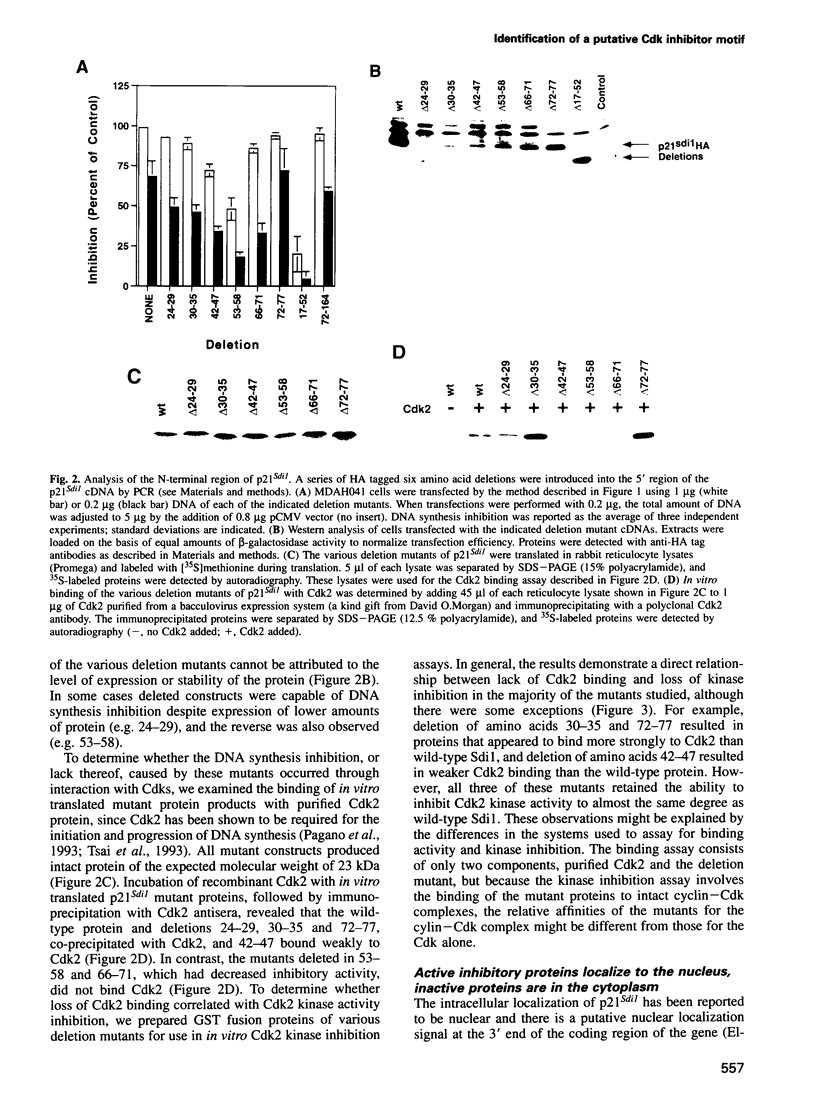
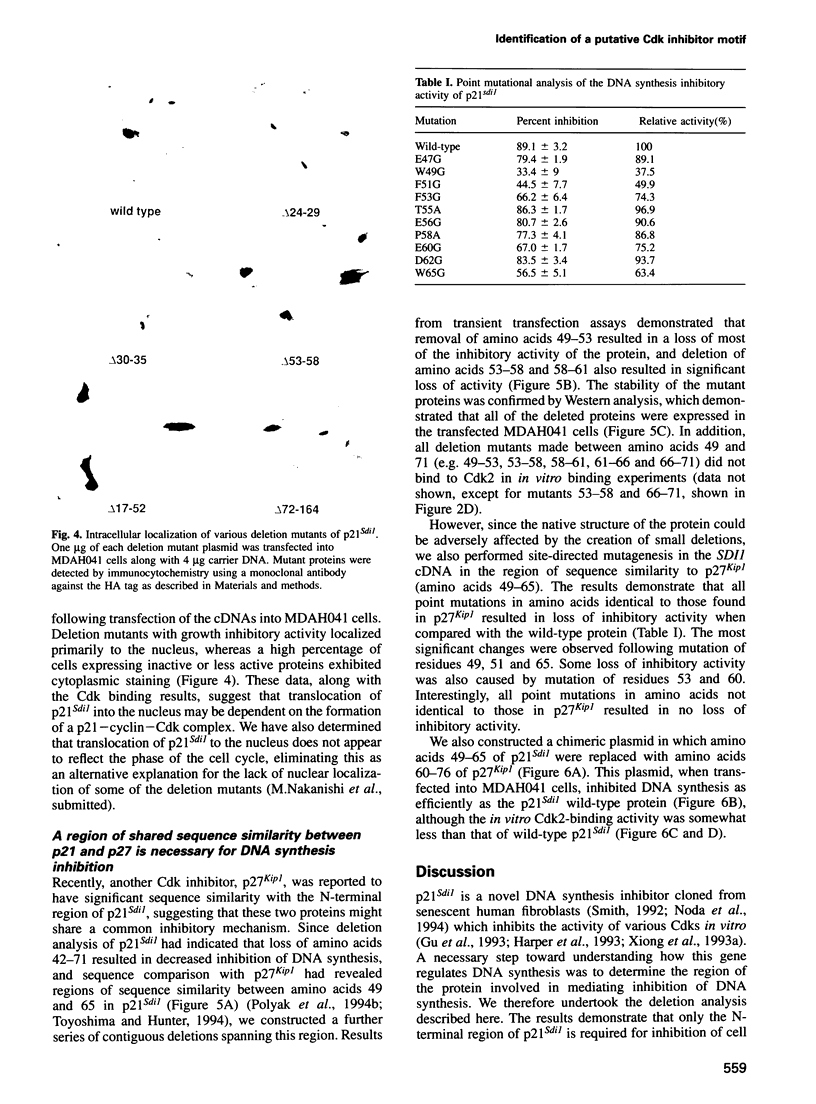
Abstract
The cloning of the negative growth regulatory gene, p21Sdi1, has led to the convergence of the fields of cellular senescence, cell cycle regulation and tumor suppression. This gene was first cloned as an inhibitor of DNA synthesis that was overexpressed in terminally non-dividing senescent human fibroblasts (SD11) and later as a p53 transactivated gene (WAF1) and a Cdk-interacting protein (CIP1, p21) that inhibited cyclin-dependent kinase activity. To identify the active region(s) of p21Sdi1, cDNA constructs encoding various deleted forms of the protein were analyzed. Amino acids 22-71 were found to be the minimal region required for DNA synthesis inhibition. Amino acids 49-71 were involved in binding to Cdk2, and constructs deleted in this region expressed proteins that were unable to inhibit Cdk2 kinase activity in vitro. The latter stretch of amino acids shared sequence similarity with amino acids 60-76 of the p27Kip1 protein, another Cdk inhibitor. Point mutations made in p21Sdi1 in this region confirmed that amino acids common to both proteins were involved in DNA synthesis inhibition. Additionally, a chimeric protein, in which amino acids 49-65 of p21Sdi1 were substituted with amino acids 60-76 of p27Kip1, had almost the same DNA synthesis inhibitory activity as the wild-type protein. The results indicate that the region of sequence similarity between p21Sdi1 and p27Kip1 encodes an inhibitory motif characteristic of this family of Cdk inhibitors.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blow J. J., Nurse P. A cdc2-like protein is involved in the initiation of DNA replication in Xenopus egg extracts. Cell. 1990 Sep 7;62(5):855–862. doi: 10.1016/0092-8674(90)90261-c. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F., Roberts J., Weintraub H. Simple and complex cell cycles. Annu Rev Cell Biol. 1989;5:341–396. doi: 10.1146/annurev.cb.05.110189.002013. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Nuclear targeting sequences--a consensus? Trends Biochem Sci. 1991 Dec;16(12):478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- Flores-Rozas H., Kelman Z., Dean F. B., Pan Z. Q., Harper J. W., Elledge S. J., O'Donnell M., Hurwitz J. Cdk-interacting protein 1 directly binds with proliferating cell nuclear antigen and inhibits DNA replication catalyzed by the DNA polymerase delta holoenzyme. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8655–8659. doi: 10.1073/pnas.91.18.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bustos J., Heitman J., Hall M. N. Nuclear protein localization. Biochim Biophys Acta. 1991 Mar 7;1071(1):83–101. doi: 10.1016/0304-4157(91)90013-m. [DOI] [PubMed] [Google Scholar]
- Girard F., Strausfeld U., Fernandez A., Lamb N. J. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell. 1991 Dec 20;67(6):1169–1179. doi: 10.1016/0092-8674(91)90293-8. [DOI] [PubMed] [Google Scholar]
- Gu Y., Turck C. W., Morgan D. O. Inhibition of CDK2 activity in vivo by an associated 20K regulatory subunit. Nature. 1993 Dec 16;366(6456):707–710. doi: 10.1038/366707a0. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- MacGregor G. R., Caskey C. T. Construction of plasmids that express E. coli beta-galactosidase in mammalian cells. Nucleic Acids Res. 1989 Mar 25;17(6):2365–2365. doi: 10.1093/nar/17.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maridor G., Gallant P., Golsteyn R., Nigg E. A. Nuclear localization of vertebrate cyclin A correlates with its ability to form complexes with cdk catalytic subunits. J Cell Sci. 1993 Oct;106(Pt 2):535–544. doi: 10.1242/jcs.106.2.535. [DOI] [PubMed] [Google Scholar]
- Michaely P., Bennett V. The ANK repeat: a ubiquitous motif involved in macromolecular recognition. Trends Cell Biol. 1992 May;2(5):127–129. doi: 10.1016/0962-8924(92)90084-z. [DOI] [PubMed] [Google Scholar]
- Noda A., Ning Y., Venable S. F., Pereira-Smith O. M., Smith J. R. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994 Mar;211(1):90–98. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Pagano M., Pepperkok R., Lukas J., Baldin V., Ansorge W., Bartek J., Draetta G. Regulation of the cell cycle by the cdk2 protein kinase in cultured human fibroblasts. J Cell Biol. 1993 Apr;121(1):101–111. doi: 10.1083/jcb.121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano M., Pepperkok R., Verde F., Ansorge W., Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992 Mar;11(3):961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira-Smith O. M., Smith J. R. Phenotype of low proliferative potential is dominant in hybrids of normal human fibroblasts. Somatic Cell Genet. 1982 Nov;8(6):731–742. doi: 10.1007/BF01543015. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. Human cyclins A and B1 are differentially located in the cell and undergo cell cycle-dependent nuclear transport. J Cell Biol. 1991 Oct;115(1):1–17. doi: 10.1083/jcb.115.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K., Kato J. Y., Solomon M. J., Sherr C. J., Massague J., Roberts J. M., Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994 Jan;8(1):9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- Polyak K., Lee M. H., Erdjument-Bromage H., Koff A., Roberts J. M., Tempst P., Massagué J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994 Jul 15;78(1):59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- Reed S. I. The role of p34 kinases in the G1 to S-phase transition. Annu Rev Cell Biol. 1992;8:529–561. doi: 10.1146/annurev.cb.08.110192.002525. [DOI] [PubMed] [Google Scholar]
- Scott J. D., Fischer E. H., Takio K., Demaille J. G., Krebs E. G. Amino acid sequence of the heat-stable inhibitor of the cAMP-dependent protein kinase from rabbit skeletal muscle. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5732–5736. doi: 10.1073/pnas.82.17.5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M., Hannon G. J., Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993 Dec 16;366(6456):704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- Silver P. A. How proteins enter the nucleus. Cell. 1991 Feb 8;64(3):489–497. doi: 10.1016/0092-8674(91)90233-o. [DOI] [PubMed] [Google Scholar]
- Toyoshima H., Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994 Jul 15;78(1):67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- Tsai L. H., Lees E., Faha B., Harlow E., Riabowol K. The cdk2 kinase is required for the G1-to-S transition in mammalian cells. Oncogene. 1993 Jun;8(6):1593–1602. [PubMed] [Google Scholar]
- Waga S., Hannon G. J., Beach D., Stillman B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature. 1994 Jun 16;369(6481):574–578. doi: 10.1038/369574a0. [DOI] [PubMed] [Google Scholar]
- Wen W., Taylor S. S. High affinity binding of the heat-stable protein kinase inhibitor to the catalytic subunit of cAMP-dependent protein kinase is selectively abolished by mutation of Arg133. J Biol Chem. 1994 Mar 18;269(11):8423–8430. [PubMed] [Google Scholar]
- Xiong Y., Hannon G. J., Zhang H., Casso D., Kobayashi R., Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993 Dec 16;366(6456):701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Zhang H., Beach D. D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell. 1992 Oct 30;71(3):505–514. doi: 10.1016/0092-8674(92)90518-h. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Zhang H., Beach D. Subunit rearrangement of the cyclin-dependent kinases is associated with cellular transformation. Genes Dev. 1993 Aug;7(8):1572–1583. doi: 10.1101/gad.7.8.1572. [DOI] [PubMed] [Google Scholar]
- Yin Y., Tainsky M. A., Bischoff F. Z., Strong L. C., Wahl G. M. Wild-type p53 restores cell cycle control and inhibits gene amplification in cells with mutant p53 alleles. Cell. 1992 Sep 18;70(6):937–948. doi: 10.1016/0092-8674(92)90244-7. [DOI] [PubMed] [Google Scholar]
- Zindy F., Lamas E., Chenivesse X., Sobczak J., Wang J., Fesquet D., Henglein B., Bréchot C. Cyclin A is required in S phase in normal epithelial cells. Biochem Biophys Res Commun. 1992 Feb 14;182(3):1144–1154. doi: 10.1016/0006-291x(92)91851-g. [DOI] [PubMed] [Google Scholar]
- el-Deiry W. S., Harper J. W., O'Connor P. M., Velculescu V. E., Canman C. E., Jackman J., Pietenpol J. A., Burrell M., Hill D. E., Wang Y. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994 Mar 1;54(5):1169–1174. [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]







