Abstract
Interleukin 3 (IL-3) and granulocyte-macrophage colony stimulating factor (GM-CSF) exert their biological functions through acting on a specific receptor which consists of a ligand-specific alpha subunit and the shared common beta subunit. Inhibition by genistein of a subset of IL-3/GM-CSF-mediated signals, including c-myc induction, resulted in the abrogation of DNA synthesis, however, IL-3 still protected cells from apoptotic cell death. Conversely, a C-terminal truncated form of the GM-CSF receptor, which is missing a critical cytoplasmic region required for activation of the Ras/Raf-1/MAP kinase pathway, induced DNA synthesis, but failed to prevent cell death in response to GM-CSF. Consequently, cells died by apoptosis in the presence of GM-CSF, despite displaying a transient mitogenic response. However, expression of activated Ras protein complemented defective signalling through the mutant receptor and supported long-term proliferation in concert with GM-CSF. These results indicate that IL-3 and GM-CSF prevent apoptosis of hematopoietic cells by activating a signalling pathway distinct from the induction of DNA synthesis and that long-term cell proliferation requires the activation of both pathways.
Full text
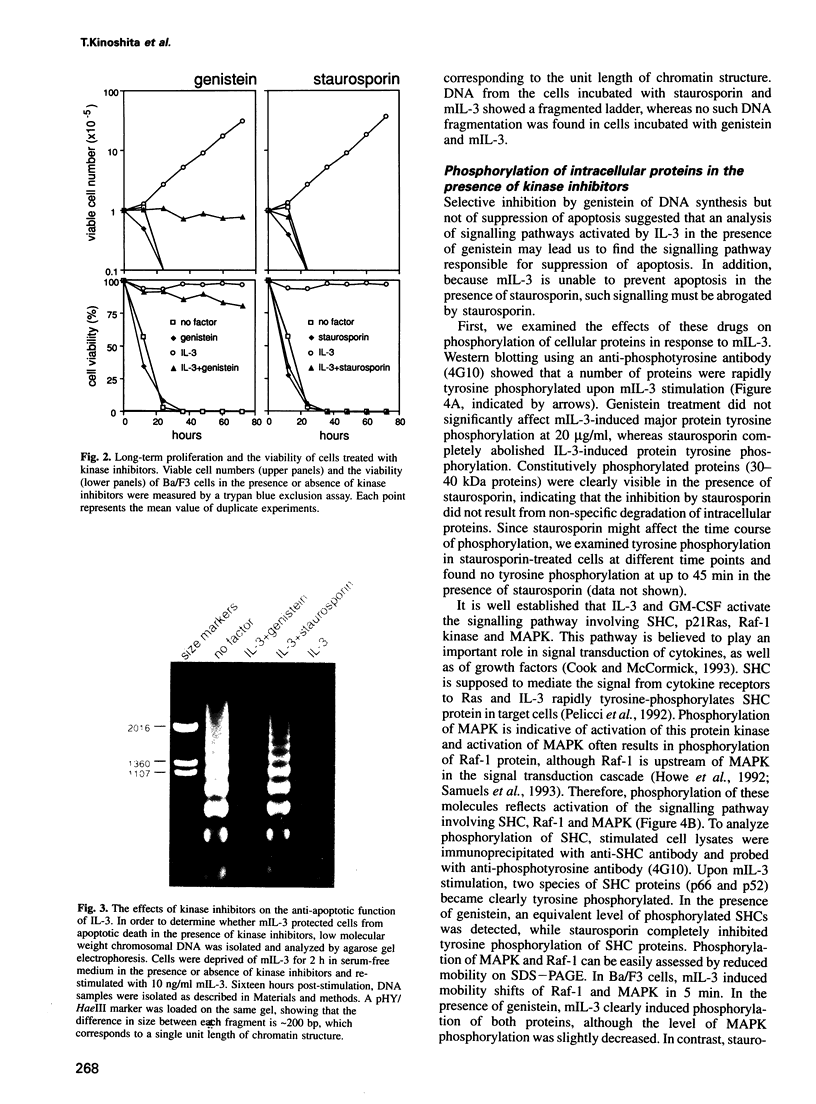
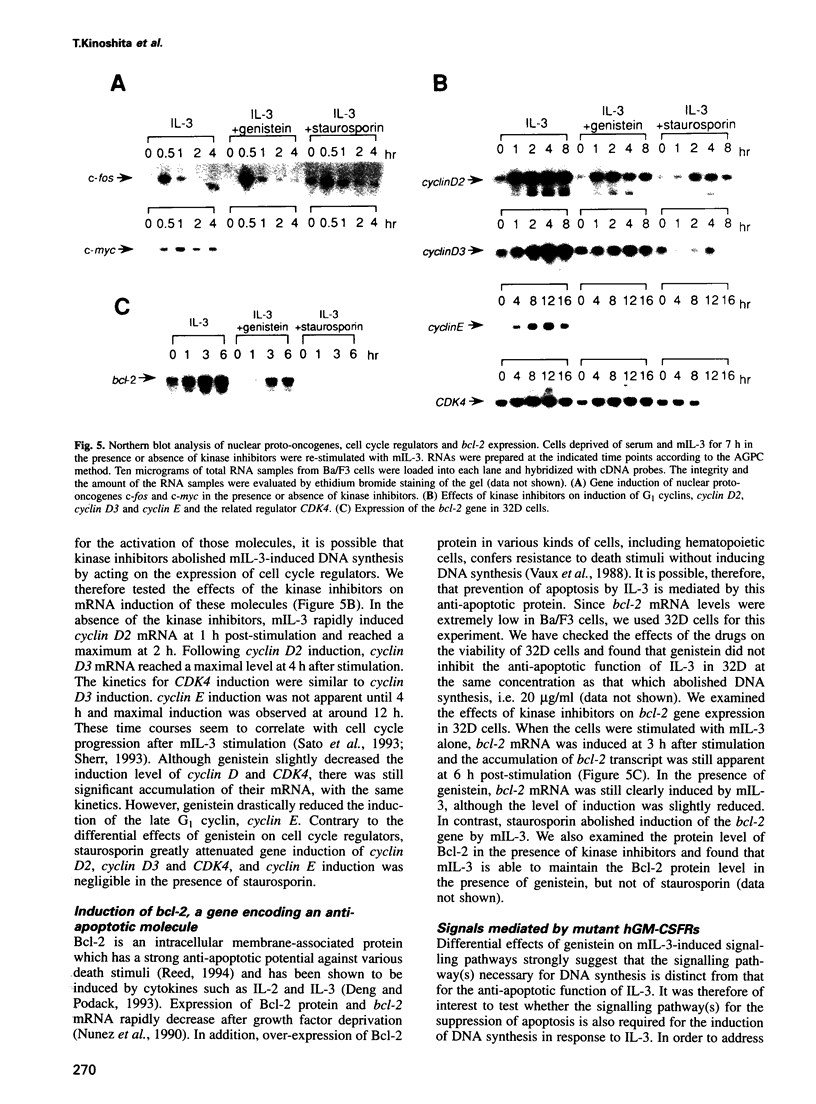
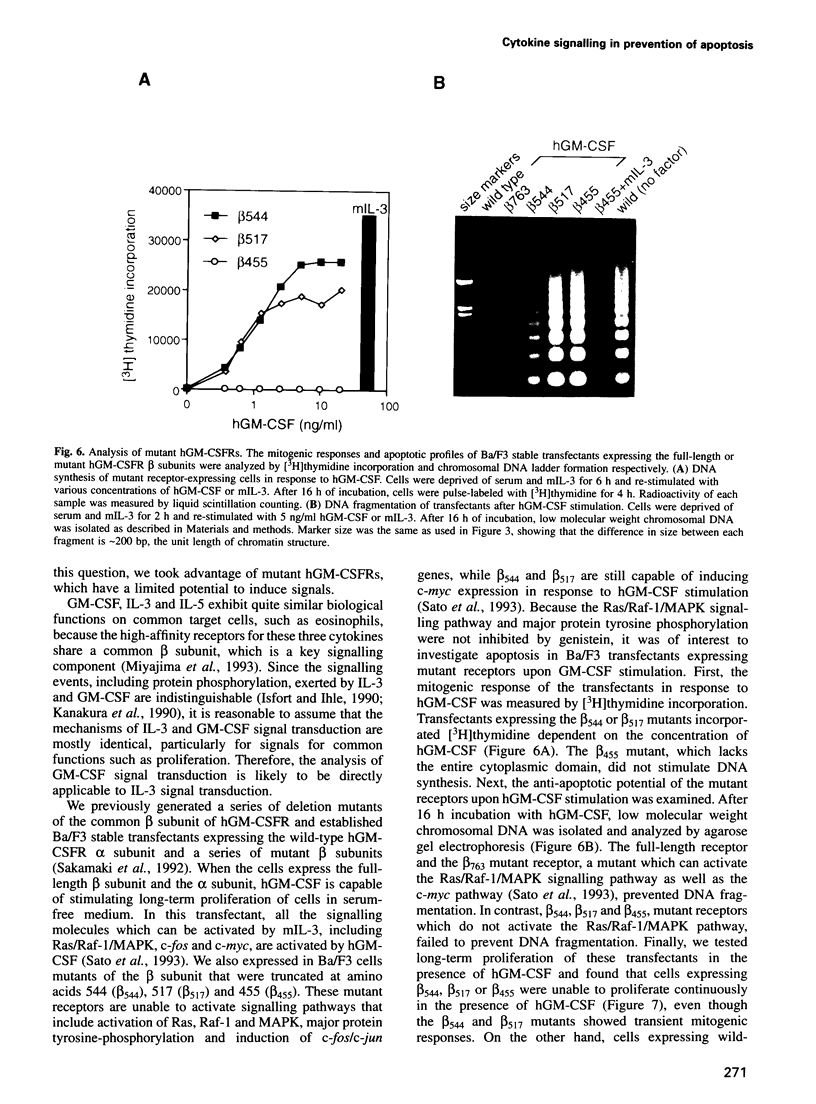
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Allen P. D., Bustin S. A., Newland A. C. The role of apoptosis (programmed cell death) in haemopoiesis and the immune system. Blood Rev. 1993 Mar;7(1):63–73. doi: 10.1016/0268-960x(93)90025-y. [DOI] [PubMed] [Google Scholar]
- Ando K., Ajchenbaum-Cymbalista F., Griffin J. D. Regulation of G1/S transition by cyclins D2 and D3 in hematopoietic cells. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9571–9575. doi: 10.1073/pnas.90.20.9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K. I., Lee F., Miyajima A., Miyatake S., Arai N., Yokota T. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- Blasi E., Mathieson B. J., Varesio L., Cleveland J. L., Borchert P. A., Rapp U. R. Selective immortalization of murine macrophages from fresh bone marrow by a raf/myc recombinant murine retrovirus. Nature. 1985 Dec 19;318(6047):667–670. doi: 10.1038/318667a0. [DOI] [PubMed] [Google Scholar]
- Chiba T., Kishi A., Sugiyama M., Amanuma H., Machide M., Nagata Y., Todokoro K. Functionally essential cytoplasmic domain of the erythropoietin receptor. Biochem Biophys Res Commun. 1992 Aug 14;186(3):1236–1241. doi: 10.1016/s0006-291x(05)81538-4. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collins M. K., Marvel J., Malde P., Lopez-Rivas A. Interleukin 3 protects murine bone marrow cells from apoptosis induced by DNA damaging agents. J Exp Med. 1992 Oct 1;176(4):1043–1051. doi: 10.1084/jem.176.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S. J., McCormick F. Inhibition by cAMP of Ras-dependent activation of Raf. Science. 1993 Nov 12;262(5136):1069–1072. doi: 10.1126/science.7694367. [DOI] [PubMed] [Google Scholar]
- Cormier F., Ponting I. L., Heyworth C. M., Dexter T. M. Serum-free culture of enriched murine haemopoietic stem cells. I: Effect of haemopoietic growth factors on proliferation. Growth Factors. 1991;4(3):157–164. doi: 10.3109/08977199109104811. [DOI] [PubMed] [Google Scholar]
- Deng G., Podack E. R. Suppression of apoptosis in a cytotoxic T-cell line by interleukin 2-mediated gene transcription and deregulated expression of the protooncogene bcl-2. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2189–2193. doi: 10.1073/pnas.90.6.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath I., Schweighoffer F., Rey I., Multon M. C., Boiziau J., Duchesne M., Tocqué B. Cloning of a Grb2 isoform with apoptotic properties. Science. 1994 May 13;264(5161):971–974. doi: 10.1126/science.8178156. [DOI] [PubMed] [Google Scholar]
- Fukunaga R., Ishizaka-Ikeda E., Nagata S. Growth and differentiation signals mediated by different regions in the cytoplasmic domain of granulocyte colony-stimulating factor receptor. Cell. 1993 Sep 24;74(6):1079–1087. doi: 10.1016/0092-8674(93)90729-a. [DOI] [PubMed] [Google Scholar]
- Howe L. R., Leevers S. J., Gómez N., Nakielny S., Cohen P., Marshall C. J. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992 Oct 16;71(2):335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- Isfort R. J., Ihle J. N. Multiple hematopoietic growth factors signal through tyrosine phosphorylation. Growth Factors. 1990;2(2-3):213–220. doi: 10.3109/08977199009071507. [DOI] [PubMed] [Google Scholar]
- Ishida Y., Agata Y., Shibahara K., Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992 Nov;11(11):3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. D., Burne J. F., King M. P., Miyashita T., Reed J. C., Raff M. C. Bcl-2 blocks apoptosis in cells lacking mitochondrial DNA. Nature. 1993 Jan 28;361(6410):365–369. doi: 10.1038/361365a0. [DOI] [PubMed] [Google Scholar]
- Kanakura Y., Druker B., Cannistra S. A., Furukawa Y., Torimoto Y., Griffin J. D. Signal transduction of the human granulocyte-macrophage colony-stimulating factor and interleukin-3 receptors involves tyrosine phosphorylation of a common set of cytoplasmic proteins. Blood. 1990 Aug 15;76(4):706–715. [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Miyajima A., Kitamura T., Harada N., Yokota T., Arai K. Cytokine receptors and signal transduction. Annu Rev Immunol. 1992;10:295–331. doi: 10.1146/annurev.iy.10.040192.001455. [DOI] [PubMed] [Google Scholar]
- Miyajima A., Mui A. L., Ogorochi T., Sakamaki K. Receptors for granulocyte-macrophage colony-stimulating factor, interleukin-3, and interleukin-5. Blood. 1993 Oct 1;82(7):1960–1974. [PubMed] [Google Scholar]
- Murakami M., Narazaki M., Hibi M., Yawata H., Yasukawa K., Hamaguchi M., Taga T., Kishimoto T. Critical cytoplasmic region of the interleukin 6 signal transducer gp130 is conserved in the cytokine receptor family. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11349–11353. doi: 10.1073/pnas.88.24.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez G., London L., Hockenbery D., Alexander M., McKearn J. P., Korsmeyer S. J. Deregulated Bcl-2 gene expression selectively prolongs survival of growth factor-deprived hemopoietic cell lines. J Immunol. 1990 May 1;144(9):3602–3610. [PubMed] [Google Scholar]
- Peitsch M. C., Polzar B., Stephan H., Crompton T., MacDonald H. R., Mannherz H. G., Tschopp J. Characterization of the endogenous deoxyribonuclease involved in nuclear DNA degradation during apoptosis (programmed cell death). EMBO J. 1993 Jan;12(1):371–377. doi: 10.1002/j.1460-2075.1993.tb05666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicci G., Lanfrancone L., Grignani F., McGlade J., Cavallo F., Forni G., Nicoletti I., Grignani F., Pawson T., Pelicci P. G. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell. 1992 Jul 10;70(1):93–104. doi: 10.1016/0092-8674(92)90536-l. [DOI] [PubMed] [Google Scholar]
- Quelle F. W., Sato N., Witthuhn B. A., Inhorn R. C., Eder M., Miyajima A., Griffin J. D., Ihle J. N. JAK2 associates with the beta c chain of the receptor for granulocyte-macrophage colony-stimulating factor, and its activation requires the membrane-proximal region. Mol Cell Biol. 1994 Jul;14(7):4335–4341. doi: 10.1128/mcb.14.7.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Social controls on cell survival and cell death. Nature. 1992 Apr 2;356(6368):397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- Rajotte D., Haddad P., Haman A., Cragoe E. J., Jr, Hoang T. Role of protein kinase C and the Na+/H+ antiporter in suppression of apoptosis by granulocyte macrophage colony-stimulating factor and interleukin-3. J Biol Chem. 1992 May 15;267(14):9980–9987. [PubMed] [Google Scholar]
- Rao P., Mufson R. A. Human interleukin-3 stimulates a phosphatidylcholine specific phospholipase C and protein kinase C translocation. Cancer Res. 1994 Feb 1;54(3):777–783. [PubMed] [Google Scholar]
- Reed J. C. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994 Jan;124(1-2):1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamaki K., Miyajima I., Kitamura T., Miyajima A. Critical cytoplasmic domains of the common beta subunit of the human GM-CSF, IL-3 and IL-5 receptors for growth signal transduction and tyrosine phosphorylation. EMBO J. 1992 Oct;11(10):3541–3549. doi: 10.1002/j.1460-2075.1992.tb05437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels M. L., Weber M. J., Bishop J. M., McMahon M. Conditional transformation of cells and rapid activation of the mitogen-activated protein kinase cascade by an estradiol-dependent human raf-1 protein kinase. Mol Cell Biol. 1993 Oct;13(10):6241–6252. doi: 10.1128/mcb.13.10.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N., Sakamaki K., Terada N., Arai K., Miyajima A. Signal transduction by the high-affinity GM-CSF receptor: two distinct cytoplasmic regions of the common beta subunit responsible for different signaling. EMBO J. 1993 Nov;12(11):4181–4189. doi: 10.1002/j.1460-2075.1993.tb06102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Minami Y., Kono T., Yamada K., Kawahara A., Taniguchi T., Kaziro Y. Interleukin 2-induced activation of Ras requires two domains of interleukin 2 receptor beta subunit, the essential region for growth stimulation and Lck-binding domain. J Biol Chem. 1992 Dec 15;267(35):25423–25427. [PubMed] [Google Scholar]
- Schwartz L. M., Osborne B. A. Programmed cell death, apoptosis and killer genes. Immunol Today. 1993 Dec;14(12):582–590. doi: 10.1016/0167-5699(93)90197-S. [DOI] [PubMed] [Google Scholar]
- Sherr C. J. Mammalian G1 cyclins. Cell. 1993 Jun 18;73(6):1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- Shibuya H., Yoneyama M., Ninomiya-Tsuji J., Matsumoto K., Taniguchi T. IL-2 and EGF receptors stimulate the hematopoietic cell cycle via different signaling pathways: demonstration of a novel role for c-myc. Cell. 1992 Jul 10;70(1):57–67. doi: 10.1016/0092-8674(92)90533-i. [DOI] [PubMed] [Google Scholar]
- Shirouzu M., Fujita-Yoshigaki J., Ito Y., Koide H., Nishimura S., Yokoyama S. A glutamic acid residue at position 31 of Ras protein is essential to the signal transduction for neurite outgrowth of PC12 cells and the stimulation of GTPase activity by GAPRas. Oncogene. 1992 Mar;7(3):475–480. [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Bath M. L., Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990 Nov 22;348(6299):331–333. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- Troppmair J., Cleveland J. L., Askew D. S., Rapp U. R. v-Raf/v-Myc synergism in abrogation of IL-3 dependence: v-Raf suppresses apoptosis. Curr Top Microbiol Immunol. 1992;182:453–460. doi: 10.1007/978-3-642-77633-5_57. [DOI] [PubMed] [Google Scholar]
- Vaux D. L., Cory S., Adams J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988 Sep 29;335(6189):440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Muto A., Yokota T., Miyajima A., Arai K. Differential regulation of early response genes and cell proliferation through the human granulocyte macrophage colony-stimulating factor receptor: selective activation of the c-fos promoter by genistein. Mol Biol Cell. 1993 Oct;4(10):983–992. doi: 10.1091/mbc.4.10.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. T., Smith C. A., Spooncer E., Dexter T. M., Taylor D. R. Haemopoietic colony stimulating factors promote cell survival by suppressing apoptosis. Nature. 1990 Jan 4;343(6253):76–79. doi: 10.1038/343076a0. [DOI] [PubMed] [Google Scholar]
- Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991 Jul 25;352(6333):345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]
- von Boehmer H., Teh H. S., Kisielow P. The thymus selects the useful, neglects the useless and destroys the harmful. Immunol Today. 1989 Feb;10(2):57–61. doi: 10.1016/0167-5699(89)90307-1. [DOI] [PubMed] [Google Scholar]