Abstract
Natural killer (NK) cells are key regulators of innate defense against mouse cytomegalovirus (MCMV). Like NK cells, NKT cells also produce high levels of IFNγ rapidly after MCMV infection. However, whether similar mechanisms govern activation of these two cell-types, and the significance of NKT cells for host resistance, remain unknown. Here we show that although both NKT and NK cells are activated via cytokines, their particular cytokine requirements differ significantly, in vitro and in vivo. IL-12 is required for NKT cell activation in vitro, but is not sufficient, while NK cells have the capacity to be activated more promiscuously in response to individual cytokines from innate cells. In line with these results, GM-CSF-derived DC activated only NK cells upon MCMV infection, consistent with their virtual lack of IL-12 production, while Flt3L-derived DC produced IL-12 and activated both NK and NKT. In vivo, NKT cell activation was abolished in IL-12−/− mice infected with MCMV, while NK cells were still activated. In turn, splenic NK cell activation was more IL-18 dependent. The differential requirements for IL-12 and IL-18 correlated with the levels of cytokine receptor expression by NK and NKT cells. Finally, mice lacking NKT cells showed reduced control of MCMV, and depleting NK cells further enhanced viral replication. Taken together, our results show that NKT and NK cells have differing requirements for cytokine-mediated activation and both can contribute non-redundantly to MCMV defense, revealing that these two innate lymphocyte subsets function together to fine-tune antiviral responses.
INTRODUCTION
Host defense to mouse cytomegalovirus (MCMV, a β-herpesvirus) involves multiple cell types of both the innate and adaptive immune systems (1). Two innate-like lymphocyte populations, natural killer (NK) cells and natural killer T (NKT) cells; are major producers of IFNγ early during MCMV infection (2, 3). NKT cells are a T lymphocyte subset that is characterized by expression of an invariant T cell antigen receptor (TCR) α chain, formed by a Vα14 to Jα18 rearrangement in mice. When paired with several β chains, prominently Vβ8.2, this α chain imparts specificity for glycolipids presented by CD1d, a class I-like antigen-presenting molecule. These cells are commonly referred to as Type I or invariant natural killer T (iNKT) cells. The iNKT cell TCR is capable of recognizing several types of glycolipid antigens derived from microbial, environmental or endogenous sources (4-7). While NK cells are truly innate lymphocytes, we refer to iNKT cells as innate-like cells, because although they are bona fide T cells that mature in the thymus, they carry out very rapid effector responses.
In addition to TCR/CD1d-dependent activation of iNKT cells, antigen-independent activation of these lymphocytes can also occur, for example in response to viruses or TLR ligands, and this typically results in their exclusive production of IFNγ, while TCR stimulations results in IL-4 and other cytokines as well. This ‘indirect’ iNKT cell activation has been shown to involve dendritic cells (DC), and potentially other antigen presenting cells (APC), which produce cytokines in response TLR-triggering (3, 8-10). To this point, the key cytokines promoting iNKT cell activation in these experimental systems have been IL-12, IL-18, and type I interferons (IFN-I), with IL-12 playing a dominant role. This also holds true in the case of MCMV infection, with IL-12−/− mice being severely compromised for iNKT activation, while IL-18 or IFN-I signaling-deficient mice show only very modest reductions (3, 10). In these cases, where no foreign lipid antigen is present to contribute to iNKT activation, IL-12 may synergize with TCR recognition of self-antigens presented by CD1d (11). However, iNKT cells are activated normally in MCMV-infected mice where CD1d expression is lacking or blocked (3, 10), providing evidence for a purely cytokine-driven activation process.
Despite being distinct cell lineages, NK and iNKT cells are similar in some respects, including the shared expression of NK receptors, dependence upon IL-15 signaling, overlapping tissue localization and the ability to rapidly release copious amounts of cytokines, particularly IFNγ, following infection. Consistent with these shared properties, both cell types have been implicated in the immune response to viral, bacterial, and parasitic infections. Further linkage comes from evidence showing that iNKT cells promote the secondary activation of NK cells through iNKT cell expression of CD40L, which mediates the activation of APC (9, 12). Taken together, these data suggest that iNKT and NK cells could play similar, and potentially redundant, roles in innate defense to infection.
The critical role of NK-mediated protection during MCMV infection has been studied extensively (13, 14). In C57BL/6 (B6) mice, recognition of the MCMV m157 protein by NK cells expressing the Ly49H activating receptor results in enhanced control of early replication (15). In turn, the lack of a Ly49H homologue in BALB/c mice results in significantly enhanced levels of early MCMV replication (16). At very early times of MCMV infection (8-12h), a burst of IFN-I synthesis emanating from infected stromal cells promotes the activation of NK cell cytolytic activity (17-19). By ~36h, MCMV has completed its first replication cycle, and subsequently triggers DC populations to produce IL-12 and IL-18, in addition to a second wave of IFN-I (20, 21). While iNKT do show markers of activation at 12h (3), it is only at ~36h when a large proportion of NK and iNKT produce IFNγ (2, 3, 10). Notably, plasmacytoid DC (pDC) are the primary source of innate cytokines at 36h leading to NK and iNKT IFNγ production, through TLR9-dependent recognition of MCMV (3, 22, 23).
In this study, we explored the regulation of NK and iNKT cells by APC-produced cytokines, and determined if they have distinct roles in antiviral control. We find that iNKT cells and NK cells do have different cytokine-mediated activation requirements, and they contribute to MCMV innate defense in a non-redundant fashion.
MATERIALS AND METHODS
Ethics Statement
This study was carried out in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocols were approved by the Institutional Animal Care and Use Committee of the La Jolla Institute for Allergy & Immunology (AP112-MK2-0410 and AP087-CB2-0110). Our institute and animal care committee adheres to NIH OLAW (office of lab animal welfare) and AAALAC guidelines. Our institute's OLAW assurance number is A3779-01 and our AAALAC accreditation number is #000840.
Mice
C57BL/6 (B6), B6.129S1-Il12btm1Jm/J (IL-12p40−/−), B6.129P2-Il18tm1Aki/J (IL-18−/−) and BALB/c mice were purchased from The Jackson Laboratory. CD1d1–/– mice were a kind gift from Dr. L. Van Kaer (Vanderbilt University, Nashville TN). Tlr9cpg1 mice were a kind gift from Dr. B. Beutler (UT Southwestern, Dallas TX). BALB/c Jα18−/− mice were a kind gift from Dr. M. Taniguchi (Riken Research Center for Allergy and Immunology, Yokohoma, Japan), and were maintained as Jα18+/− heterozygotes. Jα18+/+ and Jα18−/− littermates were used for MCMV infection experiments. 4get mice on the B6 background were a kind gift from Dr. R. Locksley (University of California San Francisco, San Francisco CA). All mice were housed in specific pathogen-free conditions.
Reagents and Abs
mAbs to the following mouse antigens were purchased from BD Biosciences, as purified or conjugates to FITC, Alexa 488, phycoerythrin (PE), PerCP- cyanin (Cy)5.5, PE-Cy7, allophycocyanin, Becton Dickenson Horizon V450, or V500: TCR-β, CD11b, CD8α, NK1.1, CD11c, CD44, CD25, CD69, CD4, CD212 (IL-12 receptor β 1), IL-4, TNF, DX5, and IFNγ. A mAb to CD218a (IL-18 receptor α) conjugated to Alexa Fluor 647 was purchased from BioLegend. PE-conjugated αGalCer-CD1d tetramers were generated in our laboratory as previously described (9) and used to stain cell suspensions. Recombinant mouse IL-12 and IL-18 were purchased from R&D Systems. IFNβ was purchased from PBL Interferon Source.
No touch iNKT cell isolation
Spleens from 4get mice were isolated at 8-10 weeks of age and dissociated into single cell suspensions and RBC were lysed using red cell lysing buffer (Sigma-Aldrich), washed, filtered and counted. Single cell preparations were stained with a custom cocktail of biotin-labeled Abs containing anti-CD8α, CD11b, CD19, CD24, CD62L, B220, F4/80, Gr-1, and Ter119. Labeled cells were loaded onto a RoboSep cell separation system using a custom enrichment reagent kit and protocol according to the manufacturer's instructions (Stemcell Technologies, Vancouver BC). iNKT cells were enriched up to 20% and eGFP bright cells were then sorted by FACSAria II (BD Biosciences). Sorted cells were 99% GFP+ with the purity of the cells collected being typically >91% iNKT cells, as assessed using CD1d tetramers loaded with αGalCer (Supplementary Fig. 1).
Cell preparation and cell culture
All cell preparations were conducted using complete IMDM (Invitrogen Life Technologies) supplemented with 10% FBS, penicillin-streptomycin-glutamine, and 2-ME. DCs were prepared using 2 systems. For GM-CSF derived DCs, bone marrow was harvested and cultured in 10 cm bacterial dishes in complete IMDM supplemented with 20 ng/ml recombinant mouse GM-CSF (provided by Kyowa Hakko Kirin). On day 4, half of the media was replaced with complete IMDM containing GM-CSF (20 ng/ml). GM-CSF derived BMDCs were harvested on Day 7. For Flt3L derived DCs, bone marrow was harvested and cultured with 100 ng/ml recombinant human Flt3L (Amgen) for 8 days in T75 flasks at a concentration of 45×106 cells/25 ml. Special attention was paid not to disturb the cultures during the entire incubation period. DCs were treated with 10 μg/ml type B CpG ODN 1826, control ODN 1982 (Alexis Biochemicals), infected with MCMV Smith strain (ATCC VR-1399) at a multiplicity of infection of 3, or mock infected, for 2 h. A total of 2.5 × 105 BMDCs were cultured with or without 5 × 104 sorted iNKT cells or NK cells for 48 h in a volume of 200ul in a round bottom 96 well plate at 37 ° C and 5% CO2 before cytokine detection as described (24). Cell-cell contact assays were performed by culturing 5 × 104 sorted iNKT cells or NK cells with 200ul of conditioned BM DCs culture supernatant. MCMV stocks were prepared from NIH-3T3 fibroblasts. NK Cells were purified from splenocytes using NK1.1 PE labeled mAb followed by positive selection on RoboSep cell separation system with a PE Selection kit (Stemcell Technologies) and then sorted for NK1.1+ TCRβ− cells on a FACSAria II. NK cells were typically >98% pure.
ELISA
A standard sandwich ELISA was performed to measure mouse IFNγ, IL-12p70, (R&D Systems), IL-18 (MBL International Corporation) and Type I IFNs (PBL Interferon Source) following the manufacturer's instructions.
Viral infections
Salivary gland stocks of MCMV Smith strain were prepared essentially as described (25). BALB/c mice were infected with 1 × 104 PFU of virus in 500 μl of PBS via i.p. injection. 4 days post-infection, mice were killed by CO2 asphyxiation and organs were harvested and immediately snap frozen in liquid nitrogen. To determine viral titers, organs were weighed, homogenized and serial dilutions of homogenates were added to monolayers of NIH 3T3 fibroblasts plated in 24 well plates. Plates were spun at 524x g for 10 min prior to incubation at 37°, which increased the sensitivity of the plaque assays ~6-10 fold. Cells were fixed with formalin and plaques were visualized with 0.1% crystal violet and quantified.
Cell depletion
Jα18+/+ BALB/c and Jα18−/− BALB/c mice were depleted of NK cells by injecting 50 μl of anti-asialo-GM1 rabbit polyclonal antibody (Wako) on day -1 of MCMV infection. NK cell depletion was verified in individual mice by flow cytometry after bleeding and just prior to infection, using a DX5-specific mAb, and non-specific cellular depletion was not observed. Additionally, control mice were antibody depleted of CD8+ T cells (clone 2.43). Depletion was verified by flow cytometric staining with anti-CD8 clone 53-6.7 after bleeding and just prior to infection.
Real-Time PCR Analysis
Total RNA was isolated from sorted splenic iNKT cells and NK cells using an RNeasy Plus kit (QIAGEN). After DNase I treatment (Ambion), RNA was reverse transcribed using an iScript cDNA synthesis kit (Bio-Rad). Real-time PCR was performed using SYBR Green (Bio-Rad) on a LightCycler 480 Real-Time PCR System with PCR primers pairs for il-12rb1, il-12rb2, il-18r1, il-18rab, ifnar1, and ifnar2 from SABiosciences RT2 qPCR Primer Assays (QIAGEN). Target gene expression was assessed by comparative cycling threshold analysis with L32 mRNA expression as a control. Data are presented in arbitrary units.
Flow cytometry and intracellular cytokine staining
Lymphocytes were stained with αGalCer/CD1d tetramers labeled with streptavidinallophycocyanin, anti-NK1.1-PerCp PE-cyanin (PECy)5, anti-CD8-PECy7, anti-CD11b-PECy7, anti-TCRβ-allophycocyanin-AF750, and CD25-FITC. All Abs and isotype controls, except anti-TCRβ-allophycocyanin-AF750 (eBioscience), were purchased from BD Biosciences. Cells were fixed and permeabilized using Cytofix/Cytoperm buffer and stained for intracellular IFNγ with PE-labeled clone XMG1.2. The data were collected on a LSR II flow cytometer (BD Biosciences) and analyzed using FlowJo software (Tree Star).
Statistics
Differences between groups were evaluated using appropriate statistical tests as recommended by the La Jolla Institute bioinformatics core facility. Results are expressed as mean ± SD, except where differently indicated. p-values of <0.05 were considered significant.
RESULTS
Infected DC subtypes differ in their activation of i NKT cells
We have previously demonstrated in co-cultures that MCMV infection of Flt3L-expanded bone marrow DC (Flt3L BMDC) promoted purified iNKT cells to secrete IFNγ, but not other cytokines, such as the IL-4 these cells secrete when activated with glycolipid antigens. This did not occur when DCs from IL-12 p40 deficient mice were infected. Together with other studies indicating that IL-12 participates in the activation of iNKT cells (9, 24, 26, 27), these data strongly implicated the action of IL-12 rather than IL-23 in promoting iNKT cell effector function.
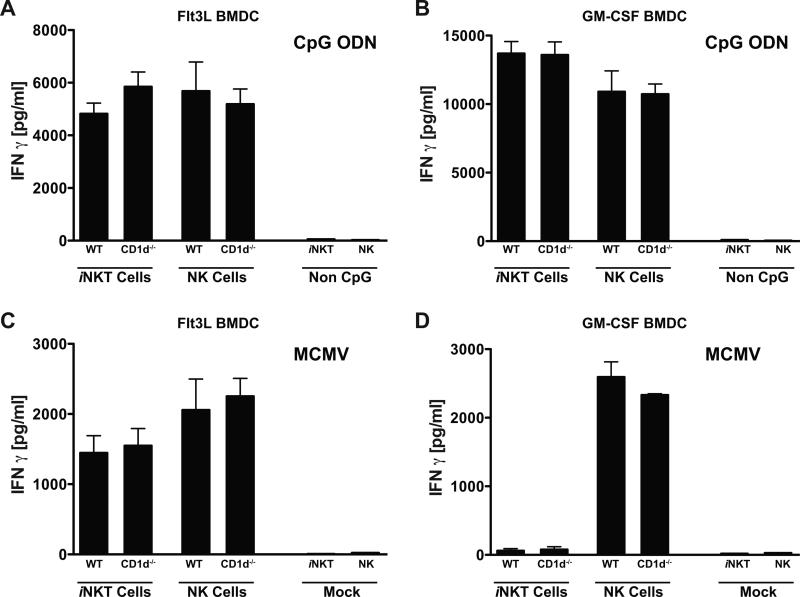
To test whether other DC types can activate iNKT cells upon MCMV infection, DC derived from GM-CSF treated bone marrow cultures were also analyzed. GM-CSF or Flt3L BMDCs were infected with MCMV, treated with CpG oligodeoxynucleotides (ODN), because the activation of IL-12 secretion was shown to be TLR9 dependent, or mock treated prior to the addition of purified iNKT cells or NK cells, and IFNγ production in the culture supernatants was measured ~48h later. To assess whether GM-CSF BMDCs activated iNKT cells in a CD1d-dependent manner, Cd1d1−/− BMDC were also tested. In addition, GM-CSF and Flt3L BMDCs generated from Tlr9cpg/cpg mice were included as negative controls. For Flt3L BMDCs, MCMV infection and CpG ODN treatment resulted in the production of IFNγ by both iNKT cells and NK cells (Fig. 1a, c). This activation by MCMV and CpG ODN was independent of CD1d expression, but required TLR9 (Supplementary Fig. 2), as expected based on our previous observations (3). CpG ODN treatment of GM-CSF BMDCs also resulted in TLR9-dependent IFNγ production by iNKT and NK cells, and again was independent of CD1d (Fig. 1b and Supplementary Fig. 2). However, when GM-CSF BMDCs were infected with MCMV, only NK cells were induced to secrete IFNγ, while iNKT cells were unresponsive (Fig. 1d).
Figure 1.
IFNγ production by iNKT and NK cells in DC co-cultures. Flt3L (A) or GM-CSF (B) derived BMDC from the indicated mouse strains were treated with CpG-ODN or control treated and then cultured with purified iNKT cells or NK cells for 48h. Flt3L (C) or GM-CSF (D) derived BMDC from the indicated mouse strains were mock or MCMV infected and cultured with purified iNKT cells or NK cells for 48h. Supernatants were analyzed for IFNγ by ELISA, and shown is a representative experiment of 6 performed. ELISA results represent the mean of one experiment with three replicate cultures measured in triplicate. Error bars represent SEM (n = 9 for each set of BMDC).
Cell contact is not required for i NKT cell activation by MCMV infected DC
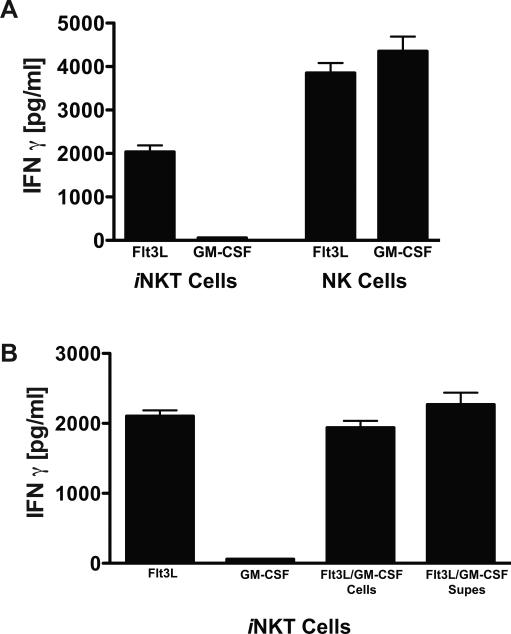
MCMV has developed numerous mechanisms to avoid detection by the immune system (1). We have previously observed that MCMV-infected GM-CSF BMDCs potently inhibit T cell proliferation through viral modulation of cosignaling pathways (28), raising the possibility that iNKT cell activation may be similarly suppressed by MCMV. To test this, or any other mechanism dependent on cell contact, supernatants from MCMV infected Flt3L or GM-CSF derived BMDCs were evaluated for their ability to activate iNKT cells. Supernatants from Flt3L BMDCs activated both NK cells and iNKT cells, indicating that cell contact was not required for the induction of IFNγ synthesis by these innate lymphocytes. By contrast, the supernatant from GM-CSF BMDCs only activated NK cells but not iNKT cells (Fig. 2a). To assess if a soluble factor released from MCMV-infected GM-CSF BMDCs was inhibiting iNKT cell activation, Flt3L and GM-CSF BMDC-derived supernatants were mixed prior to iNKT cell addition. Even at a 10:1 (GM-CSF:Flt3L) ratio of BMDC supernatants, iNKT cells were still activated, demonstrating the absence of a potent inhibitor, and therefore strongly suggesting an activating factor was absent in the supernatants derived from MCMV-infected GM-CSF BMDCs (Fig. 2b).
Figure 2.
Activation of iNKT and NK cells by MCMV-infected DC does not require cell-cell contact. (A), IFNγ production by iNKT cells or NK cells cultured for 48h with supernatants from Flt3L or GM-CSF BMDC infected with MCMV. (B), iNKT cells were cultured with individual supernatants, a 1:1 mixture of the two APC types (Flt3L/GM-CSF cells) or with a 10:1 ratio of supernatants from MCMV-infected DC (Flt3L/GM-CSF supes). IFNγ ELISA results represent the mean of one experiment with 2 replicate cultures measured in triplicate. Error bars represent SEM (n = 6 for each culture condition). Shown is a representative experiment of 3 performed.
Infected DC subtypes produce different cytokines
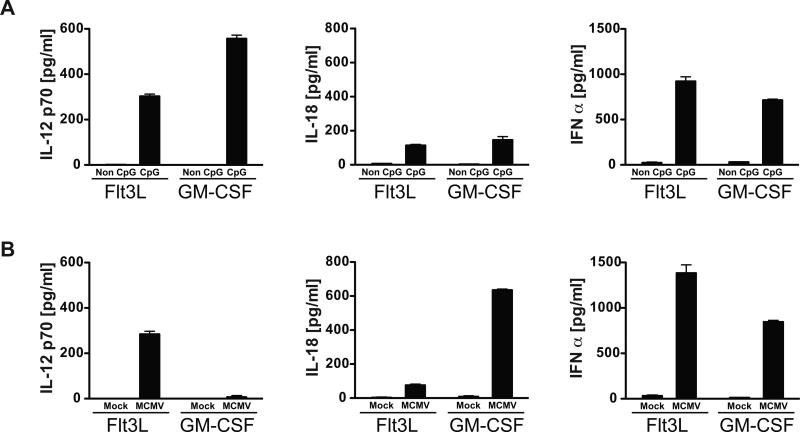
Our results indicated that a secreted factor(s) required for activation of iNKT cells was absent in supernatants from GM-CSF BMDCs infected with MCMV. Our previous work, and that of others, has indicated that IL-12, IL-18 and IFN-I can all contribute to the activation of iNKT cells (3, 9, 10), suggesting that the absence of one or more of these factors could be responsible. To address this, Flt3L and GM-CSF BMDCs were treated with CpG ODN and/or infected with MCMV prior to isolation of supernatants for analysis of IL-12p70, IL-18 and IFNα by ELISA. Following stimulation with CpG ODN, both Flt3L and GM-CSF DCs produced approximately similar amounts of these cytokines (Fig. 3a). However, when GM-CSF derived BMDCs were infected with MCMV, virtually no IL-12 was produced. By contrast, IFNα production was similar for the two infected DC types, and the amount of IL-18 was significantly higher from infected GM-CSF DCs compared to Flt3L DC (Fig. 3b). Importantly, use of an MCMV-GFP virus indicated that the infection efficiency of these two BMDC cultures was similar (data not shown), a fact also reflected by the high level production of IL-18 and IFNα by both BMDC types. In summary, although GM-CSF DCs have the capacity to produce IL-12 upon TLR9 activation with CpG ODN, they produced virtually none when exposed to MCMV, despite producing both IFN-I and IL-18 at high levels in response to viral infection.
Figure 3.
Cytokine production by DCs in response to CpG or MCMV. (A), Flt3L or GM-CSF BMDC were treated with CpG or control ODN (Non) for 48h, and supernatants were analyzed for IL-12 p70, IL-18 or IFNα by ELISA. (B), Same as in (A), but DC subtypes were mock or MCMV infected prior to analysis of cytokine levels by ELISA. Data are representative of 4 independent experiments. Results show the mean of one experiment with 3 replicate cultures of DC measured in duplicate for ELISA. Error bars represent SEM (n = 6 for each culture condition).
iNKT cells and NK cells differ in their sensitivity to cytokines
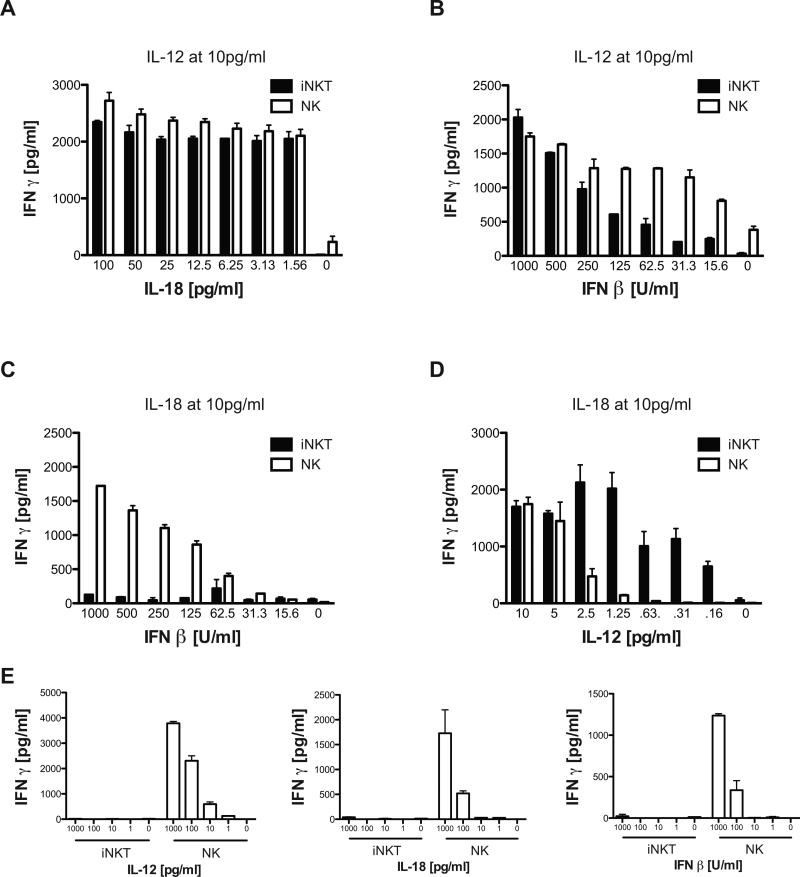
The data obtained from MCMV infection of the two DC subtypes suggested that iNKT cells are more dependent on IL-12 for their activation, while for NK cells, IL-12 may not be essential if other cytokines from innate immune cells are present. To test this, IFNγ production by purified NK and iNKT cell populations was measured after treatment with varying amounts of recombinant cytokines in the absence of DC. When the concentrations of IL-12 or IL-18 were limiting (<10 pg/ml), these two cytokines functioned synergistically to activate both iNKT cells and NK cells to produce IFNγ. For example, when iNKT cells and NK cells were cultured with 10 pg/ml of IL-12, both cell types produced large, and roughly equivalent amounts of IFNγ across a wide range of IL-18 concentrations (Fig 4a). These data suggest that this dose of IL-12, with IL-18 at or below the detection limit of the ELISA assay, induced a maximal response of both iNKT and NK cells. In contrast, fixing the IL-18 at 10 pg/ml and titrating in very limiting amounts of IL-12 (~0.1-10 pg/ml), revealed that iNKT cells were even more sensitive than NK cells to synergistic activation by combinations of these two cytokines (Fig 4d).
Figure 4.
Activation threshold of purified iNKT and NK cells to cytokines. (A-B), Purified iNKT cells or NK cells were cultured with 10 pg/ml IL-12 and serially diluted amounts of IL-18 (A) or IFNβ (B). C-D, Purified iNKT cells or NK cells were cultured with 10 pg/ml IL-18 and serially diluted IFNβ (C) or IL-12 (D). E, Purified iNKT cells or NK cells were cultured with varying doses of IL-12, IL-18 or IFNβ. 48h later, supernatants were analyzed for IFNγ by ELISA. Data are representative of 3 independent experiments and show the mean of one experiment with 3 replicate cultures measured in triplicate for ELISA. Error bars represent SEM (n = 9 for each culture condition).
When a dose titration of IFNβ was performed in the presence of 10 pg/ml IL-12, similar to what was done for IL-18, an increase in IFNγ production by both NK cells and iNKT cells was again observed. However, in these circumstances, NK cells were more sensitive to the synergistic effects of IFNβ and IL-12 than iNKT cells (Fig. 4b). IFNβ alone could not induce IFNγ secretion from iNKT cells, regardless of the dose used, while it could promote NK cells to secrete IFNγ when added at higher levels (Fig. 4e, right panel). Surprisingly, a similar observation was made with IL-18 and IL-12 (Fig. 4e). Neither of these cytokines alone, even at 1ng/ml, could activate purified iNKT cells to secrete significant levels of IFNγ. In contrast, NK cells activated by IL-18 or IL-12 alone produced high levels of IFNγ, even at doses as low as 100 pg/ml and 10 pg/ml, respectively (Fig. 4e). To attempt and exclude a potential contribution of anti-NK1.1 mAb treatment on ‘priming’ NK cell activation during the purification process, several of these experiments were repeated using DX5+CD3- cells isolated from spleens, and similar results were obtained (data not shown). Additionally, NK and iNKT cells were purified by cell sorting from BALB/c mice using similar antibody staining panels to discriminate them (+/− TCRβ expression). As we observed for C57BL/6 mice, BALB/c NK cells produced IFNγ when single innate cell-derived cytokines were added to cultures, while iNKT remained unresponsive even at high doses of either IL-12 or IL-18 alone (data not shown).
We also determined if iNKT cells secrete IFNγ in response to IFNβ and IL-18. Purified iNKT and NK cells were cultured with 10pg/ml of IL-18, a dose that synergizes with IL-12 to induce their robust activation (Fig. 4d), together with varying amounts of IFNβ. Interestingly, iNKT cells were not activated in response to the combination of IL-18 + IFNβ, while NK cells were markedly stimulated (Fig. 4c). IFNγ secretion by NK and iNKT cells has been associated with the ability of IL-12 to induce STAT4 activation (29, 30). It has been previously demonstrated that NK cells express a high basal level of STAT4 bound to the IFN-I receptor, and upon exposure to IFN-I, STAT4 is displaced leading to IFNγ secretion (29). In agreement with these findings, when NK cells were exposed to high doses of IFNβ, IL-12, or IL-18, STAT4 phosphorylation was detectable within 1 hr (Supplementary Fig. 3a). Meanwhile, STAT4 phosphorylation in iNKT cells was also detectable at high doses of IL-12 (1 ng/ml), giving a bimodal pattern seen in an earlier study (30). However, this was absent at lower IL-12 concentrations (10pg/ml), which still robustly activated STAT4 phosphorylation in NK cells, and no STAT4 phosphorylation was observed in iNKT cells cultured with high doses of IL-18 or IFNβ alone (Supplementary Fig. 3b). However, STAT4 phosphorylation was detectable in iNKT cells at low IL-12 concentrations when combined with IL-18 or IFNβ. Consequently, the pattern of STAT4 phosphorylation in these two cell types reflects their requirements for the induction of IFNγ synthesis. Taken together, these results indicate iNKT cells and NK cells have different cytokine requirements to activate their production of IFNγ. These results also explain the inability of MCMV infected GM-CSF BMDCs to activate iNKT cells, despite high levels of virus-induced IFNβ and IL-18 production, as IL-12 production was absent.
Differential sensitivity of NK and iNKT cells to IL-12 in vivo
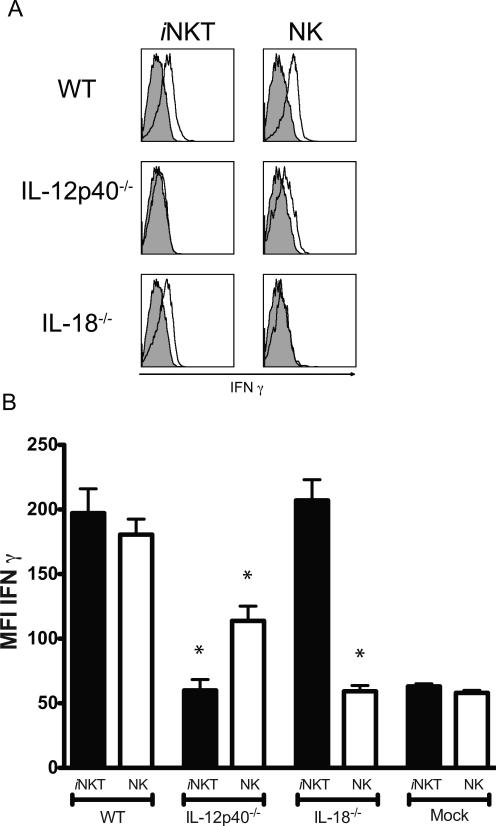
The amounts of IL-12 and IL-18 induced at ~36h after MCMV infection in the sera are in the ng/ml range (31), far above the limiting amounts used to stimulate purified iNKT and NK cells in our in vitro experiments shown in figure 4. IFNβ also reaches high levels in the serum at 36h, but also exhibits a much earlier peak at 6-8h in response to MCMV infection of splenic stromal cells (32). Because purified NK cells produced IFNγ in response to treatment with any one of the single cytokines at ng/ml doses (Fig. 4e), we postulated that in mice genetically deficient for only one of the cytokines, when compared to iNKT cells, the NK cells might show enhanced activation upon MCMV infection in vivo. Consistent with previous results (3, 10), in MCMV infected mice iNKT cell IFNγ synthesis was strictly dependent on IL-12 when measured by intracellular cytokine staining on cells analyzed directly ex vivo. Although the NK cell response was decreased in the absence of IL-12, these cells still produced measurable levels of IFNγ in the spleen following MCMV infection (Fig. 5a, b). In contrast, spleen NK cells were unable to produce IFNγ in the absence of IL-18, while iNKT cell IFNγ synthesis was not diminished (Fig. 5). Therefore, the cytokine requirements for the activation of NK cells to secrete IFNγ in vivo may be more stringent than when these cells are purified and cultured with cytokine. Interestingly, NK cells isolated from the livers of infected mice retained the ability to secrete IFNγ in the absence of IL-18, in agreement with previous studies (31), although the response was reduced compared to WT controls (data not shown).
Figure 5.
iNKT and NK cell activation following MCMV infection in vivo. (A), IFNγ expression by NK and iNKT cells from the spleen of indicated mouse strains 36h post-infection with MCMV (open histograms) compared with isotype controls (shaded histograms). Histograms are representative plots of three independent experiments of 3–8 mice per group. B, IFNγ mean fluorescence intensity (MFI) of 3–8 mice per group 36h post-infection with MCMV from a minimum of three independent experiments. Statistically significant differences using the equal variance Student t test are indicated with an asterisk (*, p≤ 0.01) comparing the responses of either WT NK or iNKT cells, respectively, to the corresponding responses in either of the mutant strains. Error bars represent SEM (n = 3–8 for each group).
Expression of cytokine receptors by NK and i NKT
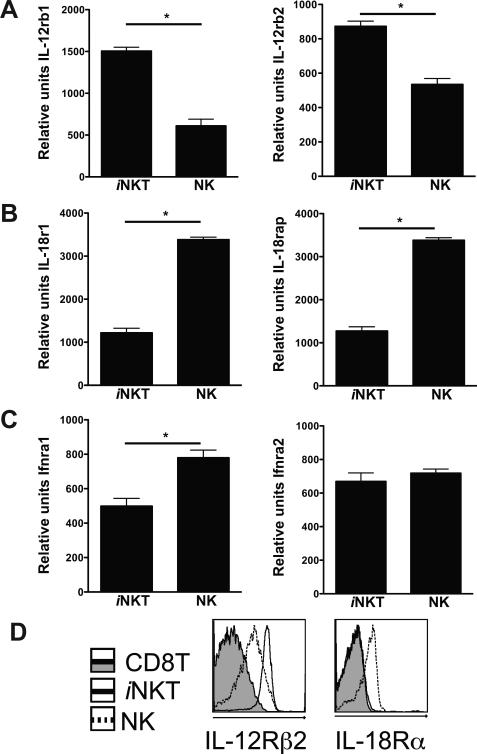
One possible explanation for the differing sensitivity of NK cells and iNKT cells to combinations of IL-12, IL-18 and IFN-I could be varying levels of cytokine receptor expression. Quantitative PCR analysis of cytokine receptor mRNA levels, as well as protein expression for those receptors where antibodies are available, was performed in these two cell populations. When compared to NK cells, both il-12rb1 and il-12rb2 expression levels were higher in freshly purified iNKT cells (~2 and 3 fold, respectively) (Fig. 6a), while il-18rb1 and il-18rap expression was approximately three-fold lower (Fig. 6b). Expression of ifnar1 was approximately two-fold higher in NK cells compared to iNKT cells, although the two cell populations expressed similar levels of ifnar2 (Fig. 6c). To verify if the increase in mRNA expression correlated with protein, IL-12Rβ2 and IL-18Rα expression was analyzed by flow cytometry on iNKT and NK cells (Fig. 6d). While IL-12Rβ2 was readily detectable on tetramer− /CD3− NK cells, the highest level of expression was on tetramer+ / CD3+ iNKT cells. IL-12Rβ1 receptor expression was not detectable on MHC class I-reactive CD8+ αβ or γδ T cells. Expression of the IL-18Rα chain was detectable on NK cells, but this was not the case for either iNKT cells, conventional αβ T cells or γδ T cells in the spleen (Fig 6d) (30, 33). Analysis of IL-12 and IL-18 receptor chain expression in iNKT and NK cells isolated from BALB/c mice revealed identical results to those seen in C57BL/6, suggesting that the differential sensitivity of these two cell populations to these innate cytokines may be a general character (data not shown). These trends in the amounts of cytokine receptor expression are consistent with the increased sensitivity of iNKT cells to IL-12 in the presence of IL-18, and with the enhanced ability of NK cells to respond to IL-18 + IFN-I. However, the results do not exclude the contribution of additional factors to the differential cytokine sensitivity of the two lymphocyte populations.
Figure 6.
Cytokine receptor expression by iNKT and NK cells. (A-C), Purified splenic iNKT or NK cells were analyzed by quantitative real-time PCR for their expression of IL-12 (A), IL-18 (B) and IFN-I (C) receptor mRNAs. Bar graphs show each receptor chain or receptor associated protein mRNA levels relative to the L32 ribosomal protein gene expression. Differences between groups were evaluated for statistical significance by the two-tailed unpaired Student t test. Data are the mean of 2 independent experiments performed in duplicate. Error bars represent SEM (n=4); =p<0.05. (D), Flow cytometric analysis of CD19− splenocytes for IL-12Rβ2 (left panel) and IL-18Rα (right panel) expression on tetramer+ iNKT cells (solid line), tetramer− NK cells (dashed line) and tetramer− αβ and γδ CD8+ T cells (shaded histogram). Histograms are representative plots of 3 mice per group from 2 independent experiments.
i NKT cells contribute to anti-viral innate defense
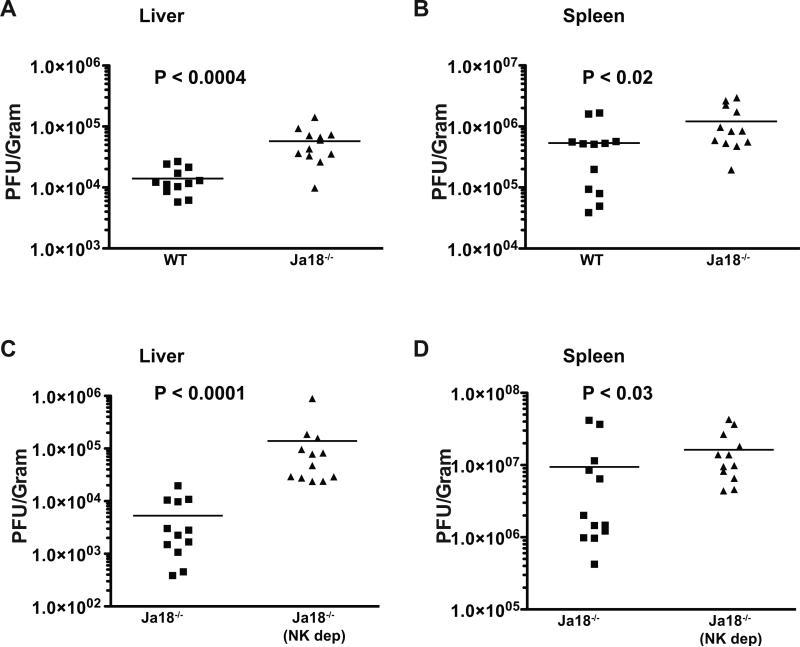
Previous reports have indicated that mice genetically deficient for iNKT cells (Jα18−/−) can control MCMV replication normally (10, 34). However, this has only been examined in B6 mice, which have very robust NK cell-mediated MCMV defenses due to expression of the Ly49H activating receptor that interacts with the viral m157 protein (35, 36). In contrast, NK cell-mediated defense against MCMV in BALB/c mice differs significantly from that in B6 mice due to a different expression pattern of activating and inhibitory Ly49 family receptors (37), including a lack of Ly49H. Such differences, including the lack of LY49H, also pertain to many other inbred strains and wild mice as well (38). Consequently, we tested if iNKT cells contribute to MCMV innate defense in BALB/c mice lacking Ly49H by analyzing viral replication in the spleen and liver of Jα18−/− mice and WT littermate controls. We found that Jα18−/− mice had approximately 3-5 fold higher levels of MCMV replication in both these organs, indicating that iNKT cells do play a role in the early control of MCMV replication in some mouse strains (Fig. 7a, b). In separate experiments, we compared MCMV replication in Jα18−/− BALB/c mice that were depleted of NK cells using anti-asialo-GM1 antisera. Depleting NK cells in mice that lack iNKT cells resulted in a further enhancement of viral replication, which was more dramatic in the liver than the spleen (Fig. 7c, d). Since anti-asialo-GM1 antisera can potentially deplete activated CD8+ T cells, we verified that MCMV replication was unaltered in BALB/c mice at day 4 following CD8 depletion using CD8-specific Abs (Supplementary Fig. 4). These data establish that iNKT cells contribute significantly, and non-redundantly, to early or innate defense to MCMV in BALB/c mice.
Figure 7.
iNKT and NK cells mediate non-redundant, innate defense against MCMV. Groups of BALB/c Jα18+/+ iNKT cell-sufficient (WT) or BALB/c Jα18−/− iNKT cell-deficient littermate control mice were infected with MCMV, and viral pfu levels were determined in the liver (A), and spleen (B) 4 days later. (C-D), BALB/c Jα18−/− iNKT cell-deficient mice were depleted of NK cells (NKdep), or not, and subsequently infected with MCMV. MCMV replication levels in the liver (C), and spleen (D) were determined 4 days later. For (A-D), results are representative of 12 mice per group from a minimum of 2 independent experiments. Error bars represent SEM (n = 12 for each group). Differences between groups were evaluated for statistical significance by the two-tailed unpaired Student t test. Note that the data shown in (C) and (D) are from experiments carried out at separate times from those in (A) and (B), and therefore the absolute values of pfu cannot be directly compared.
DISCUSSION
The importance of NK cells for innate immune responses to viral infections is well established, but while iNKT cells also can be activated after exposure to viruses, in the absence of synthetic glycolipid antigen activation, there are relatively few examples in which these responses are protective. Consistent with an important anti-viral role for iNKT cells, however, is the uncontrolled infection in response to the Varicella-zoster vaccine in rare individuals lacking iNKT cells or CD1d and the finding that several viruses down regulate CD1d expression, suggesting evolutionary pressure for an immune evasion mechanism targeting iNKT cells (39-45). Here we show that NK cells and iNKT cells exhibit cytokine-induced activation in response to infection with MCMV, but that their cytokine requirements for activation differ, and that both cell types contribute to host protection. These data not only definitively show that iNKT and NK cells are differentially activated in the context of pathogen infection, perhaps in response to distinct APC subsets or other influences, but indicate they may play distinct roles in the control of infection.
iNKT cells express an invariant TCR that recognizes glycolipids, raising the issue as to how they participate in the immune response to viruses. However, several additional modes of activation allow iNKT cells to be activated by microbes in the absence of a microbial glycolipid presented by CD1d. First, activation can result from the presentation of self-glycolipid antigens by CD1d. The relevant self antigens may including phospholipids and various types of glycosphingolipids (46), but this response also requires IL-12 production by activated APC. iNKT cells activated in this way produce predominantly IFNγ, in contrast to the diverse Th1 and Th2 cytokines that are elicited by strong glycolipid agonists for the TCR (e.g. αGalCer) (6, 27). An exception to the requirement for IL-12 is one study in which APC were activated with CpG ODN; in this case IFN-I was required as the co-factor for iNKT cell activation mediated by self antigen (8).
Second, an additional pathway for iNKT cell activation is purely cytokine driven, and does not require concomitant TCR engagement with any antigen presented by CD1d. In studying MCMV infection, we have shown that activation by TLR9-mediated signaling leads to IL-12 release by APC, resulting in iNKT cell production of IFNγ by ~36h after infection. CD1d expression was not required and IL-18 had a minimal role (3). Wesley et al. reported similar results, but they observed a contribution of IFN-I for optimal iNKT cell activation during MCMV infection. In addition, we have previously seen that iNKT cells increase expression of the activation marker CD69 early as 12h after MCMV infection, which is strictly dependent upon IFN-I produced by MCMV-infected splenic stromal cells (47). The activated iNKT cells do not produce IFNγ at this early time, however, at least as assessed by intracellular cytokine staining directly ex vivo, although it remains to be determined if lower amounts of IFNγ are produced or if other effector functions are acquired. Interestingly, this ‘initial’ IFN-I produced by stromal cells at 8-12h of MCMV infection is sufficient to promote IFNγ production by some NK cells (47), consistent with our results that IFNβ alone can induce IFNγ-production by purified NK cells but not iNKT cells (Fig. 4).
The engagement of activating NK receptors, such as NK1.1, CD94/NKG2C or NKG2D, provides a third, TCR-independent pathway for iNKT cell activation that is illustrative of their innate- or NK cell-like function (48, 49) (50). However, because productive MCMV infection inhibits cell surface expression of ligands for activating NK receptors (51, 52), it is likely this mechanism of iNKT cell activation would be muted or absent when directly encountering virus infected cells.
Although the critical importance of IL-12 for iNKT cell activation has been established, here we have characterized in detail the cytokine activation requirements for iNKT cells compared to NK cells. Interestingly, IL-12 alone did induce iNKT cells to produce IFNγ in vitro. This is in agreement with our earlier findings that both IL-12 and IL-18 were required for iNKT cell stimulation in response to LPS in vitro or in vivo (9). Furthermore, activation induced arrest of iNKT cells in liver sinusoids was also most evident when IL-12 and IL-18 were injected together (53). By contrast, our earlier studies of the response to MCMV demonstrated the importance of IL-12, but the absence of IL-18 had only a marginal effect (3). Here we show that either IL-18 or IFN-I can synergize with IL-12 to activate iNKT cells in vitro. Therefore, in the context of MCMV infection, when high levels of IFN-I are produced, it is likely that IL-18 is largely redundant for iNKT cell activation.
We also now show that the cytokine requirements for NK cell stimulation in vitro to produce IFNγ are different, and generally less stringent, than those for iNKT cells. This is most noticeable in the ability of NK cells to respond to single cytokines when added at higher concentrations, as well as their responses to combinations of either IL-18 + IFNβ or IL-12 + IFNβ. The only exception to this trend was that iNKT cells were more responsive to IL-18 in the presence of low IL-12 levels. The differential cytokine sensitivity of these two cell types correlated with the expression of cytokine receptors, suggesting this could be a contributing factor. Furthermore, our in vivo data are generally consistent with the in vitro results. iNKT cells were unable to produce IFNγ in IL-12p40−/− mice, while NK cells remained partially responsive, presumably due to suboptimal activation by IL-18 and IF–I. Somewhat surprisingly, however, production of IFNγ by NK cells in vivo was found to be highly dependent on IL-18 in the spleen. Based on our in vitro experiments, we would not have predicted this result, as other cytokines from innate cells are present at the time NK cells were analyzed. However, this finding is consistent with previous work showing IL-18 is required for NK cell production of IFNγ in the spleen and for expansion of Ly49H+ NK cells in vivo, as well as for IFNγ production by NK cells co-cultured with MCMV-infected GM-CSF BMDCs (31, 54, 55). It should be noted that while the in vitro work defines the capacity/potential for NK or iNKT cells to respond to minimal cytokine concentrations, negative regulatory influences would be diminished in this set up. For example, when cytokines are added to purified NK cells, the influence of cell surface molecules expressed by APCs acting on NK cell inhibitory receptors will be minimized. Furthermore, local cytokine concentrations may not reflect serum concentrations, and the existence of such local effects is supported by the organ-specific requirement for IL-18 for MCMV-mediated NK cell activation in the spleen, but not the liver (31).
Using two common methods for generating DC populations in vitro, we found that GM-CSF BMDCs were capable of activating NK cells but not iNKT cells when infected with MCMV. The defining difference was not in the efficiency of the infection, or in their ability to generally detect virus and subsequently produce cytokines. Instead, the defect was in the absence of IL-12 in the supernatant following MCMV infection. We do not know why GM-CSF BMDCs do not produce IL-12 after MCMV infection, as they are competent to do so after exposure to CpG ODN. Regardless, as microbes evolve they are able to evade detection in numerous ways, including the blocking of production of critical cytokines from innate cells, and our results are consistent with previous work showing that productive infection of GM-CSF BMDCs with MCMV inhibits their ability to produce IL-12 in response to secondary TLR activation (56). The results from microarray analysis of gene expression suggest GM-CSF BMDCs are likely most similar to inflammatory monocytes (57). However, the phenotype of DC subsets changes rapidly after infection, and it is uncertain if there is a DC population in vivo after MCMV infection that truly corresponds to GM-CSF BMDCs. Nevertheless, it is certain from many studies that the cytokines produced by innate cells following infection will depend on the infecting agent and the cell subset that is activated. Therefore, extrapolating the MCMV results to other infections, it seems reasonable to propose that circumstances exist where the balance of activated APC types and cytokines produced could favor the preferential activation of NK or iNKT cells.
There are only a few examples where iNKT cells have been shown to affect viral clearance in the absence of pharmacologic activation by glycolipid antigen (58), and therefore a key issue is whether iNKT cell responses are important for limiting MCMV. We observed that viral replication was elevated in BALB/c mice that lack iNKT cells, especially in the liver. Depletion of NK cells from Jα18−/− BALB/c mice led to a further increase in replication. These data indicate that NK cell activation after infection is not completely dependent on iNKT cells, although in some cases activation of iNKT cells can contribute to NK cell stimulation, and furthermore, considering the effects of NK cell depletion in iNKT cell deficient mice, also that the NK cell contribution to viral clearance is quantitatively quite similar to iNKT cell-mediated control. Therefore, NK cells and iNKT cells each play unique roles in antiviral defense in mice. Importantly, previous work has shown that B6 mice lacking iNKT cells do not show enhanced replication of MCMV, and we have also seen similar results in Jα18−/− mice generated in this strain (data not shown). This could be due to several reasons, including the more robust, Ly49H-dependent NK cell response in B6 mice, or perhaps the differential effector cytokine production by iNKT cells in BALB/c mice compared to B6 mice. As HCMV infection impacts the NK cell repertoire differentially in people of varying genetic backgrounds, perhaps it is not surprising that iNKT cells show a differential importance in controlling MCMV replication in mouse strains, as this is certainly the case for MCMV and NK cells (59). Interestingly, as noted above, a drastic reduction in the number of iNKT cells and decreased CD1d expression has been associated in a few cases with disseminated Varicella infection following the administration of the vaccine strain (39, 40). Therefore we must consider the possibility that iNKT cell activity is particularly important in humans for control of infections by the herpesviridae.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge A. Khurana for assistance with the generation of CD1d tetramers, Josh Chong for assistance with plaque assays, Shelby Johnson for assistance with cell isolation and cell sorting and Petra Krause with scientific and technical assistance for gene quantification.
This work was supported by NIH grants RO1 AI69296 and R37 AI71922 (M.K.), R21 AI076864 (C.A.B., M.K.), F32 AI80087 (A.T.)
Abbreviations used in this paper
- BMDC
bone marrow-derived dendritic cells
- APC
antigen presenting cells
- Flt3L
fms-related tyrosine kinase 3 ligand
- GM-CSF
granulocyte macrophage colony stimulating factor
- i
invariant
- ICCS
intracellular cytokine staining
- IFN-I
type I interferon
- MCMV
mouse cytomegalovirus
- NK
natural killer
- ODN
oligodeoxynucleotide
- pDC
plasmacytoid DC
REFERENCES
- 1.Loewendorf A, Benedict CA. Modulation of host innate and adaptive immune defenses by cytomegalovirus: Timing is everything. J. Intern. Med. 2010;267:483–501. doi: 10.1111/j.1365-2796.2010.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orange JS, Wang B, Terhorst C, Biron CA. Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J. Exp. Med. 1995;182:1045–1056. doi: 10.1084/jem.182.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tyznik AJ, Tupin E, Nagarajan NA, Her MJ, Benedict CA, Kronenberg M. Cutting edge: the mechanism of invariant NKT cell responses to viral danger signals. J. Immunol. 2008;181:4452–4456. doi: 10.4049/jimmunol.181.7.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu. Rev. Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 5.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu. Rev. Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 6.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu. Rev. Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 7.Wingender G, Rogers P, Batzer G, Lee MS, Bai D, Pei B, Khurana A, Kronenberg M, Horner AA. Invariant NKT cells are required for airway inflammation induced by environmental antigens. J. Exp. Med. 2011;208:1151–1162. doi: 10.1084/jem.20102229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paget C, Mallevaey T, Speak AO, Torres D, Fontaine J, Sheehan KC, Capron M, Ryffel B, Faveeuw C, Leite de Moraes M, Platt F, Trottein F. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007;27:597–609. doi: 10.1016/j.immuni.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J. Immunol. 2007;178:2706–2713. doi: 10.4049/jimmunol.178.5.2706. [DOI] [PubMed] [Google Scholar]
- 10.Wesley JD, Tessmer MS, Chaukos D, Brossay L. NK cell-like behavior of Valpha14i NK T cells during MCMV infection. PLoS Pathog. 2008;4:e1000106. doi: 10.1371/journal.ppat.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen NR, Tatituri RV, Rivera A, Watts GF, Kim EY, Chiba A, Fuchs BB, Mylonakis E, Besra GS, Levitz SM, Brigl M, Brenner MB. Innate recognition of cell wall beta-glucans drives invariant natural killer T cell responses against fungi. Cell Host Microbe. 2011;10:437–450. doi: 10.1016/j.chom.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tyznik AJ, Farber E, Girardi E, Birkholz A, Li Y, Chitale S, So R, Arora P, Khurana A, Wang J, Porcelli SA, Zajonc DM, Kronenberg M, Howell AR. Glycolipids that elicit IFN-gamma-biased responses from natural killer T cells. Chem. Biol. 2011;18:1620–1630. doi: 10.1016/j.chembiol.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salazar-Mather TP, Hokeness KL. Cytokine and chemokine networks: pathways to antiviral defense. Curr. Top. Microbiol. Immunol. 2006;303:29–46. doi: 10.1007/978-3-540-33397-5_2. [DOI] [PubMed] [Google Scholar]
- 14.Pyzik M, Gendron-Pontbriand EM, Vidal SM. The impact of Ly49-NK cell-dependent recognition of MCMV infection on innate and adaptive immune responses. J. Biomed. Biotechnol. 2011;2011:641702. doi: 10.1155/2011/641702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Babic M, Krmpotic A, Jonjic S. All is fair in virus-host interactions: NK cells and cytomegalovirus. Trends Mol. Med. 2011;17:677–685. doi: 10.1016/j.molmed.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scalzo AA, Brown MG, Chu DT, Heusel JW, Yokoyama WM, Forbes CA. Development of intra-natural killer complex (NKC) recombinant and congenic mouse strains for mapping and functional analysis of NK cell regulatory loci. Immunogenetics. 1999;49:238–241. doi: 10.1007/s002510050486. [DOI] [PubMed] [Google Scholar]
- 17.Loewendorf A, Benedict CA. Modulation of host innate and adaptive immune defenses by cytomegalovirus: timing is everything. J. Intern. Med. 2010;267:483–501. doi: 10.1111/j.1365-2796.2010.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider K, Loewendorf A, De Trez C, Fulton J, Rhode A, Shumway H, Ha S, Patterson G, Pfeffer K, Nedospasov SA, Ware CF, Benedict CA. Lymphotoxin-mediated crosstalk between B cells and splenic stroma promotes the initial type I interferon response to cytomegalovirus. Cell Host Microbe. 2008;3:67–76. doi: 10.1016/j.chom.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shellam GR, Allan JE, Papadimitriou JM, Bancroft GJ. Increased Susceptibility to Cytomegalovirus-Infection in Beige Mutant Mice. Proc. Natl. Acad. Sci. USA. 1981;78:5104–5108. doi: 10.1073/pnas.78.8.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalod M, Hamilton T, Salomon R, Salazar-Mather TP, Henry SC, Hamilton JD, Biron CA. Dendritic cell responses to early murine cytomegalovirus infection: subset functional specialization and differential regulation by interferon alpha/beta. J. Exp. Med. 2003;197:885–898. doi: 10.1084/jem.20021522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zucchini N, Bessou G, Robbins SH, Chasson L, Raper A, Crocker PR, Dalod M. Individual plasmacytoid dendritic cells are major contributors to the production of multiple innate cytokines in an organ-specific manner during viral infection. Int. Immunol. 2008;20:45–56. doi: 10.1093/intimm/dxm119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity. 2010;33:955–966. doi: 10.1016/j.immuni.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, Alexopoulou L, Flavell RA, Beutler B. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc. Natl. Acad. Sci. U S A. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, 3rd, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, Schneewind O, Walker D, Beutler B, Teyton L, Savage PB, Bendelac A. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 25.Reddehase MJ, Weiland F, Munch K, Jonjic S, Luske A, Koszinowski UH. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J. Virol. 1985;55:264–273. doi: 10.1128/jvi.55.2.264-273.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat. Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 27.Brigl M, Tatituri RV, Watts GF, Bhowruth V, Leadbetter EA, Barton N, Cohen NR, Hsu FF, Besra GS, Brenner MB. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J. Exp. Med. 2011;208:1163–1177. doi: 10.1084/jem.20102555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benedict CA, Loewendorf A, Garcia Z, Blazar BR, Janssen EM. Dendritic cell programming by cytomegalovirus stunts naive T cell responses via the PD-L1/PD-1 pathway. J. Immunol. 2008;180:4836–4847. doi: 10.4049/jimmunol.180.7.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyagi T, Gil MP, Wang X, Louten J, Chu WM, Biron CA. High basal STAT4 balanced by STAT1 induction to control type 1 interferon effects in natural killer cells. J. Exp. Med. 2007;204:2383–2396. doi: 10.1084/jem.20070401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brigl M, Tatituri RVV, Watts GFM, Bhowruth V, Leadbetter EA, Barton N, Cohen NR, Hsu FF, Besra GS, Brenner MB. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J. Exp. Med. 2011;208:1163–1177. doi: 10.1084/jem.20102555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pien GC, Satoskar AR, Takeda K, Akira S, Biron CA. Cutting edge: selective IL-18 requirements for induction of compartmental IFN-gamma responses during viral infection. J. Immunol. 2000;165:4787–4791. doi: 10.4049/jimmunol.165.9.4787. [DOI] [PubMed] [Google Scholar]
- 32.Schneider K, Loewendorf A, De Trez C, Fulton J, Rhode A, Shumway H, Ha S, Patterson G, Pfeffer K, Nedospasov SA, Ware CF, Benedict CA. Lymphotoxin-mediated crosstalk between B cells and splenic stroma promotes the initial type I interferon response to cytomegalovirus. Cell Host Microbe. 2008;3:67–76. doi: 10.1016/j.chom.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kastenmuller W, Torabi-Parizi P, Subramanian N, Lammermann T, Germain RN. A Spatially-Organized Multicellular Innate Immune Response in Lymph Nodes Limits Systemic Pathogen Spread. Cell. 2012;150:1235–1248. doi: 10.1016/j.cell.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Dommelen SL, Tabarias HA, Smyth MJ, Degli-Esposti MA. Activation of natural killer (NK) T cells during murine cytomegalovirus infection enhances the antiviral response mediated by NK cells. J. Virol. 2003;77:1877–1884. doi: 10.1128/JVI.77.3.1877-1884.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 36.Brown MG, Dokum AO, Heusel JW, Smith HR, Beckman DL, Blattenberger EA, Dubbelde CE, Stone LR, Sclazo AA, Yokoyama WM. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science. 2001;292:934–937. doi: 10.1126/science.1060042. [DOI] [PubMed] [Google Scholar]
- 37.Pyzik M, Charbonneau B, Gendron-Pontbriand EM, Babic M, Krmpotic A, Jonjic S, Vidal SM. Distinct MHC class I-dependent NK cell-activating receptors control cytomegalovirus infection in different mouse strains. J. Exp. Med. 2011;208:1105–1117. doi: 10.1084/jem.20101831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown MG, Scalzo AA. NK gene complex dynamics and selection for NK cell receptors. Sem. Immunol. 2008;20:361–368. doi: 10.1016/j.smim.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banovic T, Yanilla M, Simmons R, Robertson I, Schroder WA, Raffelt NC, Wilson YA, Hill GR, Hogan P, Nourse CB. Disseminated Varicella Infection Caused by Varicella Vaccine Strain in a Child With Low Invariant Natural Killer T Cells and Diminished CD1d Expression. J. Infect. Dis. 2011;204:1893–1901. doi: 10.1093/infdis/jir660. [DOI] [PubMed] [Google Scholar]
- 40.Levy O, Orange JS, Hibberd P, Steinberg S, LaRussa P, Weinberg A, Wilson SB, Shaulov A, Fleisher G, Geha RS, Bonilla FA, Exley M. Disseminated varicella infection due to the vaccine strain of varicella-zoster virus, in a patient with a novel deficiency in natural killer T cells. J. Infect. Dis. 2003;188:948–953. doi: 10.1086/378503. [DOI] [PubMed] [Google Scholar]
- 41.Moll M, Andersson SK, Smed-Sorensen A, Sandberg JK. HIV-1 Vpu Interferes With CD1d Recycling From Endosomal Compartments and Inhibits Lipid Antigen Presentation in Productively Infected Dendritic Cells. Scand. J. Immunol. 2010;71:530–530. doi: 10.1182/blood-2009-09-243667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raftery MJ, Hitzler M, Winau F, Giese T, Plachter B, Kaufmann SHE, Schonrich G. n. Inhibition of CD1 antigen presentation by human cytomegalovirus. J. Virol. 2008;82:4308–4319. doi: 10.1128/JVI.01447-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raftery MJ, Winau F, Giese T, Kaufmann SHE, Schaible UE, Schonrich G. n. Viral danger signals control CD1d de novo synthesis and NKT cell activation. Eur. J. Immunol. 2008;38:668–679. doi: 10.1002/eji.200737233. [DOI] [PubMed] [Google Scholar]
- 44.Webb TJR, Litavecz RA, Khan MA, Du WJ, Gervay-Hague J, Renukaradhya GJ, Brutkiewicz RR. Inhibition of CD1d1-mediated antigen presentation by the vaccinia virus B1R and H5R molecules. Eur. J. Immunol. 2006;36:2595–2600. doi: 10.1002/eji.200636024. [DOI] [PubMed] [Google Scholar]
- 45.Yuan WM, Dasgupta A, Cresswell P. Herpes simplex virus evades natural killer T cell recognition by suppressing CD1d recycling. Nat. Immunol. 2006;7:835–842. doi: 10.1038/ni1364. [DOI] [PubMed] [Google Scholar]
- 46.Brennan PJ, Tatituri RV, Brigl M, Kim EY, Tuli A, Sanderson JP, Gadola SD, Hsu FF, Besra GS, Brenner MB. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat. Immunol. 2011;12:1202–1211. doi: 10.1038/ni.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verma S, Wang Q, Chodaczek G, Benedict CA. Lymphoid tissue stromal cells coordinate innate defense to cytomegalovirus. J. Virol. 2013;87:6201–6210. doi: 10.1128/JVI.00113-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vilarinho S, Ogasawara K, Nishimura S, Lanier LL, Baron JL. Blockade of NKG2D on NKT cells prevents hepatitis and the acute immune response to hepatitis B virus. Proc. Natl. Acad. Sci. U S A. 2007;104:18187–18192. doi: 10.1073/pnas.0708968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arase H, Arase N, Saito T. Interferon gamma production by natural killer (NK) cells and NK1.1+ T cells upon NKR-P1 cross-linking. J. Exp. Med. 1996;183:2391–2396. doi: 10.1084/jem.183.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benlagha K, Weiss A, Beavis A, Teyton L, Bendelac A. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J. Exp. Med. 2000;191:1895–1903. doi: 10.1084/jem.191.11.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krmpotic A, Hasan M, Loewendorf A, Saulig T, Halenius A, Lenac T, Polic B, Bubic I, Kriegeskorte A, Pernjak-Pugel E, Messerle M, Hengel H, Busch DH, Koszinowski UH, Jonjic S. NK cell activation through the NKG2D ligand MULT-1 is selectively prevented by the glycoprotein encoded by mouse cytomegalovirus gene m145. J. Exp. Med. 2005;201:211–220. doi: 10.1084/jem.20041617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lodoen M, Ogasawara K, Hamerman JA, Arase H, Houchins JP, Mocarski ES, Lanier LL. NKG2D-mediated natural killer cell protection against cytomegalovirus is impaired by viral gp40 modulation of retinoic acid early inducible 1 gene molecules. J. Exp. Med. 2003;197:1245–1253. doi: 10.1084/jem.20021973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Velazquez P, Cameron TO, Kinjo Y, Nagarajan N, Kronenberg M, Dustin ML. Cutting edge: activation by innate cytokines or microbial antigens can cause arrest of natural killer T cell patrolling of liver sinusoids. J. Immunol. 2008;180:2024–2028. doi: 10.4049/jimmunol.180.4.2024. [DOI] [PubMed] [Google Scholar]
- 54.Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, Degli-Esposti MA. Functional interactions between dendritic cells and NK cells during viral infection. Nat. Immunol. 2003;4:175–181. doi: 10.1038/ni880. [DOI] [PubMed] [Google Scholar]
- 55.Andoniou CE, van Dommelen SL, Voigt V, Andrews DM, Brizard G, Asselin- Paturel C, Delale T, Stacey KJ, Trinchieri G, Degli-Esposti MA. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat. Immunol. 2005;6:1011–1019. doi: 10.1038/ni1244. [DOI] [PubMed] [Google Scholar]
- 56.Andrews DM, Andoniou CE, Granucci F, Ricciardi-Castagnoli P, Degli- Esposti MA. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nat. Immunol. 2001;2:1077–1084. doi: 10.1038/ni724. [DOI] [PubMed] [Google Scholar]
- 57.Robbins SH, Walzer T, Dembele D, Thibault C, Defays A, Bessou G, Xu H, Vivier E, Sellars M, Pierre P, Sharp FR, Chan S, Kastner P, Dalod M. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Bio.l. 2008;9:R17. doi: 10.1186/gb-2008-9-1-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat. Rev. Microbiol. 2007;5:405–417. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- 59.Xie XF, Dighe A, Clark P, Sabastian P, Buss S, Brown MG. Deficient major histocompatibility complex-linked innate murine cytomegalovirus immunity in MA/My.L-H2(b) mice and viral downregulation of H-2(k) class I proteins. J. Virol. 2007;81:229–236. doi: 10.1128/JVI.00997-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.