffin cells. EMBO J. 1993 Oct;12(10):3747–3752. doi: 10.1002/j.1460-2075.1993.tb06052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naor Z., Dan-Cohen H., Hermon J., Limor R. Induction of exocytosis in permeabilized pituitary cells by alpha- and beta-type protein kinase C. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4501–4504. doi: 10.1073/pnas.86.12.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Zucker R. S. Multiple calcium-dependent processes related to secretion in bovine chromaffin cells. Neuron. 1993 Jan;10(1):21–30. doi: 10.1016/0896-6273(93)90238-m. [DOI] [PubMed] [Google Scholar]
- Nishizaki T., Walent J. H., Kowalchyk J. A., Martin T. F. A key role for a 145-kDa cytosolic protein in the stimulation of Ca(2+)-dependent secretion by protein kinase C. J Biol Chem. 1992 Nov 25;267(33):23972–23981. [PubMed] [Google Scholar]
- Podbilewicz B., Mellman I. ATP and cytosol requirements for transferrin recycling in intact and disrupted MDCK cells. EMBO J. 1990 Nov;9(11):3477–3487. doi: 10.1002/j.1460-2075.1990.tb07556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez L., Stirling C. J., Woodman P. G. Multiple N-ethylmaleimide-sensitive components are required for endosomal vesicle fusion. Mol Biol Cell. 1994 Jul;5(7):773–783. doi: 10.1091/mbc.5.7.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D., Burgoyne R. D. SNAP-25 is present in a SNARE complex in adrenal chromaffin cells. FEBS Lett. 1994 Sep 5;351(2):207–210. doi: 10.1016/0014-5793(94)00833-7. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Orci L. Molecular dissection of the secretory pathway. Nature. 1992 Jan 30;355(6359):409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- Sarafian T., Aunis D., Bader M. F. Loss of proteins from digitonin-permeabilized adrenal chromaffin cells essential for exocytosis. J Biol Chem. 1987 Dec 5;262(34):16671–16676. [PubMed] [Google Scholar]
- Sarafian T., Pradel L. A., Henry J. P., Aunis D., Bader M. F. The participation of annexin II (calpactin I) in calcium-evoked exocytosis requires protein kinase C. J Cell Biol. 1991 Sep;114(6):1135–1147. doi: 10.1083/jcb.114.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavo G., Benfenati F., Poulain B., Rossetto O., Polverino de Laureto P., DasGupta B. R., Montecucco C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992 Oct 29;359(6398):832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- Schiavo G., Poulain B., Rossetto O., Benfenati F., Tauc L., Montecucco C. Tetanus toxin is a zinc protein and its inhibition of neurotransmitter release and protease activity depend on zinc. EMBO J. 1992 Oct;11(10):3577–3583. doi: 10.1002/j.1460-2075.1992.tb05441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavo G., Santucci A., Dasgupta B. R., Mehta P. P., Jontes J., Benfenati F., Wilson M. C., Montecucco C. Botulinum neurotoxins serotypes A and E cleave SNAP-25 at distinct COOH-terminal peptide bonds. FEBS Lett. 1993 Nov 29;335(1):99–103. doi: 10.1016/0014-5793(93)80448-4. [DOI] [PubMed] [Google Scholar]
- Shone C. C., Hambleton P., Melling J. A 50-kDa fragment from the NH2-terminus of the heavy subunit of Clostridium botulinum type A neurotoxin forms channels in lipid vesicles. Eur J Biochem. 1987 Aug 17;167(1):175–180. doi: 10.1111/j.1432-1033.1987.tb13320.x. [DOI] [PubMed] [Google Scholar]
- Söllner T., Bennett M. K., Whiteheart S. W., Scheller R. H., Rothman J. E. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993 Nov 5;75(3):409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Söllner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J. E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993 Mar 25;362(6418):318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Tagaya M., Wilson D. W., Brunner M., Arango N., Rothman J. E. Domain structure of an N-ethylmaleimide-sensitive fusion protein involved in vesicular transport. J Biol Chem. 1993 Feb 5;268(4):2662–2666. [PubMed] [Google Scholar]
- Thomas P., Wong J. G., Almers W. Millisecond studies of secretion in single rat pituitary cells stimulated by flash photolysis of caged Ca2+. EMBO J. 1993 Jan;12(1):303–306. doi: 10.1002/j.1460-2075.1993.tb05657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VON EULER U. S., FLODING I. A fluorimetric micromethod for differential estimation of adrenaline and noradrenaline. Acta Physiol Scand Suppl. 1955;33(118):45–56. [PubMed] [Google Scholar]
- Walent J. H., Porter B. W., Martin T. F. A novel 145 kd brain cytosolic protein reconstitutes Ca(2+)-regulated secretion in permeable neuroendocrine cells. Cell. 1992 Sep 4;70(5):765–775. doi: 10.1016/0092-8674(92)90310-9. [DOI] [PubMed] [Google Scholar]
- Whiteheart S. W., Griff I. C., Brunner M., Clary D. O., Mayer T., Buhrow S. A., Rothman J. E. SNAP family of NSF attachment proteins includes a brain-specific isoform. Nature. 1993 Mar 25;362(6418):353–355. doi: 10.1038/362353a0. [DOI] [PubMed] [Google Scholar]
- Whiteheart S. W., Rossnagel K., Buhrow S. A., Brunner M., Jaenicke R., Rothman J. E. N-ethylmaleimide-sensitive fusion protein: a trimeric ATPase whose hydrolysis of ATP is required for membrane fusion. J Cell Biol. 1994 Aug;126(4):945–954. doi: 10.1083/jcb.126.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. W., Whiteheart S. W., Wiedmann M., Brunner M., Rothman J. E. A multisubunit particle implicated in membrane fusion. J Cell Biol. 1992 May;117(3):531–538. doi: 10.1083/jcb.117.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. W., Wilcox C. A., Flynn G. C., Chen E., Kuang W. J., Henzel W. J., Block M. R., Ullrich A., Rothman J. E. A fusion protein required for vesicle-mediated transport in both mammalian cells and yeast. Nature. 1989 Jun 1;339(6223):355–359. doi: 10.1038/339355a0. [DOI] [PubMed] [Google Scholar]
- Wu Y. N., Vu N. D., Wagner P. D. Anti-(14-3-3 protein) antibody inhibits stimulation of noradrenaline (norepinephrine) secretion by chromaffin-cell cytosolic proteins. Biochem J. 1992 Aug 1;285(Pt 3):697–700. doi: 10.1042/bj2850697. [DOI] [PMC free article] [PubMed] [Google Scholar]