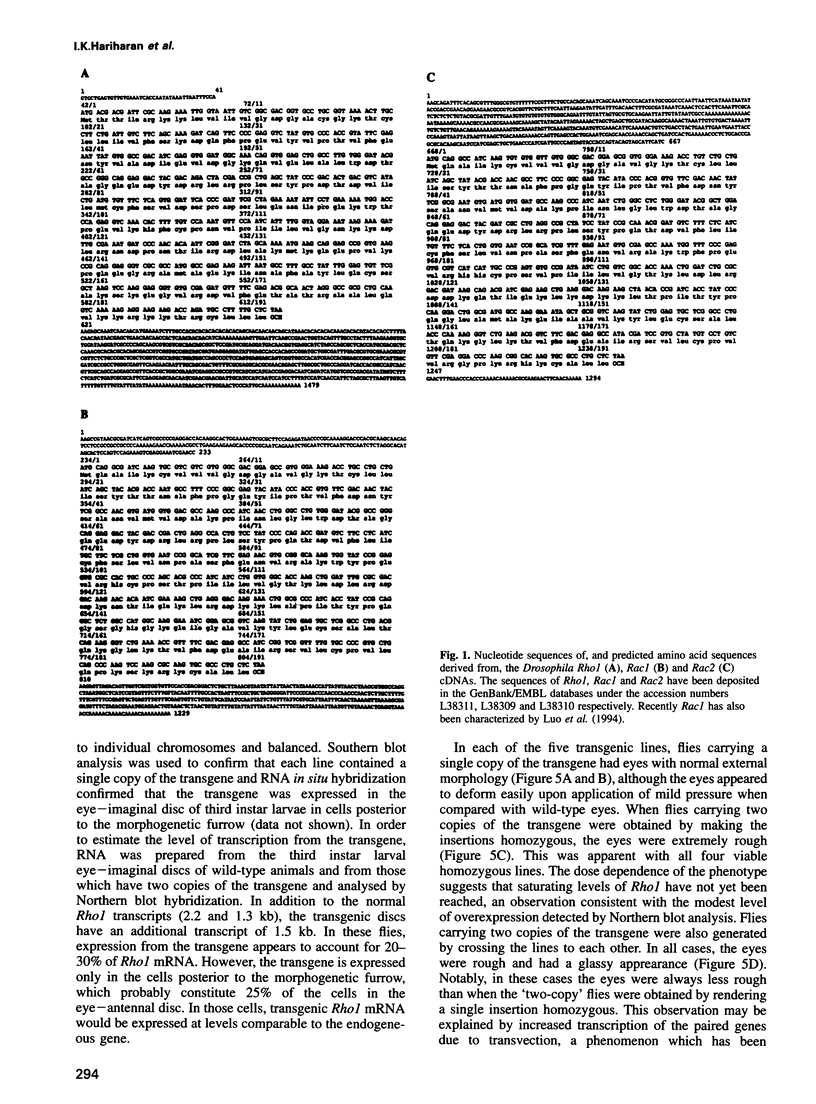
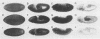Abstract
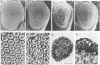
The rho family of GTPases has been implicated in regulating changes in cell morphology in response to extracellular signals. We have cloned three widely expressed members of this family from Drosophila melanogaster; a rho homologue (Rho1) and two rac homologues (Rac1 and Rac2). Flies harbouring a Rho1 transgene that is specifically expressed in the eye exhibit a dramatic dose dependent disruption of normal eye development. Flies bearing at least two copies of the transgene display a severe rough eye phenotype characterized by missing secondary and tertiary pigment cells, a substantial reduction in the number of photoreceptor cells and a grossly abnormal morphology of the rhabdomeres. Cell fate determination in the imaginal disc occurs normally and abnormalities become manifest late in pupariation, coincident with the phase when the cells undergo major morphological changes. This phenotype is modified by mutations at several other loci that have been implicated in signal transduction, but not by mutations in ras pathway components.
Full text
PDF

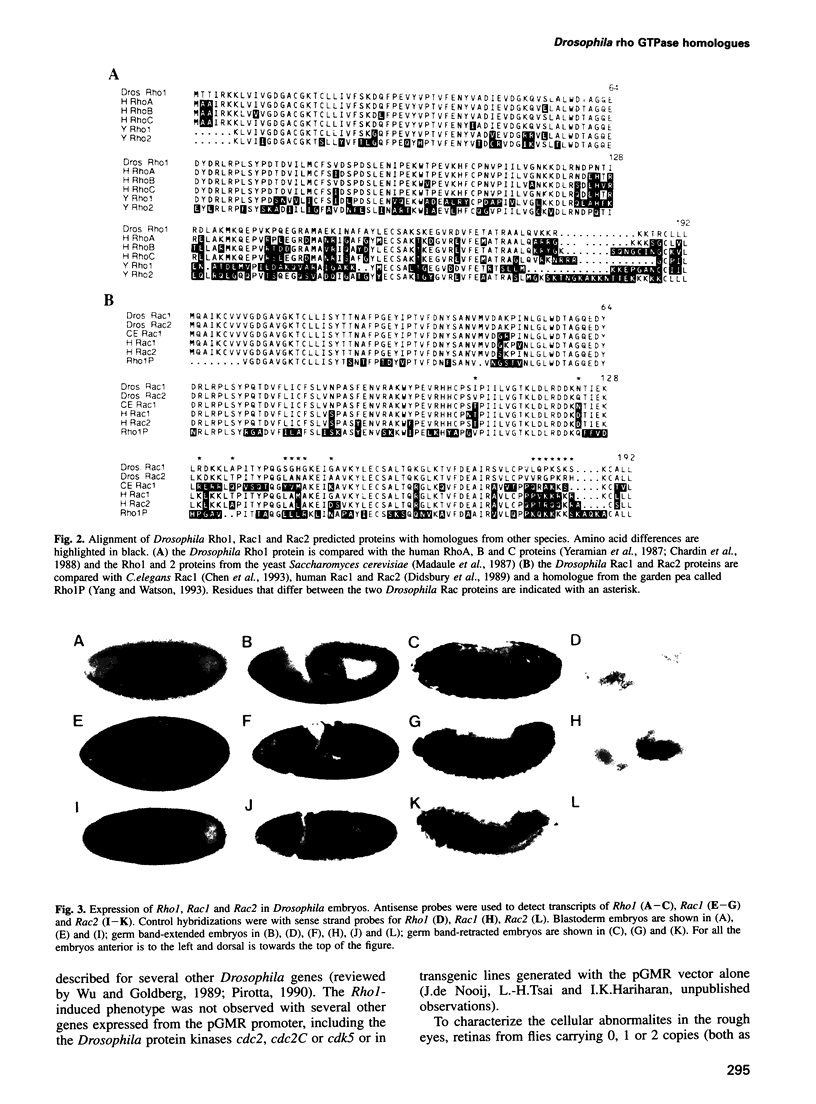
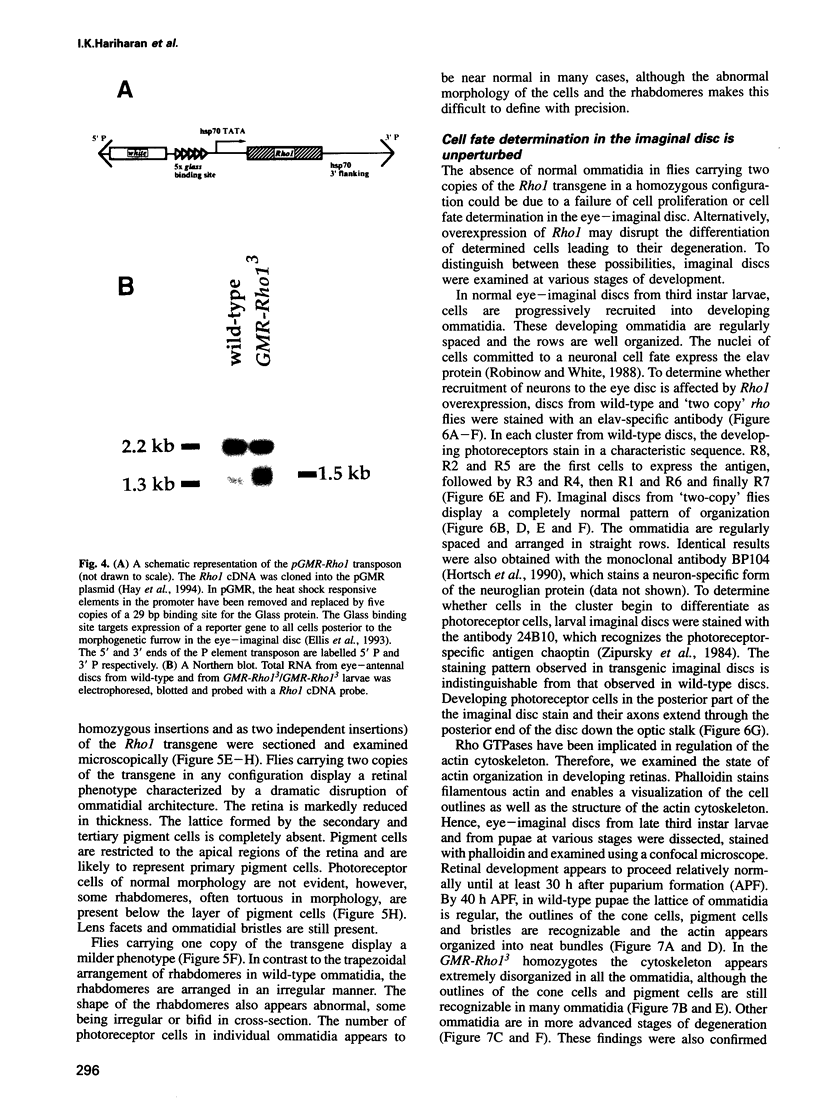
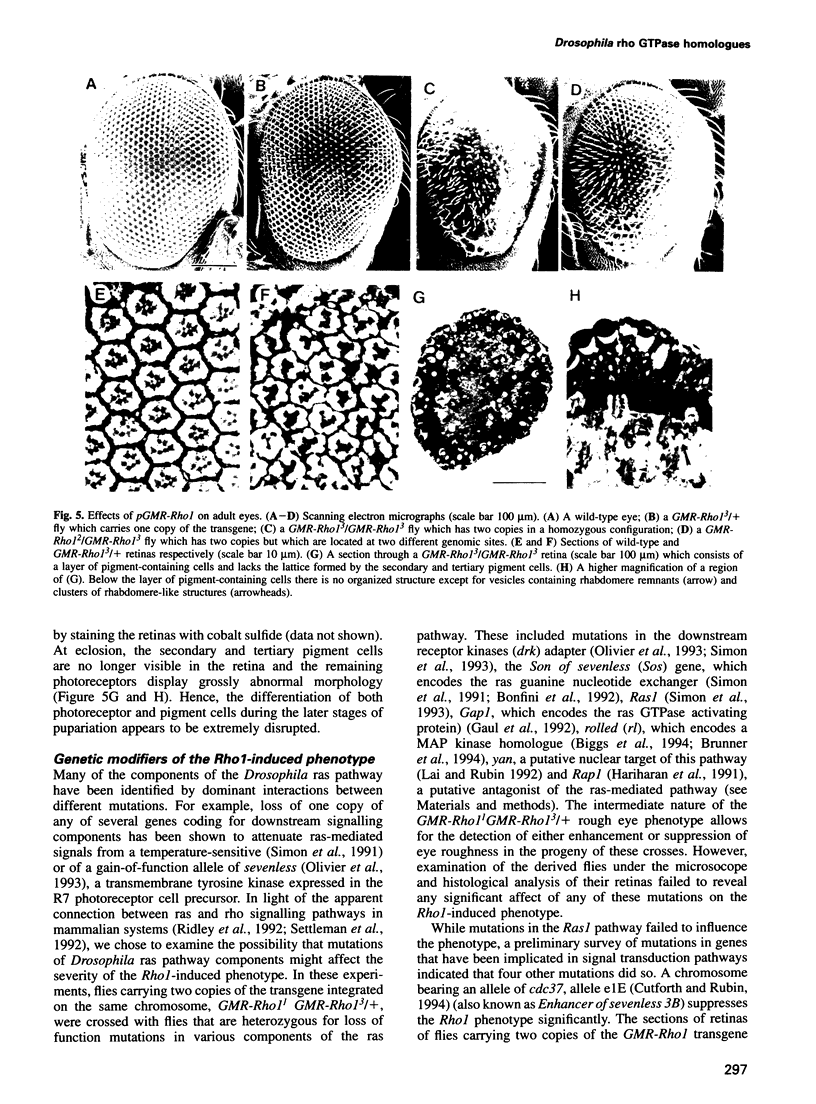

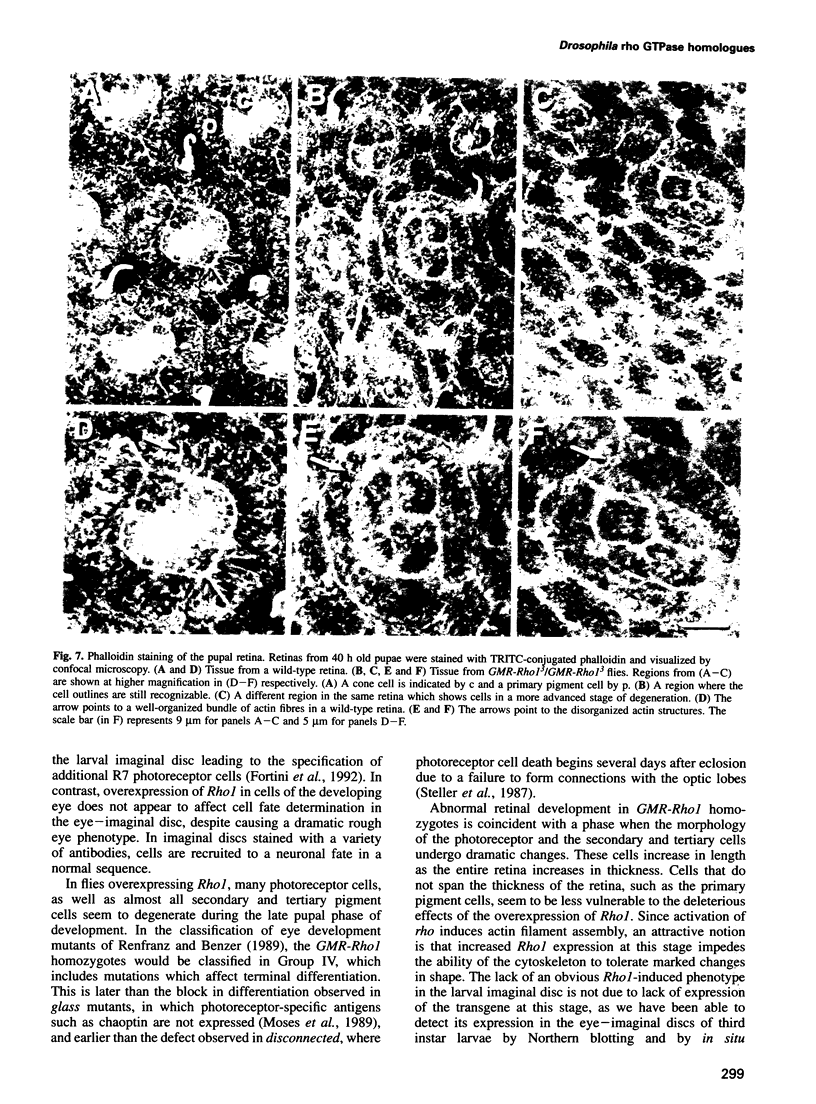



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avraham H., Weinberg R. A. Characterization and expression of the human rhoH12 gene product. Mol Cell Biol. 1989 May;9(5):2058–2066. doi: 10.1128/mcb.9.5.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Biggs W. H., 3rd, Zavitz K. H., Dickson B., van der Straten A., Brunner D., Hafen E., Zipursky S. L. The Drosophila rolled locus encodes a MAP kinase required in the sevenless signal transduction pathway. EMBO J. 1994 Apr 1;13(7):1628–1635. doi: 10.1002/j.1460-2075.1994.tb06426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfini L., Karlovich C. A., Dasgupta C., Banerjee U. The Son of sevenless gene product: a putative activator of Ras. Science. 1992 Jan 31;255(5044):603–606. doi: 10.1126/science.1736363. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990 Nov 8;348(6297):125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Brunner D., Oellers N., Szabad J., Biggs W. H., 3rd, Zipursky S. L., Hafen E. A gain-of-function mutation in Drosophila MAP kinase activates multiple receptor tyrosine kinase signaling pathways. Cell. 1994 Mar 11;76(5):875–888. doi: 10.1016/0092-8674(94)90362-x. [DOI] [PubMed] [Google Scholar]
- Cagan R. L., Ready D. F. The emergence of order in the Drosophila pupal retina. Dev Biol. 1989 Dec;136(2):346–362. doi: 10.1016/0012-1606(89)90261-3. [DOI] [PubMed] [Google Scholar]
- Carthew R. W., Rubin G. M. seven in absentia, a gene required for specification of R7 cell fate in the Drosophila eye. Cell. 1990 Nov 2;63(3):561–577. doi: 10.1016/0092-8674(90)90452-k. [DOI] [PubMed] [Google Scholar]
- Chardin P., Madaule P., Tavitian A. Coding sequence of human rho cDNAs clone 6 and clone 9. Nucleic Acids Res. 1988 Mar 25;16(6):2717–2717. doi: 10.1093/nar/16.6.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Lim H. H., Lim L. A new member of the ras superfamily, the rac1 homologue from Caenorhabditis elegans. Cloning and sequence analysis of cDNA, pattern of developmental expression, and biochemical characterization of the protein. J Biol Chem. 1993 Jan 5;268(1):320–324. [PubMed] [Google Scholar]
- Costa M., Wilson E. T., Wieschaus E. A putative cell signal encoded by the folded gastrulation gene coordinates cell shape changes during Drosophila gastrulation. Cell. 1994 Mar 25;76(6):1075–1089. doi: 10.1016/0092-8674(94)90384-0. [DOI] [PubMed] [Google Scholar]
- Cutforth T., Rubin G. M. Mutations in Hsp83 and cdc37 impair signaling by the sevenless receptor tyrosine kinase in Drosophila. Cell. 1994 Jul 1;77(7):1027–1036. doi: 10.1016/0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]
- Dickson B., Sprenger F., Morrison D., Hafen E. Raf functions downstream of Ras1 in the Sevenless signal transduction pathway. Nature. 1992 Dec 10;360(6404):600–603. doi: 10.1038/360600a0. [DOI] [PubMed] [Google Scholar]
- Didsbury J., Weber R. F., Bokoch G. M., Evans T., Snyderman R. rac, a novel ras-related family of proteins that are botulinum toxin substrates. J Biol Chem. 1989 Oct 5;264(28):16378–16382. [PubMed] [Google Scholar]
- Ellis M. C., O'Neill E. M., Rubin G. M. Expression of Drosophila glass protein and evidence for negative regulation of its activity in non-neuronal cells by another DNA-binding protein. Development. 1993 Nov;119(3):855–865. doi: 10.1242/dev.119.3.855. [DOI] [PubMed] [Google Scholar]
- Fischer-Vize J. A., Rubin G. M., Lehmann R. The fat facets gene is required for Drosophila eye and embryo development. Development. 1992 Dec;116(4):985–1000. doi: 10.1242/dev.116.4.985. [DOI] [PubMed] [Google Scholar]
- Fortini M. E., Simon M. A., Rubin G. M. Signalling by the sevenless protein tyrosine kinase is mimicked by Ras1 activation. Nature. 1992 Feb 6;355(6360):559–561. doi: 10.1038/355559a0. [DOI] [PubMed] [Google Scholar]
- Gaul U., Mardon G., Rubin G. M. A putative Ras GTPase activating protein acts as a negative regulator of signaling by the Sevenless receptor tyrosine kinase. Cell. 1992 Mar 20;68(6):1007–1019. doi: 10.1016/0092-8674(92)90073-l. [DOI] [PubMed] [Google Scholar]
- Greenwald I., Rubin G. M. Making a difference: the role of cell-cell interactions in establishing separate identities for equivalent cells. Cell. 1992 Jan 24;68(2):271–281. doi: 10.1016/0092-8674(92)90470-w. [DOI] [PubMed] [Google Scholar]
- Habets G. G., Scholtes E. H., Zuydgeest D., van der Kammen R. A., Stam J. C., Berns A., Collard J. G. Identification of an invasion-inducing gene, Tiam-1, that encodes a protein with homology to GDP-GTP exchangers for Rho-like proteins. Cell. 1994 May 20;77(4):537–549. doi: 10.1016/0092-8674(94)90216-x. [DOI] [PubMed] [Google Scholar]
- Hafen E., Basler K., Edstroem J. E., Rubin G. M. Sevenless, a cell-specific homeotic gene of Drosophila, encodes a putative transmembrane receptor with a tyrosine kinase domain. Science. 1987 Apr 3;236(4797):55–63. doi: 10.1126/science.2882603. [DOI] [PubMed] [Google Scholar]
- Hall A. The cellular functions of small GTP-binding proteins. Science. 1990 Aug 10;249(4969):635–640. doi: 10.1126/science.2116664. [DOI] [PubMed] [Google Scholar]
- Hariharan I. K., Carthew R. W., Rubin G. M. The Drosophila roughened mutation: activation of a rap homolog disrupts eye development and interferes with cell determination. Cell. 1991 Nov 15;67(4):717–722. doi: 10.1016/0092-8674(91)90066-8. [DOI] [PubMed] [Google Scholar]
- Harrison S. D., Travers A. A. The tramtrack gene encodes a Drosophila finger protein that interacts with the ftz transcriptional regulatory region and shows a novel embryonic expression pattern. EMBO J. 1990 Jan;9(1):207–216. doi: 10.1002/j.1460-2075.1990.tb08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart M. J., Eva A., Evans T., Aaronson S. A., Cerione R. A. Catalysis of guanine nucleotide exchange on the CDC42Hs protein by the dbl oncogene product. Nature. 1991 Nov 28;354(6351):311–314. doi: 10.1038/354311a0. [DOI] [PubMed] [Google Scholar]
- Hay B. A., Wolff T., Rubin G. M. Expression of baculovirus P35 prevents cell death in Drosophila. Development. 1994 Aug;120(8):2121–2129. doi: 10.1242/dev.120.8.2121. [DOI] [PubMed] [Google Scholar]
- Hortsch M., Bieber A. J., Patel N. H., Goodman C. S. Differential splicing generates a nervous system-specific form of Drosophila neuroglian. Neuron. 1990 May;4(5):697–709. doi: 10.1016/0896-6273(90)90196-m. [DOI] [PubMed] [Google Scholar]
- Karpen G. H., Spradling A. C. Analysis of subtelomeric heterochromatin in the Drosophila minichromosome Dp1187 by single P element insertional mutagenesis. Genetics. 1992 Nov;132(3):737–753. doi: 10.1093/genetics/132.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel B. E., Heberlein U., Rubin G. M. The homeo domain protein rough is expressed in a subset of cells in the developing Drosophila eye where it can specify photoreceptor cell subtype. Genes Dev. 1990 May;4(5):712–727. doi: 10.1101/gad.4.5.712. [DOI] [PubMed] [Google Scholar]
- Knaus U. G., Heyworth P. G., Evans T., Curnutte J. T., Bokoch G. M. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science. 1991 Dec 6;254(5037):1512–1515. doi: 10.1126/science.1660188. [DOI] [PubMed] [Google Scholar]
- Lai Z. C., Rubin G. M. Negative control of photoreceptor development in Drosophila by the product of the yan gene, an ETS domain protein. Cell. 1992 Aug 21;70(4):609–620. doi: 10.1016/0092-8674(92)90430-k. [DOI] [PubMed] [Google Scholar]
- Luo L., Liao Y. J., Jan L. Y., Jan Y. N. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 1994 Aug 1;8(15):1787–1802. doi: 10.1101/gad.8.15.1787. [DOI] [PubMed] [Google Scholar]
- Madaule P., Axel R., Myers A. M. Characterization of two members of the rho gene family from the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1987 Feb;84(3):779–783. doi: 10.1073/pnas.84.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses K., Ellis M. C., Rubin G. M. The glass gene encodes a zinc-finger protein required by Drosophila photoreceptor cells. Nature. 1989 Aug 17;340(6234):531–536. doi: 10.1038/340531a0. [DOI] [PubMed] [Google Scholar]
- Olivier J. P., Raabe T., Henkemeyer M., Dickson B., Mbamalu G., Margolis B., Schlessinger J., Hafen E., Pawson T. A Drosophila SH2-SH3 adaptor protein implicated in coupling the sevenless tyrosine kinase to an activator of Ras guanine nucleotide exchange, Sos. Cell. 1993 Apr 9;73(1):179–191. doi: 10.1016/0092-8674(93)90170-u. [DOI] [PubMed] [Google Scholar]
- Parks S., Wieschaus E. The Drosophila gastrulation gene concertina encodes a G alpha-like protein. Cell. 1991 Jan 25;64(2):447–458. doi: 10.1016/0092-8674(91)90652-f. [DOI] [PubMed] [Google Scholar]
- Pirrotta V. Transvection and long-distance gene regulation. Bioessays. 1990 Sep;12(9):409–414. doi: 10.1002/bies.950120903. [DOI] [PubMed] [Google Scholar]
- Renfranz P. J., Benzer S. Monoclonal antibody probes discriminate early and late mutant defects in development of the Drosophila retina. Dev Biol. 1989 Dec;136(2):411–429. doi: 10.1016/0012-1606(89)90267-4. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992 Aug 7;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Paterson H. F., Johnston C. L., Diekmann D., Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992 Aug 7;70(3):401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Robinow S., White K. The locus elav of Drosophila melanogaster is expressed in neurons at all developmental stages. Dev Biol. 1988 Apr;126(2):294–303. doi: 10.1016/0012-1606(88)90139-x. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Schejter E. D., Shilo B. Z. The Drosophila EGF receptor homolog (DER) gene is allelic to faint little ball, a locus essential for embryonic development. Cell. 1989 Mar 24;56(6):1093–1104. doi: 10.1016/0092-8674(89)90642-9. [DOI] [PubMed] [Google Scholar]
- Settleman J., Albright C. F., Foster L. C., Weinberg R. A. Association between GTPase activators for Rho and Ras families. Nature. 1992 Sep 10;359(6391):153–154. doi: 10.1038/359153a0. [DOI] [PubMed] [Google Scholar]
- Simon M. A., Bowtell D. D., Dodson G. S., Laverty T. R., Rubin G. M. Ras1 and a putative guanine nucleotide exchange factor perform crucial steps in signaling by the sevenless protein tyrosine kinase. Cell. 1991 Nov 15;67(4):701–716. doi: 10.1016/0092-8674(91)90065-7. [DOI] [PubMed] [Google Scholar]
- Simon M. A., Dodson G. S., Rubin G. M. An SH3-SH2-SH3 protein is required for p21Ras1 activation and binds to sevenless and Sos proteins in vitro. Cell. 1993 Apr 9;73(1):169–177. doi: 10.1016/0092-8674(93)90169-q. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982 Oct 22;218(4570):341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Steller H., Fischbach K. F., Rubin G. M. Disconnected: a locus required for neuronal pathway formation in the visual system of Drosophila. Cell. 1987 Sep 25;50(7):1139–1153. doi: 10.1016/0092-8674(87)90180-2. [DOI] [PubMed] [Google Scholar]
- Tautz D., Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989 Aug;98(2):81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Tomlinson A., Ready D. F. Cell fate in the Drosophila ommatidium. Dev Biol. 1987 Sep;123(1):264–275. doi: 10.1016/0012-1606(87)90448-9. [DOI] [PubMed] [Google Scholar]
- Tomlinson A., Ready D. F. Neuronal differentiation in Drosophila ommatidium. Dev Biol. 1987 Apr;120(2):366–376. doi: 10.1016/0012-1606(87)90239-9. [DOI] [PubMed] [Google Scholar]
- Tsuda L., Inoue Y. H., Yoo M. A., Mizuno M., Hata M., Lim Y. M., Adachi-Yamada T., Ryo H., Masamune Y., Nishida Y. A protein kinase similar to MAP kinase activator acts downstream of the raf kinase in Drosophila. Cell. 1993 Feb 12;72(3):407–414. doi: 10.1016/0092-8674(93)90117-9. [DOI] [PubMed] [Google Scholar]
- Wu C. T., Goldberg M. L. The Drosophila zeste gene and transvection. Trends Genet. 1989 Jun;5(6):189–194. doi: 10.1016/0168-9525(89)90074-7. [DOI] [PubMed] [Google Scholar]
- Yang Z., Watson J. C. Molecular cloning and characterization of rho, a ras-related small GTP-binding protein from the garden pea. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8732–8736. doi: 10.1073/pnas.90.18.8732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipursky S. L., Venkatesh T. R., Teplow D. B., Benzer S. Neuronal development in the Drosophila retina: monoclonal antibodies as molecular probes. Cell. 1984 Jan;36(1):15–26. doi: 10.1016/0092-8674(84)90069-2. [DOI] [PubMed] [Google Scholar]