Abstract
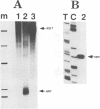
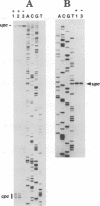
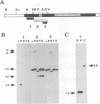
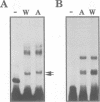
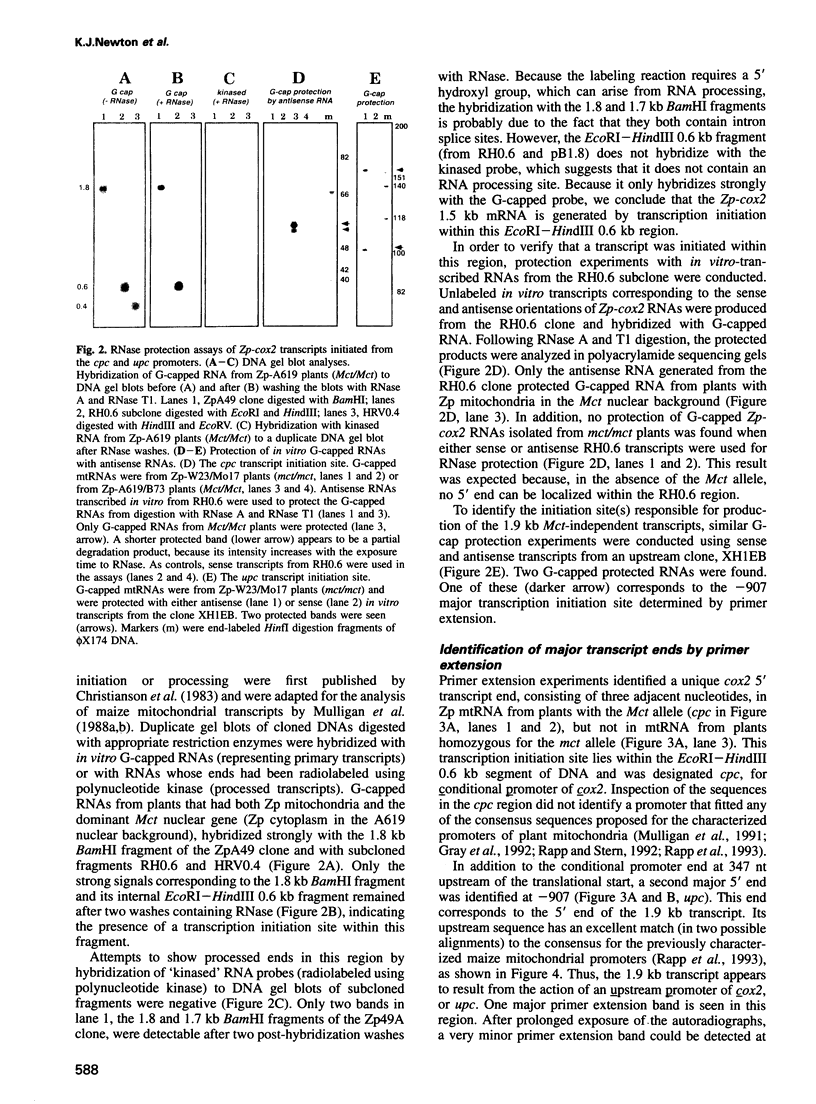
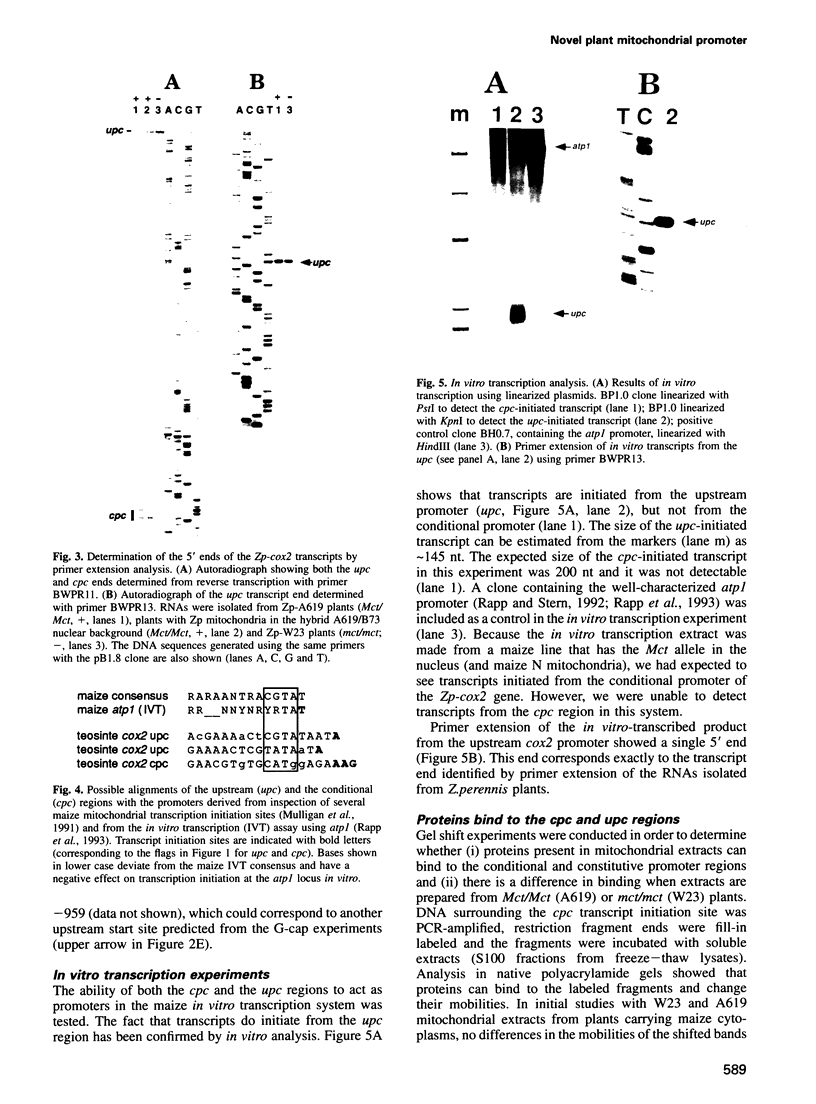
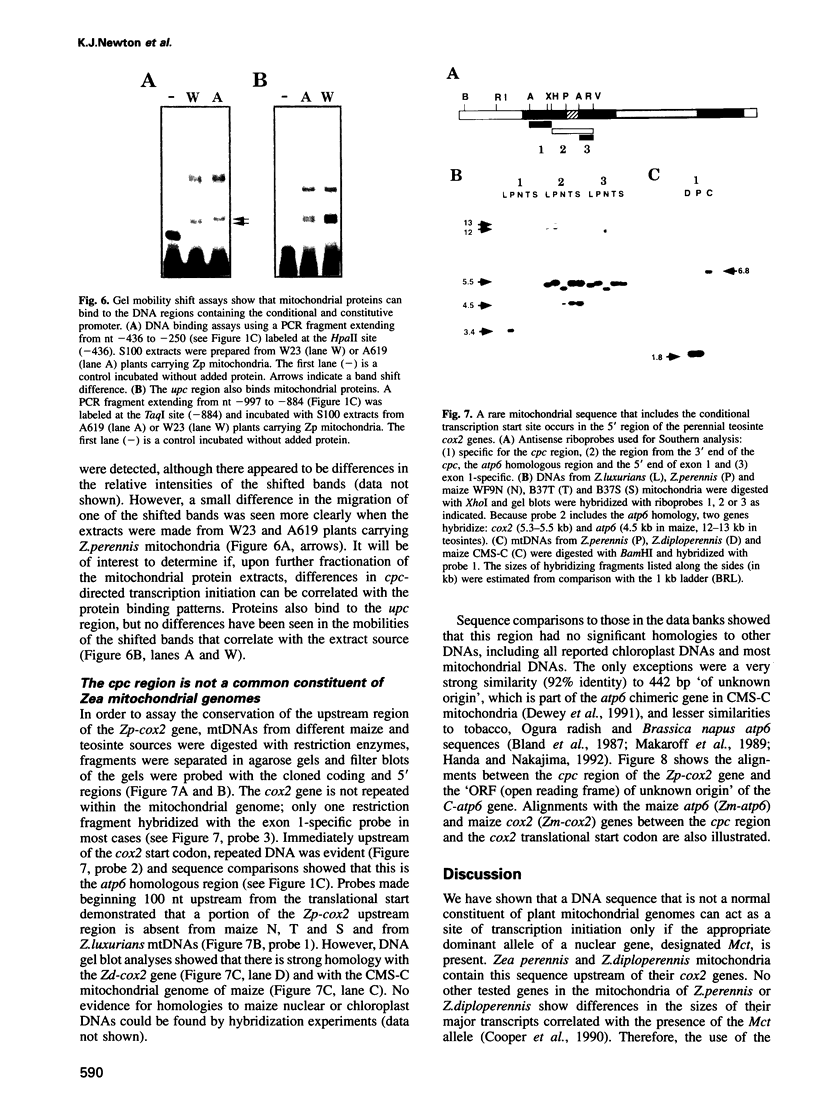
We have characterized two promoters of the cytochrome oxidase subunit 2 (cox2) gene in Zea perennis mitochondria present in maize lines. Initiation at a site 907 bases upstream of the start codon results in the major approximately 1900 nt cox2 transcript. A sequence just upstream of this site conforms to the consensus described for maize mitochondrial promoters and its transcription is correctly initiated in a maize mitochondrial in vitro transcription extract. A second transcription initiation site (-347) is used only when the dominant allele of a nuclear gene, Mct, is present and its use results in an additional, shorter major transcript. Sequences flanking the Mct-dependent transcription initiation site, which we have termed the conditional promoter of cox2 (cpc), do not fit the maize mitochondrial promoter consensus and do not function in the maize in vitro transcription extract. The cpc region does not hybridize with mitochondrial, chloroplast or nuclear DNAs from most maize or teosinte lines. However, the cpc sequence is found in the same position upstream of the cox2 gene in Zea diploperennis mtDNA and it has striking similarity to the previously reported 'ORF of unknown origin' fused to the ATPase subunit 6 gene in maize CMS-C mitochondria. cpc appears to represent a new type of mitochondrial promoter. Further analysis of both conditional and constitutive promoters should help us to better understand the control of transcription in plant mitochondria.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B., Lüke W., Hunsmann G. Improvement of PCR amplified DNA sequencing with the aid of detergents. Nucleic Acids Res. 1990 Mar 11;18(5):1309–1309. doi: 10.1093/nar/18.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland M. M., Levings C. S., 3rd, Matzinger D. F. The ATPase subunit 6 gene of tobacco mitochondria contains an unusual sequence. Curr Genet. 1987;12(7):475–481. doi: 10.1007/BF00419555. [DOI] [PubMed] [Google Scholar]
- Brown G. G., Auchincloss A. H., Covello P. S., Gray M. W., Menassa R., Singh M. Characterization of transcription initiation sites on the soybean mitochondrial genome allows identification of a transcription-associated sequence motif. Mol Gen Genet. 1991 Sep;228(3):345–355. doi: 10.1007/BF00260626. [DOI] [PubMed] [Google Scholar]
- Christianson T., Edwards J. C., Mueller D. M., Rabinowitz M. Identification of a single transcriptional initiation site for the glutamic tRNA and COB genes in yeast mitochondria. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5564–5568. doi: 10.1073/pnas.80.18.5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher D. A., Kim M., Mullet J. E. A novel light-regulated promoter is conserved in cereal and dicot chloroplasts. Plant Cell. 1992 Jul;4(7):785–798. doi: 10.1105/tpc.4.7.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. A. Transcription and replication of animal mitochondrial DNAs. Int Rev Cytol. 1992;141:217–232. doi: 10.1016/s0074-7696(08)62067-7. [DOI] [PubMed] [Google Scholar]
- Cooper P., Butler E., Newton K. J. Identification of a maize nuclear gene which influences the size and number of cox2 transcripts in mitochondria of perennial ++teosintes. Genetics. 1990 Oct;126(2):461–467. doi: 10.1093/genetics/126.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P., Newton K. J. Maize nuclear background regulates the synthesis of a 22-kDa polypeptide in Zea luxurians mitochondria. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7423–7426. doi: 10.1073/pnas.86.19.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo M. C., Fox T. D. Control of mitochondrial gene expression in Saccharomyces cerevisiae. Annu Rev Genet. 1990;24:91–113. doi: 10.1146/annurev.ge.24.120190.000515. [DOI] [PubMed] [Google Scholar]
- Covello P. S., Gray M. W. Differences in editing at homologous sites in messenger RNAs from angiosperm mitochondria. Nucleic Acids Res. 1990 Sep 11;18(17):5189–5196. doi: 10.1093/nar/18.17.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covello P. S., Gray M. W. Sequence analysis of wheat mitochondrial transcripts capped in vitro: definitive identification of transcription initiation sites. Curr Genet. 1991 Aug;20(3):245–251. doi: 10.1007/BF00326239. [DOI] [PubMed] [Google Scholar]
- Dewey R. E., Timothy D. H., Levings C. S., 3rd Chimeric mitochondrial genes expressed in the C male-sterile cytoplasm of maize. Curr Genet. 1991 Dec;20(6):475–482. doi: 10.1007/BF00334775. [DOI] [PubMed] [Google Scholar]
- Fox T. D., Leaver C. J. The Zea mays mitochondrial gene coding cytochrome oxidase subunit II has an intervening sequence and does not contain TGA codons. Cell. 1981 Nov;26(3 Pt 1):315–323. doi: 10.1016/0092-8674(81)90200-2. [DOI] [PubMed] [Google Scholar]
- Haley J., Bogorad L. Alternative promoters are used for genes within maize chloroplast polycistronic transcription units. Plant Cell. 1990 Apr;2(4):323–333. doi: 10.1105/tpc.2.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa H., Nakajima K. Different organization and altered transcription of the mitochondrial atp6 gene in the male-sterile cytoplasm of rapeseed (Brassica napus L.). Curr Genet. 1992 Feb;21(2):153–159. doi: 10.1007/BF00318475. [DOI] [PubMed] [Google Scholar]
- Hanic-Joyce P. J., Gray M. W. Accurate transcription of a plant mitochondrial gene in vitro. Mol Cell Biol. 1991 Apr;11(4):2035–2039. doi: 10.1128/mcb.11.4.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaehning J. A. Mitochondrial transcription: is a pattern emerging? Mol Microbiol. 1993 Apr;8(1):1–4. doi: 10.1111/j.1365-2958.1993.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Kumar R., Levings C. S., 3rd RNA editing of a chimeric maize mitochondrial gene transcript is sequence specific. Curr Genet. 1993 Feb;23(2):154–159. doi: 10.1007/BF00352015. [DOI] [PubMed] [Google Scholar]
- Laughnan J. R., Gabay-Laughnan S. Cytoplasmic male sterility in maize. Annu Rev Genet. 1983;17:27–48. doi: 10.1146/annurev.ge.17.120183.000331. [DOI] [PubMed] [Google Scholar]
- Lonsdale D. M., Hodge T. P., Fauron C. M. The physical map and organisation of the mitochondrial genome from the fertile cytoplasm of maize. Nucleic Acids Res. 1984 Dec 21;12(24):9249–9261. doi: 10.1093/nar/12.24.9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makaroff C. A., Apel I. J., Palmer J. D. The atp6 coding region has been disrupted and a novel reading frame generated in the mitochondrial genome of cytoplasmic male-sterile radish. J Biol Chem. 1989 Jul 15;264(20):11706–11713. [PubMed] [Google Scholar]
- Mulligan R. M., Lau G. T., Walbot V. Numerous transcription initiation sites exist for the maize mitochondrial genes for subunit 9 of the ATP synthase and subunit 3 of cytochrome oxidase. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7998–8002. doi: 10.1073/pnas.85.21.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. M., Leon P., Walbot V. Transcriptional and posttranscriptional regulation of maize mitochondrial gene expression. Mol Cell Biol. 1991 Jan;11(1):533–543. doi: 10.1128/mcb.11.1.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. M., Maloney A. P., Walbot V. RNA processing and multiple transcription initiation sites result in transcript size heterogeneity in maize mitochondria. Mol Gen Genet. 1988 Mar;211(3):373–380. doi: 10.1007/BF00425688. [DOI] [PubMed] [Google Scholar]
- Newton K. J., Coe E. H. Mitochondrial DNA changes in abnormal growth (nonchromosomal stripe) mutants of maize. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7363–7366. doi: 10.1073/pnas.83.19.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp W. D., Lupold D. S., Mack S., Stern D. B. Architecture of the maize mitochondrial atp1 promoter as determined by linker-scanning and point mutagenesis. Mol Cell Biol. 1993 Dec;13(12):7232–7238. doi: 10.1128/mcb.13.12.7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp W. D., Stern D. B. A conserved 11 nucleotide sequence contains an essential promoter element of the maize mitochondrial atp1 gene. EMBO J. 1992 Mar;11(3):1065–1073. doi: 10.1002/j.1460-2075.1992.tb05145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottmann W. H., Brears T., Hodge T. P., Lonsdale D. M. A mitochondrial gene is lost via homologous recombination during reversion of CMS T maize to fertility. EMBO J. 1987 Jun;6(6):1541–1546. doi: 10.1002/j.1460-2075.1987.tb02398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton T. B., Christopher D. A., Mullet J. E. Light-induced switch in barley psbD-psbC promoter utilization: a novel mechanism regulating chloroplast gene expression. EMBO J. 1990 Dec;9(13):4485–4494. doi: 10.1002/j.1460-2075.1990.tb07899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. B., Newton K. J. Isolation of plant mitochondrial RNA. Methods Enzymol. 1986;118:488–496. doi: 10.1016/0076-6879(86)18095-5. [DOI] [PubMed] [Google Scholar]
- Yang A. J., Mulligan R. M. RNA editing intermediates of cox2 transcripts in maize mitochondria. Mol Cell Biol. 1991 Aug;11(8):4278–4281. doi: 10.1128/mcb.11.8.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]