Abstract
The final step of cytoplasmic mRNA degradation proceeds in either a 5′-3′ direction catalysed by Xrn1 or in a 3′-5′ direction catalysed by the exosome. Dis3/Rrp44, an RNase II family protein, is the catalytic subunit of the exosome. In humans, there are three paralogues of this enzyme: DIS3, DIS3L, and DIS3L2. In this work, we identified a novel Schizosaccharomyces pombe exonuclease belonging to the conserved family of human DIS3L2 and plant SOV. Dis3L2 does not interact with the exosome components and localizes in the cytoplasm and in cytoplasmic foci, which are docked to P-bodies. Deletion of dis3l2+ is synthetically lethal with xrn1Δ, while deletion of dis3l2+ in an lsm1Δ background results in the accumulation of transcripts and slower mRNA degradation rates. Accumulated transcripts show enhanced uridylation and in vitro Dis3L2 displays a preference for uridylated substrates. Altogether, our results suggest that in S. pombe, and possibly in most other eukaryotes, Dis3L2 is an important factor in mRNA degradation. Therefore, this novel 3′-5′ RNA decay pathway represents an alternative to degradation by Xrn1 and the exosome.
Keywords: Dis3, exosome, P-bodies, RNB domain, uridylation
Introduction
Cytoplasmic mRNAs are degraded by different pathways to ensure a fast and efficient regulation of the transcriptome at the post-transcriptional level (Meyer et al, 2004; Garneau et al, 2007; Houseley and Tollervey, 2009; Balagopal et al, 2012; Schoenberg and Maquat, 2012). Two general pathways of mRNA degradation were identified using the S. cerevisiae model. Regardless of the mechanism triggering degradation, the final step of RNA decay proceeds in either 5′-3′ or 3′-5′ direction catalysed by Xrn1 exoribonuclease or the exosome complex, respectively. Deadenylation is usually the first step in both pathways. After deadenylation, the mRNA cap structure can be removed by the Dcp1–Dcp2 decapping complex. The decapping step is activated by a subset of factors that include the Lsm1-7 complex (Nissan et al, 2010). After cap removal, mRNA is accessible to the Xrn1 exonuclease which can then digest it in the 5′-3′ direction. Decapped or deadenylated mRNAs can also be degraded in the 3′-5′ direction by the exosome complex.
The exosome contributes to the processing, quality control, and turnover of a large number of cellular RNAs (Mitchell et al, 1997; Houseley et al, 2006; Schaeffer et al, 2011). Exosome degradation of cytoplasmic RNAs is facilitated by the SKI complex (Anderson and Parker, 1998). In S. cerevisiae, the cytoplasmic exosome consists of nine inactive subunits (core ring structure) plus an active ribonuclease Rrp44/Dis3 (Dziembowski et al, 2007). This is a 3′-5′ hydrolytic exonuclease belonging to the ubiquitous RNase II family of enzymes. Beyond the conserved catalytic RNB domain (Frazão et al, 2006), it additionally contains a PIN domain with endonucleolytic activity (Lebreton et al, 2008; Schaeffer et al, 2009; Schneider et al, 2009).
Xrn1 together with other proteins involved in decapping and 5′-3′ degradation can be localized in nucleoprotein aggregates named processing bodies (P-bodies) (Sheth and Parker, 2003; Decker and Parker, 2012). Part of the 5′-3′ mRNA degradation occurs inside these cytoplasmic structures. However messengers can also be stored inside P-bodies in an untranslational state to be later released to re-initiate translation (Brengues et al, 2005). Other aggregates found in the cytoplasm, which have a different composition from P-bodies, are the stress granules (Anderson and Kedersha, 2008; Decker and Parker, 2012). Stress granules are formed in response to stress, and can store mRNAs ready to re-initiate translation. The relationship between these two types of granules is unclear, as some components are found in both P-bodies and stress granules, and stress granules can be generated from P-bodies (Buchan et al, 2008).
S. cerevisiae has been an excellent model to study cytoplasmic mRNA degradation. However, there are a substantial number of processes and enzymes conserved in eukaryotes which are absent in S. cerevisiae such as small RNA induced transcript silencing (Drinnenberg et al, 2009) and post-transcriptional modifications that can target RNAs to degradation, such as CUCU sequences (Morozov et al, 2010) or short uridine stretches (Rissland and Norbury, 2009) added to 3′-ends of mRNAs.
Recent findings show that the composition of the exosome complex differs between S. cerevisiae and humans. In budding yeast, Rrp44/Dis3 localizes in the nucleus and cytoplasm and interacts with the 9-subunit exosome ring (Synowsky et al, 2009). In human genome, there are three Dis3 isoforms: DIS3, DIS3L, and DIS3L2. DIS3 is nuclear, and DIS3L and DIS3L2 are cytoplasmic (Staals et al, 2010; Tomecki et al, 2010; Astuti et al, 2012). DIS3 and DIS3L were shown to interact with the exosome ring; however, it is not clear whether DIS3L2 interacts with the exosome. DIS3L2 has a conserved catalytic RNB domain but the N-terminal PIN and CR3 domains, which facilitate interaction with the exosome (Schaeffer et al, 2012; Makino et al, 2013), are not conserved.
Mutations in the human DIS3L2 gene were found in individuals with Perlman syndrome—a congenital overgrowth disease. At the molecular level, DIS3L2 depletion was shown to cause mitotic abnormalities due to the deregulation of the expression of mitotic control proteins (Astuti et al, 2012).
Different isoforms of Dis3 protein seem to be a conserved feature among eukaryotes. In Arabidopsis thaliana, a protein called SOV, which has a conserved RNB domain, was suggested to be involved in cytoplasmic mRNA metabolism (Zhang et al, 2010).
Although there are no Dis3L2 isoforms in S. cerevisiae, we have identified the Dis3L2 homologue in S. pombe. Fission yeast seems to share more conservation in RNA metabolism with higher eukaryotes than budding yeast. For instance, RNA interference (Moazed, 2009) or transcript uridylation, which is an evolutionary conserved mechanism of gene regulation (Schmidt and Norbury, 2010; Choi et al, 2012). Therefore, S. pombe appears to be a promising model system to investigate the cellular role of Dis3L2.
Our phylogenetic analyses proved that the novel identified S. pombe nuclease belongs to the same protein family as A. thaliana SOV and human Dis3L2, and that members of this family are conserved throughout eukaryotes. Our results show that S. pombe Dis3L2 is involved in cytoplasmic mRNA degradation, and its function is independent of the exosome. This indicates that in S. pombe and possibly in most other eukaryotes, cytoplasmic mRNAs can be degraded in the 3′-5′ direction not only by the exosome complex, but alternatively by the Dis3L2 exonuclease. This discovery brings additional complexity to the current models of cytoplasmic RNA degradation.
Results
Identification of a new exoribonuclease in S. pombe
Using S. cerevisiae Dis3p/Rrp44p sequence as a query, five genes encoding proteins with RNB domain were identified in the S. pombe genome (Supplementary Figure S1). SPBC26H8 (dis3+) gene product is a homologue of S. cerevisiae Dis3p, the active component of the exosome complex involved in RNA metabolism in both cytoplasm and nucleus (Dziembowski et al, 2007). SPCC23B6.06 gene encodes the Par1 protein, a homologue of S. cerevisiae Dss1p exonuclease crucial for RNA processing and degradation in mitochondria (Dziembowski et al, 2003; Malecki et al, 2007). A similar mitochondrial function was reported for Par1 (Hoffmann et al, 2008). SPCC16C4.09 gene encodes Sts5, homologue of S. cerevisiae Ssd1p, a protein reported to be a translation repressor involved in cell wall biogenesis and polar growth (Kurischko et al, 2011). The phenotype of fission yeast sts5+ deletion suggests a similar function for this protein (Toda et al, 1996). The other two genes identified, SPAC2C4.07C and SPBC609.01, do not have obvious homologues in S. cerevisiae genome, and their functions have not previously been investigated in S. pombe.
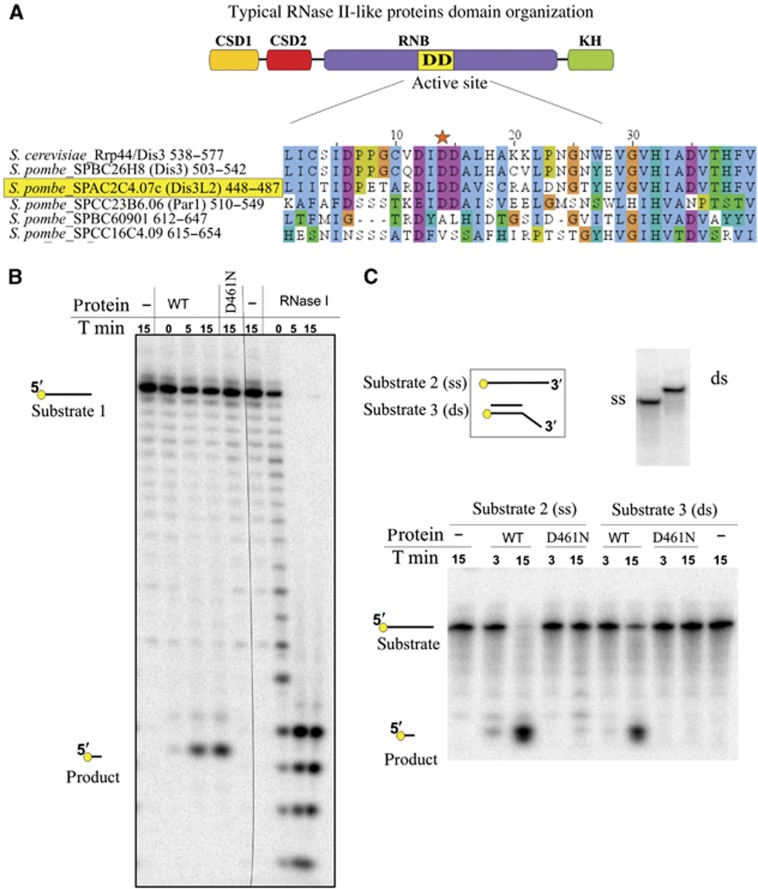
To distinguish which of the identified genes can be an active exoribonuclease, we have compared the sequences of the active sites of their RNB domains using Clustal alignment (Figure 1A) (Thompson et al, 1997). The sequence of three conserved aspartic acids in the active site of the RNB-like enzymes is essential for exonucleolytic activity (Frazão et al, 2006). Alignment results confirmed that both S. pombe proteins, Dis3 and Par1, share conservation of the active site consensus. From the other sequences analysed, only SPAC2C4.07c retained the conserved architecture of the active site, making it a candidate for an active exoribonuclease.
Figure 1.
Fission yeast gene SPAC2C4.07c encodes an active RNase II family exonuclease with no homologue in S. cerevisiae. (A) S. pombe genome encodes five proteins with RNB domain. Sequence alignment depicts exonucleolytic active site of RNB domain of fission yeast proteins, compared with S. cerevisiae Rrp44/Dis3 sequence. Conserved aspartic acid in the central part of the active site is marked with the star. (B) Product of SPAC2C4.07c gene is an active exonuclease in vitro. Around 0.5 pmol of the wild-type protein product (WT) and the mutated version (D461N) were incubated with 2 pmol of the 20-nt 5′-radioactively labelled RNA substrate 1 (see below). After indicated times (T min), reactions were stopped and products separated on a denaturing polyacrylamide gel. The same substrate was incubated with 0.1 U of RNase ONE (Promega). Migration of the reaction substrates and products is indicated. (C) Product of SPAC2C4.07c gene is able to digest RNA in the context of secondary structures. Around 0.5 pmol of WT and mutated version (D461N) was incubated with 0.2 pmol of single-stranded substrate 2 (ss) or substrate 3 (ds), which is substrate 2 annealed with DNA oligonucleotide (see below). After the indicated times, reactions were stopped and products were separated on a denaturing polyacrylamide gel. Upper panel depicts schematic representation of the RNA substrates used for the reaction (left) and the control of the annealing of the double-stranded substrate (right) on a native polyacrylamide gel. RNA substrate 1: (GUUUUGUAUAGAAAUCAAUG); RNA substrate 2: (CCCGACACCAACCACUAAAAAAAAAAAAAA); substrate 3 (hybrid DNA/RNA): (DNA oligonucleotide: AGTGGTTGGTGTCGGG/RNA oligonucleotide: substrate 2).
To confirm the exoribonucleolytic activity of SPAC2C4.07c gene product, a mutant was constructed having a single amino-acid substitution of aspartic acid to asparagine (D461N) and both protein versions were purified (Supplementary Figure S2). This substitution was reported to completely abolish the exonucleolytic activity of the enzymes of RNase II family (Amblar and Arraiano, 2005; Frazão et al, 2006). The wild-type protein digested a 5′-end labelled single-stranded RNA leaving a 3-nt 5′-labelled end product and we could not observe any reaction by-product, suggesting that degradation is due to a 3′-5′ processive exonucleolytic activity characteristic of the RNase II family (Figure 1B). Similarly, any reaction by-products were observed when an internally labelled RNA was used as a substrate, and uridine mononucleotides were detected as the main reaction products confirming processivity of the exonucleolytic reaction (Supplementary Figure S3). The wild-type protein also digested the RNA strand of a double-stranded RNA/DNA substrate having a 3′ RNA overhang (Matos et al, 2012) (Figure 1C). Our results prove that the product of SPAC2C4.07c is an active exonuclease in vitro and can degrade RNA even in the context of secondary structures. The mutated protein version (D461N) was not active (Figure 1B and C), confirming that the activity observed was specific of the SPAC2C4.07c gene product.
SPAC2C4.07c gene product belongs to conserved Dis3L2 family of exonucleases
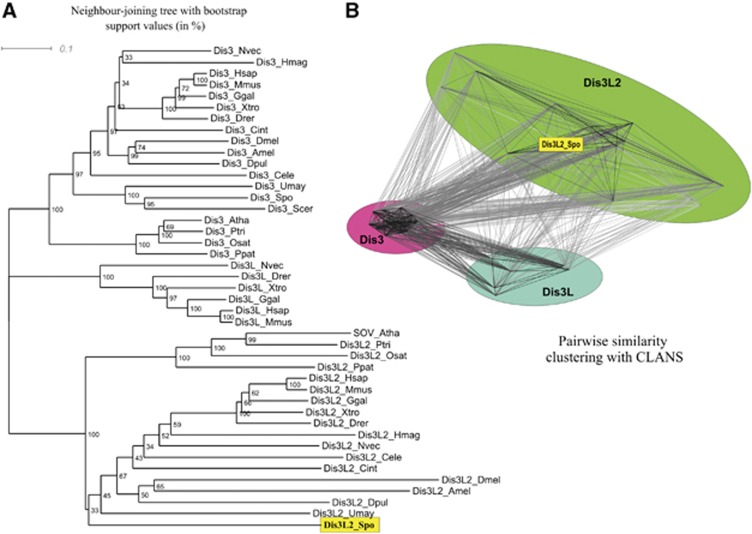
We have performed phylogenetic analysis in order to place the newly discovered S. pombe RNase in relation to the known eukaryotic proteins and to investigate conservation of Dis3 isoforms. Genomic sequences available for eukaryotic species representing different branches of the tree of life were analysed using BLASTn to find encoded proteins similar to the Dis3p of budding yeast. The sequences corresponding to mitochondrial exonucleases were discarded. Final collection of sequences (summarized in Supplementary Table 3) was subjected to phylogenetic analysis using the Neighbour Joining (NJ), Maximum Parsimony (MP), and Bayesian inference (BI) methods. The obtained trees are shown in Figure 2A (NJ) and Supplementary Figure S4 (MP and BI).
Figure 2.
Phylogenetic comparison of different Dis3 homologues in eukaryotes reveals that Dis3-like proteins can be divided into three distinct groups, corresponding to Dis3, Dis3L, and Dis3L2. Dis3 and Dis3L2 are found almost universally in Metazoa, plants, and Fungi, while proteins from the Dis3L group are found only in vertebrates and in a single invertebrate species (Nematostella vectensis). (A) A neighbour joining tree (BioNJ) of 43 eukaryotic Dis3-like proteins. Numbers correspond to percentage bootstrap support (1000 replicates) for nodes. Three main groups, corresponding to Dis3, Dis3L, and Dis3L2 proteins have 100% support, and the SPAC2C4.07c gene product of S. pombe belongs to the Dis3L2 group. (B) Clustering of the Dis3-like proteins (same set as in A) on the basis of pairwise BLAST similarity using CLANS also reveals three distinct groups of Dis3, Dis3L, and Dis3L2 sequences, with the Dis3 group forming the tightest cluster. Again, the SPAC2C4.07c gene product of S. pombe belongs to the Dis3L2 group.
Regardless of the phylogenetic inference method used, the resulting trees show the same general topology with significant support. The Dis3 homologues fall into three main branches, corresponding to the Dis3, Dis3L, and Dis3L2 proteins. Dis3 and Dis3L2 are found in all eukaryotic clades—metazoans, plants, and some fungi. A few fungal species (including S. cerevisiae) do not have Dis3L and Dis3L2, containing only one sequence of this family belonging to the Dis3 group. The presence of the third (Dis3L) isoform is restricted to metazoans and, in most cases, to vertebrates, with the intriguing exception of Nematostella vectensis (starlet sea anemone)—the only known invertebrate possessing all three isoforms of Dis3. The three groups are most likely the product of two ancient gene duplications that occurred very early during evolution.
In all phylogenetic trees (Figure 2A; Supplementary Figure S4), the SPAC2C4.07c gene product consistently clusters with the Dis3L2 proteins (including human DIS3L2 and SOV of A. thaliana), and will be henceforth called the Dis3L2 of S. pombe. Therefore, the fission yeast genome encodes two Dis3 isoforms, one from the Dis3 group and other from the Dis3L2 group.
We also performed the CLANS (CLuster ANalysis of Sequences) analysis, which clusters the sequences on the basis of pairwise BLAST scores. The results confirm the clear division of three Dis3-like protein families in eukaryotes, with the Dis3 group being the most conserved, thus tightly connected, and the Dis3L and Dis3L2 groups being more divergent (Figure 2B). Again, in this analysis the Dis3L2 of S. pombe clearly clusters with Dis3L2 group that includes human DIS3L2.
Interestingly, alignments of Dis3 homologues from different species unrevealed peculiar active site sequence conservation: all Dis3 homologues have isoleucine between the three conserved aspartic acids in the active site (DIDD), all Dis3L homologues have valine in the same site (DVDD), and all Dis3L2 have leucine (DLDD) in that site (Supplementary Figure S4C).
Dis3L2 protein localizes in the cytoplasm and foci mostly docked to P-bodies
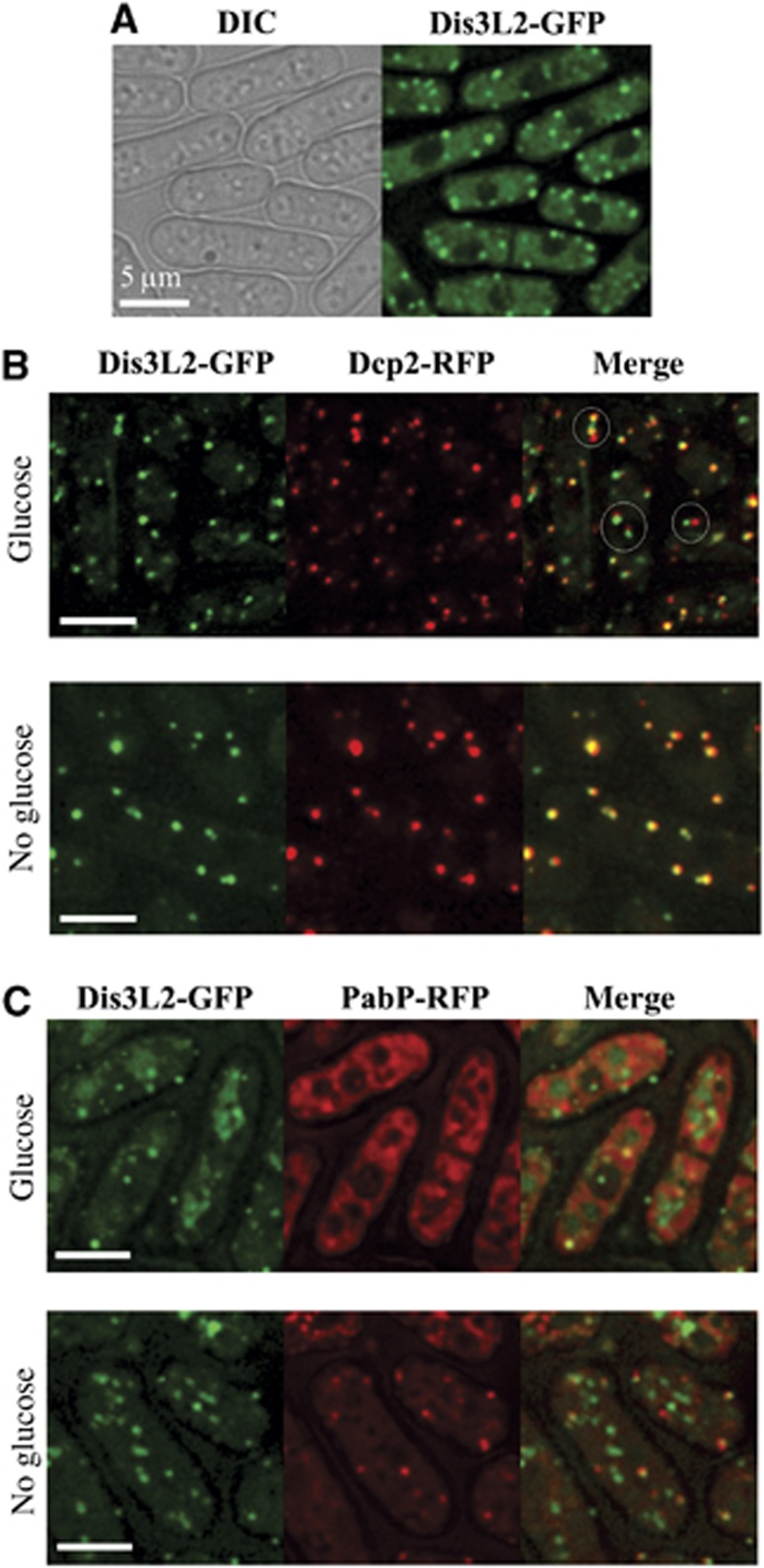
We have fused GFP to the 3′-end of S. pombe dis3l2+ in its genomic locus and analysed the localization of the fusion protein. Dis3L2-GFP, when expressed from its endogenous promoter, has a slightly different localization in the cell than when overexpressed (Matsuyama et al, 2006). The protein localizes in the cytoplasm and gives a general signal equally distributed throughout the cytosol; nevertheless, we could observe additional foci of higher fluorescence (Figure 3A; Supplementary Figure S5). The most widely studied cytoplasmic aggregates involved in mRNA metabolism are P-bodies and stress granules and we decided to check if Dis3L2 co-localized with any of these structures.
Figure 3.
Dis3L2 co-localizes with P-bodies. (A) Dis3L2 localizes in the cytoplasm and cytoplasmic foci. Cells expressing Dis3L2-GFP were grown to mid-log phase in minimal medium (EMM) and the localization of epitope-tagged protein was determined by fluorescence microscopy. (B, C) Dis3L2-GFP was examined for co-localization with Dcp2-RFP (B) or PabP-RFP (C). Cells expressing Dis3L2-GFP and Dcp2-RFP were grown to mid-log phase in minimal medium (EMM) and either immediately observed in the microscope (glucose), or deprived for glucose for 10 min and subsequently examined (no glucose) (B). Similar experiment was performed for the comparison of cells expressing Dis3L2-GFP and PabP-RFP (C) except that fission yeast was grown on full media (YES) (see Results). Examples of Dis3L2 aggregates docked to P-bodies in (B) were marked with circles. Scale bars represent 5 μm.
To check Dis3L2 co-localization with P-bodies, we used the P-body marker Dcp2 that is an active subunit of the RNA decapping complex (Ling et al, 2011). Yeast strains with Dis3L2 and Dcp2 fused with different fluorescent markers were constructed. Strains were grown in the minimal media (EMM) up to the early exponential growth phase and immediately subjected to microscopy analysis. In these conditions, most of the Dis3L2-GFP foci appear to be adjacent to Dcp2-RFP labelled P-bodies (Figure 3B; Supplementary Figure S5). In some cases, complete co-localization could also be observed. There are also a few weaker Dis3L2-GFP foci that do not co-localize with Dcp2-RFP, while the opposite situation barely happens. However, the co-localization pattern differs when we incubate the cells in glucose deprived media prior to microscopy. These conditions are known to increase the number and size of P-bodies in both S. cerevisiae and S. pombe (Teixeira et al, 2005; Nilsson and Sunnerhagen, 2011). Interestingly, after glucose deprivation the Dis3L2 and Dcp2 signals completely co-localize (Figure 3B).
It was shown for yeast that stress granules can be docked to the P-bodies in stress conditions (Buchan et al, 2008; Nilsson and Sunnerhagen, 2011). We checked the co-localization of Dis3L2 with the stress granule marker Pabp (poly(A) binding protein) (Nilsson and Sunnerhagen, 2011). Interestingly, the strain containing both fusion proteins could not grow on minimal media (data not shown). In cells grown in the rich media (YES), only a few weak stress granules could be detected (Figure 3C). The observed granules were docked or co-localized to the Dis3L2-GFP signal. However, a substantial number of Dis3L2-GFP foci did not co-localize to the stress granules. A similar localization pattern was observed in glucose deprived cells (Figure 3C).
Our results suggest that Dis3L2 can either dock or co-localize to P-bodies, as we observe a substantial number of Dis3L2 foci localizing to P-bodies even in the conditions when stress granules are hardly detected.
Dis3L2 fails to interact with the exosome complex
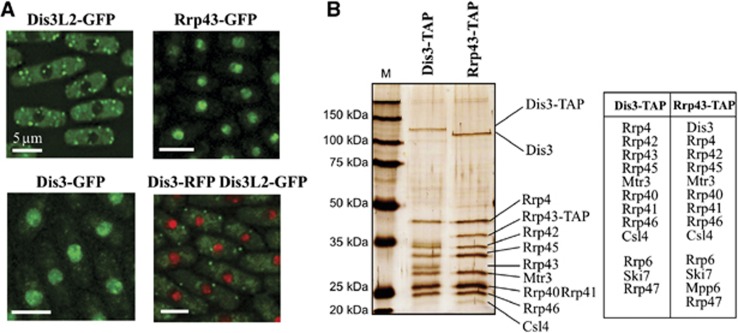
In humans, both DIS3 and DIS3L interact with the exosome ring, whereas DIS3L2 was not found in exosome pull downs, suggesting a lack of interaction (Staals et al, 2010; Tomecki et al, 2010). To check if the S. pombe Dis3L2 protein can interact with the exosome complex, we first investigated localization of the exosome ring protein Rrp43 and the active subunit Dis3. Similar to S. cerevisiae, the exosome complex is localized mainly in the nucleus (Figure 4A) (Huh et al, 2003; Yamanaka et al, 2010). The signal for both exosome proteins in cytoplasm was hardly distinguishable from the background. Co-localization of Dis3L2-GFP and Dis3-RFP showed that Dis3-RFP is mostly located in nucleus and Dis3L2-GFP in the cytoplasm. The results suggest that the exosome complex does not interact with Dis3L2 since they have different spatial distribution. However, similarly to the situation in S. cerevisiae, there is probably a small fraction of the exosome complex present in the cytosol.
Figure 4.
Dis3L2 fails to interact with the exosome complex. (A) Dis3L2 does not co-localize with the exosome complex components. Cells expressing Dis3L2-GFP, Rrp43-GFP, Dis3-GFP or both Dis3-RFP and Dis3L2-GFP were grown in the minimal media (EMM) to mid-log phase and protein localization determined by fluorescence microscopy. Scale bars represent 5 μm. (B) Dis3L2 does not co-purify with the exosome complex components. Cells expressing exosome complex components (Dis3 or Rrp43) fused with TAP tag sequence were grown to mid-log phase in rich media, subsequently tagged proteins were purified according to standard protocol (Rigaut et al, 1999). Part of each elution from the calmodulin resin was separated on SDS–PAGE gel and silver stained (gel on the left), the remaining part was subjected to mass spectrometry analysis. Table presented on the right lists all known exosome subunits and interacting proteins identified in the elutions from Dis3-TAP and Rrp43-TAP.
Since our microscopy data could not exclude the possibility that cytoplasmic fraction of the exosome interacted with Dis3L2, we decided to use Tandem Affinity Purification (TAP) (Rigaut et al, 1999) coupled to mass spectrometry analysis to identify proteins interacting with Dis3L2, Dis3 and the exosome ring. TAP tag sequence was fused with 3′-ends of dis3l2+, dis3+ and rrp43+ under their endogenous loci. Due to the difficulties with Dis3L2-TAP purification, we proceeded with the analysis of the TAP fusions of the exosome components—Rrp43 and Dis3. In both cases, after purification and analysis on silver stained protein gels, we could identify all the main exosome complex components co-purifying with the tagged proteins (Figure 4B). Parts of the fractions from the final elution were subjected to mass spectrometry analysis to identify all the co-purified proteins. As expected, we could identify all the nine main exosome subunits co-purifying with both the Rrp43-TAP and the Dis3-TAP proteins. Moreover, we identified additional proteins that were reported to interact with the exosome complex, either in the nucleus (Rrp47, Mpp6, and Rpp6) or in the cytoplasm (Ski7) (Synowsky et al, 2009; Marshall et al, 2013). Dis3L2 was not detected among the proteins identified in either sample, suggesting that this protein acts independently from the exosome complex (list of all identified proteins in Supplementary Table 4). The homologue of S. cerevisiae cytoplasmic exosome cofactor Ski7 co-purified together with both Dis3-TAP and exosome ring protein Rrp43-TAP indicating that, in S. pombe, the same 10 subunit exosome complex may act both in the cytoplasm and in the nucleus.
Dis3L2+ genetically interacts with components of cytoplasmic mRNA degradation pathway
Dis3L2 enzymatic activity, and the fact that it partially co-localizes with P-bodies, suggests a role in cytoplasmic mRNA degradation. We checked the genetic interactions between dis3l2+ and different components of mRNA degradation pathway by constructing double deletion strains. Genes chosen for the deletion together with dis3l2+ were xrn1+ encoding the exonuclease that drives degradation from the 5′-ends, ski2+ encoding a component of the Ski complex that connects the exosome to its cytoplasmic substrates, and lsm1+ encoding a protein that, in complex with six other Lsm proteins and Pat1, binds to the 3′-end of the transcripts protecting them from 3′ trimming, activating decapping and 5′-3′ degradation (He and Parker, 2001). The Lsm complex is also involved in the P-body formation (Teixeira and Parker, 2007).
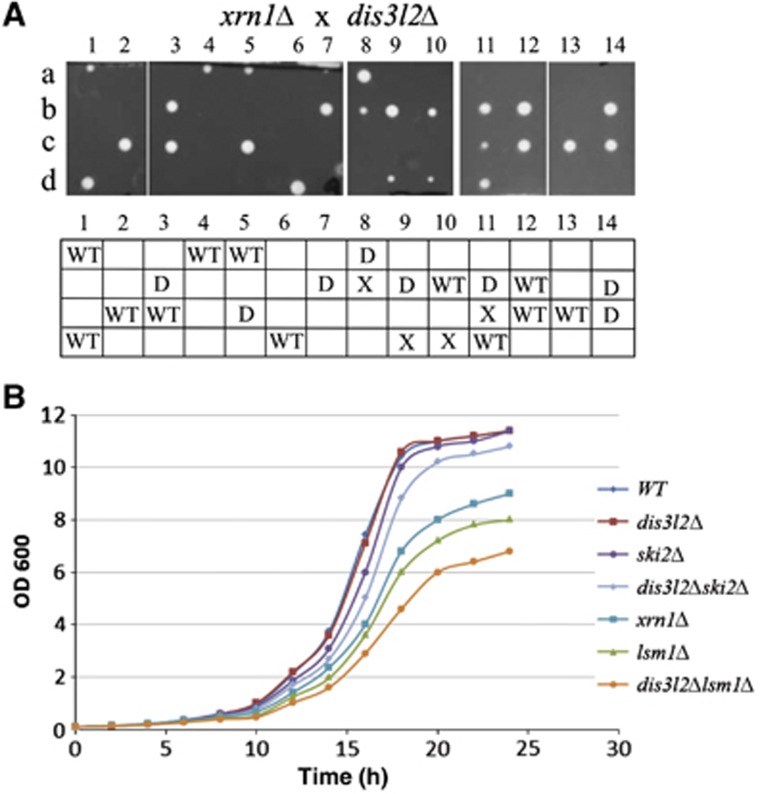
Tetrad analysis from crosses of single mutants showed that dis3l2Δ is synthetically lethal with xrn1Δ (Figure 5A). In S. cerevisiae XRN1 gene deletion is synthetically lethal with deletion of SKI complex components, crucial for 3′-5′ mRNA degradation by the exosome (Anderson and Parker, 1998). Lethality of double XRN1 SKI deletion strains is thought to be due to a blockage of both 5′-3′ and 3′-5′ RNA degradation pathways. Similarly, synthetic lethality of dis3l2Δ with xrn1Δ, together with the observed Dis3L2 3′-5′ exonucleolytic activity, suggests that dis3l2+ deletion impairs 3′-5′ mRNA degradation pathway. Lethality of double mutant suggests that cytoplasmic exosome cannot compensate for Dis3L2 function. Similarly, in the xrn1Δ strain Dis3L2 cannot fully compensate for loss of cytoplasmic exosome function since xrn1Δski2Δ mutant is inviable (Supplementary Figure S6).
Figure 5.
Genetic interactions between dis3l2+ and the components of cytoplasmic RNA degradation pathway. (A) dis3l2+ deletion is synthetically lethal with deletion of xrn1+. Haploid dis3l2+::kan cells were crossed with xrn1+::hph strain. Resulting diploids were sporulated and tetrads were dissected on YES plates. In the bottom table, the genotypes of the germinated spores are described: WT—wild-type strain, X—xrn1Δ, D—dis3l2Δ. Genotypes of the spores were analysed by their ability to grow on the selective media and by colony PCR. (B) dis3l2+ deletion enhances the growth defect of lsm1Δ strain. Overnight yeast cultures were diluted in the fresh media (EMM) to OD600 of 0.1, subsequently grown at 32°C and the OD monitored over time.
Single dis3l2+ deletion did not have much impact on S. pombe cells growth in the conditions tested (Figure 5B). Deletion of ski2+ or double deletion of dis3l2+ together with ski2+ also did not have significant effect on growth (Figure 5B). Likewise, deletions of SKI genes in S. cerevisiae have only mild effect on growth, which is due to the redundancy of the 5′ and 3′ direction degradation pathways in the cytoplasm (Muhlrad et al, 1995). Deletion of lsm1+ in S. pombe resulted in a slow growth phenotype, similarly to the situation in S. cerevisiae (Yoshikawa et al, 2011). Lsm1 is one of the decapping activators and its deletion decreases decapping rate, resulting in the accumulation of capped deadenylated transcripts (Tharun et al, 2000; Rissland and Norbury, 2009). However, deletion of lsm1+ together with dis3l2+ generated a larger growth defect than that of lsm1Δ single mutants (Figure 5B). This is in agreement with the involvement of Dis3L2 in the 3′-5′ degradation pathway, as suggested by its synthetic lethality with xrn1Δ. Since single lsm1+ deletion impairs to some extent the 5′-3′ degradation by hampering decapping, the cellular mRNA degradation in lsm1Δ mutants relies more on 3′-5′ directional decay and therefore is much more sensitive to the defects of this pathway.
Deletion of dis3l2+ in lsm1Δ mutants results in transcripts accumulation and slower degradation rates
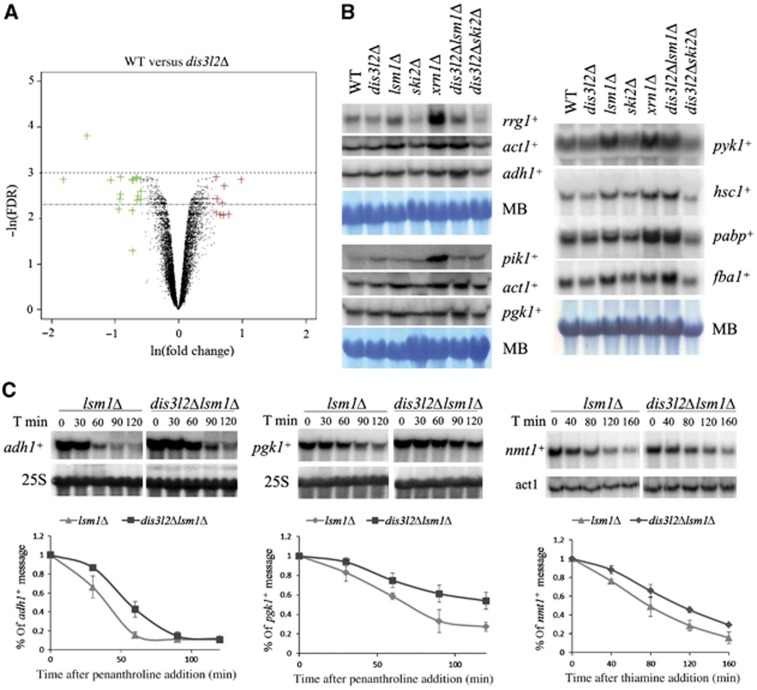
To investigate the impact of dis3l2+ deletion on mRNA metabolism, we monitored changes in mRNA steady-state levels between wild-type strain and dis3l2Δ mutant using microarray analysis (Figure 6A). The summary representation of the results using volcano plot shows no striking differences in transcripts levels between both strains, with only 9 messengers being >1.5-fold upregulated and 15 downregulated. This was in agreement with the lack of obvious growth phenotype in the single dis3l2+ deletion mutant.
Figure 6.
Dis3L2 is involved in mRNA degradation. (A) Volcano plot representation of microarray data. S. pombe mRNA fold change values (ln(fold change)) between wild-type and single dis3l2Δ deletion strains are plotted against the result significance (−ln(FDR)). Genes upregulated in dis3l2Δ >1.5 times are coloured in red and the ones downregulated >1.5 times coloured in green. The chosen significance window is indicated by dotted lines. (B) Northern blots of total RNA obtained from the wild-type and different deletion mutant strains. Loading was controlled by methylene blue (MB) staining. (C) Deletion of dis3l2+ in lsm1Δ strain background results in slower transcript degradation rates. lsm1Δ and dis3l2Δlsm1Δ yeast strains were grown in full media (adh1+ and pgk1+ messengers) or minimal media (nmt1+ messenger) until mid-log phase, and transcription was subsequently stopped by either 1,10-penanthroline (adh1+ and pgk1+) or thiamine (nmt1+) addition. Cells were harvested at the indicated time points after transcription blockage, total RNA was isolated and message decay investigated by Northern blots analysis. Upper part shows an example of Northern blot results, lower part graphs illustrate the comparison of the decay rates of the indicated transcripts in lsm1Δ and dis3l2Δlsm1Δ yeast strains. Data were collected in three independent experiments, error bars represent standard deviation.
Source data for this figure is available on the online supplementary information page.
To complement these results, we used Northern blot analysis to compare the levels of nine different cellular mRNAs derived from exponentially growing wild-type cells versus dis3l2Δ, lsm1Δ, xrn1Δ, ski2Δ single mutants and dis3l2Δlsm1Δ and dis3l2Δski2Δ double mutants (Figure 6B). Individual transcripts showed different behaviour in the mutant backgrounds investigated, revealing the complexity of the degradation pathways. In agreement with the microarray results, single dis3l2+ deletion had no significant effect on the levels of the transcripts analysed. Similarly, we noticed no significant changes in ski2Δ and dis3l2Δ ski2Δ double mutants. However, we could observe an accumulation of the mRNAs in xrn1Δ and lsm1Δ single mutants and dis3l2Δ lsm1Δ double mutants for most of the investigated transcripts. In two out of nine transcripts (pik1+, rrg1+), the steady-state levels were greatly affected by xrn1+ deletion suggesting that their degradation depends mainly on this nuclease. Regarding the lsm1Δ single and dis3l2Δ lsm1Δ double mutants, in three cases (adh1+, pabp+, and fba1+) we could clearly observe stronger mRNA accumulation in the double mutant.
Taking into account the Dis3L2 exoribonucleolytic activity, we inferred that the additive effect of dis3l2+ deletion on transcript accumulation in lsm1Δ mutants could be caused by a slower degradation rate. To test this hypothesis, we monitored mRNA degradation rate after transcription arrest with 1,10-phenanthroline (Lackner et al, 2007; Passos and Parker, 2008). We chose the adh1+ transcript as the first target for our analysis since its steady-state level was significantly affected by dis3l2+ deletion in the lsm1Δ mutant background (Figure 6B; Supplementary Figure S7).
A single dis3l2+ deletion does not impact degradation rates of the studied transcripts (Supplementary Figure S8). However, in an lsm1Δ mutant background dis3l2+ deletion causes a significantly slower degradation of adh1+ transcript (Figure 6C). We performed similar analysis for pgk1+ transcript and again, we observed impaired degradation in double mutant compared with the single lsm1Δ strain (Figure 6C). A slightly different approach was followed to monitor differences in nmt1+ transcript degradation rate: we blocked transcription of nmt1+ message by addition of thiamine to the media (Maundrell, 1990). In contrast to the treatment with phenanthroline, in these conditions cellular metabolism is not widely affected. Again, we saw slower nmt1+ transcript degradation rate in dis3l2Δ lsm1Δ double mutant compared to the lsm1Δ strain (Figure 6C). These results suggest an involvement of Dis3L2 in the degradation of the transcripts analysed.
Messenger degradation is performed by redundant pathways and the effect of a deletion in a single element does not usually provide a phenotype. Consistently, we can see the effect of dis3l2+ deletion in the background of a lsm1Δ strain, because this is impaired in 5′-3′ degradation pathway which in normal conditions masks the effects of the loss of dis3l2+.
dis3l2+ deletion results in accumulation of 3′ uridylated transcripts
To further investigate the effect of dis3l2+ deletion on messenger RNAs, we looked at the polyadenylation status of adh1+ transcript in different mutant backgrounds. Sequences of 3′-ends of adh1+ message were analysed using optimized 3′-RACE method (Poly(A) Status Examination—PASE method) (Alexander et al, 2010).
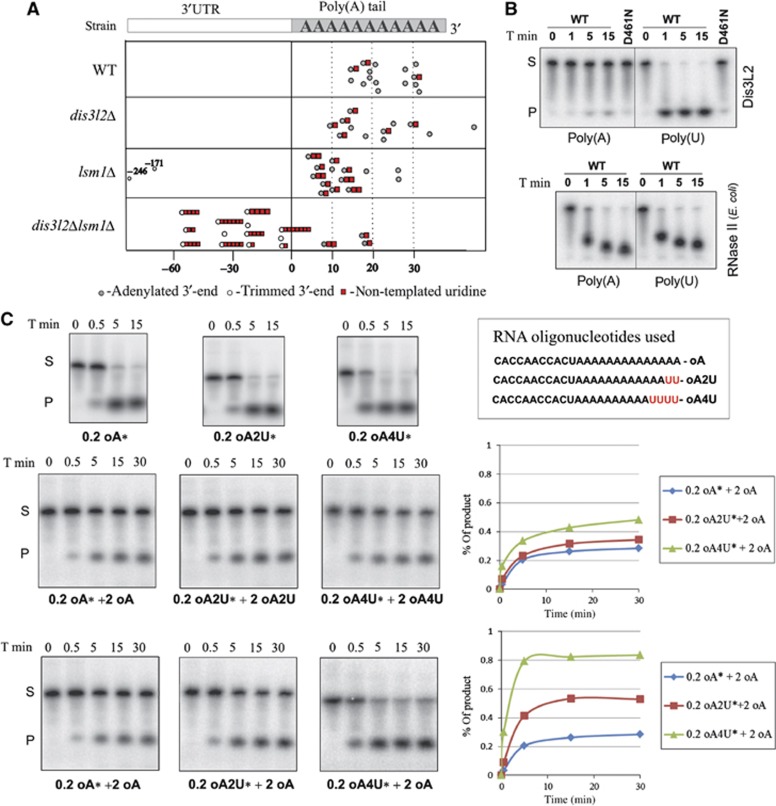
adh1+ mRNA 3′-end sequences derived from the wild-type cells were in general full-length polyadenylated transcripts. We could only detect some low level of uridylation of these polyadenylated mRNAs (3 out of 15 sequences) (Figure 7A), which was in agreement with previous data (Rissland and Norbury, 2009). Deletion of dis3l2+ did not significantly change the polyadenylation status of the transcripts. dis3l2Δ strain exhibited a slight increase in the uridylation of the polyadenylated messengers (6 out of 12); however, the sample is too small to ensure the statistical accuracy of this result. Single lsm1+ deletion resulted in the accumulation of uridylated messengers with shortened poly(A) tails, again in agreement with the results obtained (Rissland and Norbury, 2009). In lsm1Δ strain, we also detected two 3′ shortened adh1+ messengers, which could represent degradation by-products (Figure 7A).
Figure 7.
Dis3L2 preferentially degrades uridylated RNAs. (A) Deletion of dis3l2+ in Δlsm1 background results in the accumulation of trimmed and uridylated adh1+ transcripts. Each circle point in the table indicates the position of one adh1+ mRNA 3′-end. Point zero represents the standard polyadenylation site of adh1+ mRNA. Positive values correspond to the length of poly(A) tail attached to the polyadenylation site, and the negative values correspond to the 3′-end position in 3′-UTR if mRNA was trimmed. Uridylation is indicated by the red squares, and number of squares corresponds to the number of non-templated U residues detected at the 3′-end. (B) Dis3L2 exhibits stronger preference for poly(U) compared to poly(A) RNA substrate in vitro. Dis3L2 protein (WT) and mutated version (D461N) were incubated with the same amounts of radioactively labelled poly(A) or poly(U) RNAs. Reaction was stopped at the indicated time points and products were separated on denaturing polyacrylamide gels. The same substrates were incubated with bacterial RNase II. (C) Uridine residues added to the 3′-end can effectively target RNA substrates for degradation by Dis3L2 in vitro. Same amounts of Dis3L2 protein were incubated with the indicated amounts of different RNA substrates (0.2 or 2 pmol). Substrate sequence is indicated. Reactions with radioactive substrates (labelled with *) were supplemented with the substrates that were not radioactively labelled. Reactions were stopped at the indicated time points (T min) and products were separated on denaturing polyacrylamide gels. The graphs (on the right side) depict the accumulation of the product at different time points as calculated using Image Quant.
Source data for this figure is available on the online supplementary information page.
Surprisingly, we could only detect a few full-length polyadenylated messages in dis3l2Δlsm1Δ double mutants (3 out of 16), and all of them were uridylated. The rest of the sequences corresponded to the 3′-trimmed mRNAs and most of them (10 out of 13) were uridylated and not adenylated. In contrast to the previously observed uridylation, which consisted mainly on the addition of one or two U residues to poly(A) or oligo(A) tails, the U tails added to the trimmed messengers were significantly longer (up to seven U residues) (Figure 7A). The detected messages were trimmed up to around −60 position counting from the polyadenylation site (Supplementary Figure S9). In S. cerevisiae, Lsm1 was shown to protect the 3′-ends of the transcripts from trimming and 3′-5′ degradation (He and Parker, 2001). We propose that in the case of S. pombe, Dis3L2 can remove trimmed transcripts that accumulate in lsm1Δ strain. Accordingly, these transcripts can only be detected in lsm1Δdis3l2Δ strain. Extensive uridylation of these 3′-end trimmed messengers suggests that the addition of the U tails can help in the removal of these mRNAs either by attracting the binding of the components of the RNA degradation machinery or by facilitating the degradation of secondary structures, similar to that proposed for histone mRNA degradation (Mullen and Marzluff, 2008).
Dis3L2 preferentially degrades uridylated RNAs
To check if Dis3L2 has any preference towards uridylated substrates, we compared the enzyme activity over 30 nt poly(A) and poly(U) RNA substrates. Whereas Dis3L2 rapidly degrades poly(U) polymer, the enzyme is almost completely inhibited by the same amount of poly(A) (Figure 7B). When the same substrates were incubated with bacterial RNase II (the exoribonuclease that is the prototype of the RNase II family of enzymes), both of them were rapidly degraded (Figure 7B).
Uridylated 3′-ends of adh1+ message detected in 3′ RACE experiments usually contained few non-templated uridines attached directly to the sequence or to the poly(A) tail. To check if these short stretches of uridines could influence transcripts degradation by Dis3L2, we compared enzyme activity against RNA oligonucleotides with a 11-nt random sequence at the 5′-end, followed either by 14 adenines (oA), 12 adenines and 2 uridines (oA2U), or by 10 adenines and 4 uridines (oA4U) (Figure 7C). First, we performed the reactions with a small amount of each of the substrates, radioactively labelled at the 5′-end. We used 0.2 pmol of substrate per reaction and incubated it with 0.5 pmol of Dis3L2 enzyme. During the time course of the experiment, all the substrates were rapidly degraded after several seconds (Figure 7C, first row). The amounts of substrate and enzyme were comparable to the ones used in the experiment with poly(A) substrate (Figure 7B). We conclude that the addition of a random sequence to the 5′-end of the oligonucleotide in addition to poly(A) tail, caused its faster degradation compared to poly(A) polymer. The impact of 5′ sequence on the reaction is probably due to the change in the efficiency of substrate binding. The weak binding of Dis3L2 to poly(A) sequence would explain the very slow reaction rate (Figure 7B).
To better compare the efficiency of degradation of different oligouridylated substrates, we increased their concentration by supplementing each reaction with 2 pmol of non-labelled oligonucleotides of the same type. In these conditions, all oligonucleotides used were degraded with similar efficiency (Figure 7C, second row). We noticed that efficiency of the reaction slowed down with time which was due to inactivation of the enzyme (Supplementary Figure S10A). To check if uridylated substrate can out-compete non-uridylated RNA, we performed degradation experiments supplementing 0.2 pmol of each substrate (oA, oA2U, or oA4U) with 2 pmol of oA oligonucleotide. This time we observed almost two times more substrate being degraded in the case of RNA with two uridines at the 3′-end (oA2U) when compared to the non-uridylated oA substrate. Moreover, 0.2 pmol of RNA substrate with a four uridine tail (oA4U) supplemented with adenylated RNA (oA) was degraded almost completely, with an efficiency similar to the one observed for non-supplemented o4AU substrate (Figure 7C, third row). Therefore, a four uridine tail caused the enzyme to degrade almost exclusively uridylated substrate, even if non-uridylated RNA was in 10 time excess in the reaction mixture. Thus, Dis3L2 has a strong preference for oligo-uridylated substrates in vitro. In agreement with this experiment, addition of excess of either oA2U or oA4U to labelled oA inhibited oA degradation rate (Supplementary Figure S10B).
These results show that in vitro uridylation can specifically mark RNA for degradation by Dis3L2 and that adenylation inhibits enzyme activity. Together with the observed accumulation of uridylated adh1+ messengers in the dis3l2Δ lsm1Δ strain, our data strongly suggest that one of Dis3L2 cellular functions is to remove uridylated RNA substrates.
Discussion
Using BLAST search and S. cerevisiae Rrp44/Dis3 sequence as a query we have identified a new S. pombe nuclease. Phylogenetic analysis showed that the identified protein not only belongs to the RNase II family of enzymes but also is a member of the conserved Dis3L2 family. Dis3L2 proteins can be found in most of the eukaryotic genomes with S. cerevisiae being one of the few exceptions. Our in vitro studies confirmed that S. pombe Dis3L2 is an active 3′-5′ exonuclease and is capable to digest RNA even in the context of secondary structures.
Analysis of Dis3L2 function in S. pombe showed its involvement in cytoplasmic mRNA degradation, independently of the exosome. Dis3L2 is an active exoribonuclease and it can co-localize with P-bodies, where mRNA degradation occurs. Although single dis3l2+ deletion does not have an obvious phenotype, its mutation is synthetically lethal with deletion of xrn1+ exonuclease gene. Synthetic lethality of dis3l2+ with xrn1+ gene strongly supports an important physiological function for Dis3L2 in the 3′-5′ degradation pathway.
Single dis3l2+ deletion does not have an evident impact on mRNA steady-state levels or half-lives, what is not surprising taking into account the redundancy of degradation systems (Johnson and Kolodner, 1995; Anderson and Parker, 1998). However, we could observe the effect of dis3l2+ deletion in the background of lsm1Δ strain. Lsm1 is known to be a decapping activator, and its removal impairs the 5′-3′ degradation pathway (Bouveret et al, 2000; Tharun et al, 2000). In lsm1Δ background, dis3l2+ deletion causes the accumulation of transcripts and a slower degradation of the mRNAs tested. We considered this result as a direct effect of the lack of Dis3L2 exonucleolytic activity. This suggests an involvement of Dis3L2 in cytoplasmic mRNA degradation in S. pombe. Interestingly, mutation of SOV, the A. thaliana Dis3L2 homologue, gives strong phenotypes with defects on the decapping complex scaffold protein VCS (Zhang et al, 2010). Moreover, we were informed about an independent study concerning human DIS3L2 which similarly shows the involvement of this enzyme in bulk mRNA decay (Lubas et al, 2013). All these results suggest a conserved function for Dis3L2 family proteins in eukaryotes.
Our data suggest that Dis3L2 acts independently from the exosome, since it does not co-localize or co-purify with the exosome components. This is also supported by a parallel work showing that human DIS3L2 does not interact with the exosome (Lubas et al, 2013). Moreover, the N-terminal CR3 region and PIN domain responsible for tethering Dis3 proteins with the exosome are not conserved in Dis3L2 family.
Analysis of the 3′-end of adh1 transcripts showed that in the double dis3l2Δlsm1Δ deletion strain 3′-trimmed transcripts accumulate. We consider that Dis3L2 is the enzyme that is capable of removing trimmed transcripts in the single lsm1Δ deletion strain. Therefore, the accumulation of these transcripts in the double mutant is a result of the absence of the Dis3L2 exoribonuclease. Similar phenotype was observed in S. cerevisiae for LSM1 SKI2 double deletion mutant (He and Parker, 2001), which suggests that in budding yeast the exosome has taken over some of the Dis3L2 functions.
There is extensive uridylation of the trimmed transcripts detected in the dis3l2Δlsm1Δ double deletion strain. Uridine tails were added directly to the trimmed 3′-ends and they were substantially longer when compared to those present in the adenylated transcripts of the wild-type cells. It was shown that in S. pombe uridylation of both oligo- and polyadenylated messages can trigger decapping and 5′-3′ degradation, probably by attracting Lsm1 binding to the 3′-end (Rissland and Norbury, 2009). Uridylation of trimmed messengers observed in our experiments could also induce 5′-3′ decay, but their accumulation after deletion of 3′-5′ exonuclease Dis3L2 proposes other explanations. Our in vitro data suggest that Dis3L2 prefers to degrade uridylated messages, being inhibited by poly(A) sequences. Such enzyme properties make it a perfect ‘degrading machine’ able to remove not only all the non-polyadenylated decay products like 3′-trimmed transcripts observed in our experiments, but also messengers tagged for fast removal by uridylation.
Uridylation can trigger decapping and 5′-3′ degradation and even protect 3′-ends from exonucleases (Shen and Goodman, 2004; Song and Kiledjian, 2007). However, our results suggest that uridylation can also induce 3′-5′ degradation of the messengers. We hypothesize that a different function of the same process can be possible due to some spatial separation of factors responsible for 5′-3′ degradation and decapping (P-bodies), and Dis3L2, which localizes in the structures docked to P-bodies. Interestingly, in stress conditions like glucose deprivation Dis3L2 starts to completely co-localize with the P-bodies, which may be the way of accelerating degradation of uridylated RNAs. To our knowledge, this is the first time that 3′-5′ activity is seen to occur in P-bodies.
More research will be needed to discover up to what extent uridylation drives Dis3L2-dependent degradation in vivo, but it is tempting to speculate that short poly(U) tails would label the RNAs for rapid degradation by Dis3L2, resembling nuclear quality control mediated by TRAMP complex and the exosome (LaCava et al, 2005). Recent work has been revealing that uridylation of RNA forms the basis of an evolutionary conserved mechanism of gene regulation (Schmidt and Norbury, 2010). It would be interesting to check if Dis3L2 homologues from other organisms share its biochemical properties. Dis3L2 preference for uridylated RNAs suggests that proteins of this family could be involved in the degradation of growing number of cytoplasmic uridylated substrates like histone mRNAs or uridylated small non-coding RNAs (Shen and Goodman, 2004; Mullen and Marzluff, 2008; Choi et al, 2012; Thornton et al, 2012).
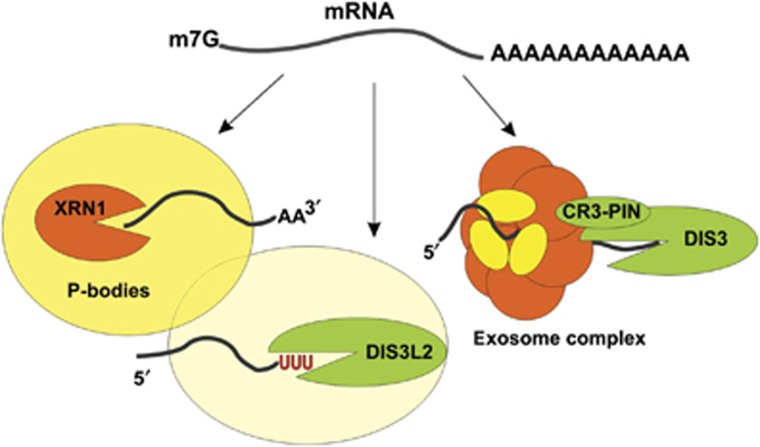
Regardless of the mechanism triggering degradation it has been widely established that the final step of RNA decay proceeds in either 5′-3′ direction (catalysed by Xrn1 exoribonuclease) or 3′-5′ direction (catalysed by the exosome complex). However, our work demonstrates that Dis3L2 is an important novel factor in RNA decay, degrading RNAs independently of the exosome. Therefore, this newly discovered 3′–5′ cytoplasmic degradation pathway should be considered as an alternative to Xrn1 and exosome degradation (Figure 8). Future research will surely uncover the details of the interplay between these three cytoplasmic mRNA degradation pathways.
Figure 8.
Three pathways of mRNA degradation in the cytoplasm. In S. pombe and possibly in most other eukaryotes, mRNAs can be degraded in the 5′-3′ direction by Xrn1 exonuclease, or in the 3′-5′ direction by either the exosome complex or Dis3L2 exonuclease, which acts independently from the exosome. Our results suggest that in S. pombe Dis3L2 degradation pathway is connected with the P-bodies.
Materials and methods
Yeast strains and media
The strains used in this work are listed in Supplementary Table 1. Strains were constructed using standard techniques (Bahler et al, 1998). Standard media and growth conditions were used throughout this work (Moreno et al, 1991). For strains construction one new plasmid (pFA6a TAP kan) was created to introduce TAP tag sequence into pFA6a kan MX6 backbone (details in Supplementary Materials). Genetic interactions were assessed through standard mating techniques at permissive temperatures, followed by tetrad dissection on YES plates. Plates were monitored after 4 days.
Microscopy
Unless stated otherwise, cells were grown at 32°C in EMM. For GFP and mRFP visualization, live cells were imaged using a Delta Vision Core System (Applied Precision) using a × 100 1.4 numerical aperture UplanSApo objective and a cascade2 EMCCD camera (Photometrics). Images were acquired at 14 z-sections separated by 0.2 μm intervals. Deconvolution was performed using the enhanced ratio method in softWoRx software. Co-localization experiments were performed using maximum intensity projections of deconvoluted images. Image J software was used for final image preparation. For nucleoid staining, cells were incubated for 10 min in 0.5 μg/ml Hoechst solution and subsequently resuspended in fresh EMM.
Dis3L2 protein purification
SPAC2C4.07c (dis3l2+) sequence was cloned into pGEX-4T-1 vector (GE Healthcare) using EcoRI and XhoI restriction sites. Point mutations were introduced into the cloned sequence using primers (Lmut_Dis3L2; R_mut_Dis3L2) which changed D461 codon for N, and introduced additional silent mutation that results in the new XhoI restriction site in the sequence. The purification of the wild-type and mutated version of Dis3l2 protein was performed in parallel (details in Supplementary data).
In vitro assays
For each assay, radioactive substrate (supplemented or not with non-labelled oligonucleotide) was mixed with around 0.5 pmol of purified proteins (either wild-type or point mutated version) and the reaction was carried out at 30°C in 40 μl of the reaction buffer: 25 mM NaCl, 5 mM MgCl2, 1 mM DTT, 10 mM Tris pH 7.5. At the indicated times, reactions were stopped by adding 2 × RNA loading buffer (95% formamide, 0.025% SDS, 5 mM EDTA) supplemented with 0.5 mg/ml of total yeast RNA. Reaction products were separated on 20% denaturing polyacrylamide gels and then visualized and calculated using Storm Scan and Image Quant systems.
TAP Tag protein purification
In all, 300 ml of cells was grown in full media (YES) to the early exponential stage (OD600∼0.6). Yeasts were then harvested, resuspended in IPP150 buffer and lysed using FastPrep-24 (MP Biomedicals). TAP tag purification was performed as described (Rigaut et al, 1999). After final elution from calmodulin, half of the elution was separated on SDS–PAGE gel, silver stained according to the protocol (Chevallet et al, 2006), and the other half was acetone precipitated and sent for mass spectrometry analysis (http://mslab-ibb.pl/).
RNA isolation
RNA was extracted by the phenol-chloroform isolation method (see details in Supplementary Materials).
RNA half-life
Two methods were used to stop transcription and monitor degradation of different messengers. First, cellular transcription was stopped using 1,10-phenanthroline (Sigma) (Lackner et al, 2007; Passos and Parker, 2008). Cells were grown in 200 ml of YES media until OD600∼0.6, subsequently spun down and resuspended back in 20 ml of prewarmed YES supplemented with 100 μg/ml of phenanthroline. At the indicated times, 2 ml of cells was spun down and pellets snap frozen.
In the other method, half-life of the thiamine messenger was monitored after its transcription was specifically blocked by addition of thiamine to the media (Maundrell, 1990). Cells were grown in 200 ml of EMM media until early exponential phase, then thiamine (Sigma) was added to the final concentration of 2 μM (time zero). Subsequently, at the indicated times, 20 ml of cells was harvested and snap frozen.
Northern blots
Equal amounts of total yeast RNA (10–15 μg) were separated in agarose formaldehyde gels. Gel preparation and blotting procedure were according to Qiagen protocols (www.qiagen.com/literature/benchguide/bg_rna_lit.aspx). RNA was transferred onto the nylon membrane (GE Healthcare), loading and transfer were monitored using methylene blue staining (Sigma) or later by hybridization with probes against rRNAs. RNA was hybridized against DNA probes, either with long PCR fragments labelled with αP32ATP using random primer DNA labelling system (Invitrogen), or with short oligonucleotides labelled with γP32ATP using PNK (Fermentas). PERFECTHYB Plus (Sigma) buffer was used for hybridization and conditions were according to manufacturer’s instructions. Primers used to prepare the probes are listed in Supplementary Table 2.
RNA 3′-RACE
We used optimized 3′-RACE method (PASE method) according to the protocol (Alexander et al, 2010). Total RNA from different mutants was used as the template for RT–PCR and PCR. Final PCR products were cloned into pGEM-T easy vector (pGEM-T Easy Vector System, Promega) and inserts were sequenced using T7 or SP6 promoters. Primers used for PASE reaction are listed in Supplementary Table 2.
Phylogenetic analysis and microarray analysis
Detailed procedures are described in Supplementary Materials.
Supplementary Material
Acknowledgments
We acknowledge Sarah Newbury for the critical reading of the manuscript. We also thank E Bouveret, A Dziembowski, and I Kühl for their kind gift of plasmids; R Matos for RNase II sample; A Aires for technical assistance and R Szczesny for critical discussions. The work at ITQB was supported by Fundação para a Ciência e Tecnologia (FCT), Portugal (including grant PEst-OE/EQB/LA0004/2011) and by grant FP7-KBBE-2011-1-289326 from European Commission. SC Viegas was recipient of an FCT Post-Doctoral Fellowship (SFRH/BPD/30766/2006). MM was recipient of a Marie Curie Individual European Fellowship (PIEF-GA-2009-254183).
Author contributions: MM planned and performed most of the experiments and prepared first version of the manuscript. SCV performed part of Northern blot analysis. TC performed tetrad dissection, part of microscopy analysis and advised in yeast techniques. PG did the phylogenetic analysis and prepared part of the manuscript concerning this topic. CD did the analysis of microarray data. MGF advised and gave support in yeast techniques and contributed to the manuscript. CMA supervised all the work performed; CMA, SCV, and MM prepared final versions of the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Alexander RD, Barrass JD, Dichtl B, Kos M, Obtulowicz T, Robert MC, Koper M, Karkusiewicz I, Mariconti L, Tollervey D, Kufel J, Bertrand E, Beggs JD (2010) RiboSys, a high-resolution, quantitative approach to measure the in vivo kinetics of pre-mRNA splicing and 3′-end processing in Saccharomyces cerevisiae. RNA 16: 2570–2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amblar M, Arraiano CM (2005) A single mutation in Escherichia coli ribonuclease II inactivates the enzyme without affecting RNA binding. FEBS J 272: 363–374 [DOI] [PubMed] [Google Scholar]
- Anderson JS, Parker RP (1998) The 3′ to 5′ degradation of yeast mRNAs is a general mechanism for mRNA turnover that requires the SKI2 DEVH box protein and 3' to 5' exonucleases of the exosome complex. EMBO J 17: 1497–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P, Kedersha N (2008) Stress granules: the Tao of RNA triage. Trends Biochem Sci 33: 141–150 [DOI] [PubMed] [Google Scholar]
- Astuti D, Morris MR, Cooper WN, Staals RH, Wake NC, Fews GA, Gill H, Gentle D, Shuib S, Ricketts CJ, Cole T, van Essen AJ, van Lingen RA, Neri G, Opitz JM, Rump P, Stolte-Dijkstra I, Muller F, Pruijn GJ, Latif F et al. (2012) Germline mutations in DIS3L2 cause the Perlman syndrome of overgrowth and Wilms tumor susceptibility. Nat Genet 44: 277–284 [DOI] [PubMed] [Google Scholar]
- Bahler J, Wu JQ, Longtine MS, Shah NG, McKenzie A 3rd, Steever AB, Wach A, Philippsen P, Pringle JR (1998) Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14: 943–951 [DOI] [PubMed] [Google Scholar]
- Balagopal V, Fluch L, Nissan T (2012) Ways and means of eukaryotic mRNA decay. Biochim Biophys Acta 1819: 593–603 [DOI] [PubMed] [Google Scholar]
- Bouveret E, Rigaut G, Shevchenko A, Wilm M, Seraphin B (2000) A Sm-like protein complex that participates in mRNA degradation. EMBO J 19: 1661–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brengues M, Teixeira D, Parker R (2005) Movement of eukaryotic mRNAs between polysomes and cytoplasmic processing bodies. Science 310: 486–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan JR, Muhlrad D, Parker R (2008) P bodies promote stress granule assembly in Saccharomyces cerevisiae. J Cell Biol 183: 441–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallet M, Luche S, Rabilloud T (2006) Silver staining of proteins in polyacrylamide gels. Nat Protoc 1: 1852–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Patena W, Leavitt AD, McManus MT (2012) Widespread RNA 3'-end oligouridylation in mammals. RNA 18: 394–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker CJ, Parker R (2012) P-bodies and stress granules: possible roles in the control of translation and mRNA degradation. Cold Spring Harb Perspect Biol 4: a012286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinnenberg IA, Weinberg DE, Xie KT, Mower JP, Wolfe KH, Fink GR, Bartel DP (2009) RNAi in budding yeast. Science 326: 544–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziembowski A, Lorentzen E, Conti E, Seraphin B (2007) A single subunit, Dis3, is essentially responsible for yeast exosome core activity. Nat Struct Mol Biol 14: 15–22 [DOI] [PubMed] [Google Scholar]
- Dziembowski A, Piwowarski J, Hoser R, Minczuk M, Dmochowska A, Siep M, van der Spek H, Grivell L, Stepien PP (2003) The yeast mitochondrial degradosome. Its composition, interplay between RNA helicase and RNase activities and the role in mitochondrial RNA metabolism. J Biol Chem 278: 1603–1611 [DOI] [PubMed] [Google Scholar]
- Frazão C, McVey CE, Amblar M, Barbas A, Vonrhein C, Arraiano CM, Carrondo MA (2006) Unravelling the dynamics of RNA degradation by ribonuclease II and its RNA-bound complex. Nature 443: 110–114 [DOI] [PubMed] [Google Scholar]
- Garneau NL, Wilusz J, Wilusz CJ (2007) The highways and byways of mRNA decay. Nat Rev Mol Cell Biol 8: 113–126 [DOI] [PubMed] [Google Scholar]
- He W, Parker R (2001) The yeast cytoplasmic LsmI/Pat1p complex protects mRNA 3' termini from partial degradation. Genetics 158: 1445–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann B, Nickel J, Speer F, Schafer B (2008) The 3′ ends of mature transcripts are generated by a processosome complex in fission yeast mitochondria. J Mol Biol 377: 1024–1037 [DOI] [PubMed] [Google Scholar]
- Houseley J, LaCava J, Tollervey D (2006) RNA-quality control by the exosome. Nat Rev Mol Cell Biol 7: 529–539 [DOI] [PubMed] [Google Scholar]
- Houseley J, Tollervey D (2009) The many pathways of RNA degradation. Cell 136: 763–776 [DOI] [PubMed] [Google Scholar]
- Huh WK, Falvo JV, Gerke LC, Carroll AS, Howson RW, Weissman JS, O’Shea EK (2003) Global analysis of protein localization in budding yeast. Nature 425: 686–691 [DOI] [PubMed] [Google Scholar]
- Johnson AW, Kolodner RD (1995) Synthetic lethality of sep1 (xrn1) ski2 and sep1 (xrn1) ski3 mutants of Saccharomyces cerevisiae is independent of killer virus and suggests a general role for these genes in translation control. Mol Cell Biol 15: 2719–2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurischko C, Kim HK, Kuravi VK, Pratzka J, Luca FC (2011) The yeast Cbk1 kinase regulates mRNA localization via the mRNA-binding protein Ssd1. J Cell Biol 192: 583–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCava J, Houseley J, Saveanu C, Petfalski E, Thompson E, Jacquier A, Tollervey D (2005) RNA degradation by the exosome is promoted by a nuclear polyadenylation complex. Cell 121: 713–724 [DOI] [PubMed] [Google Scholar]
- Lackner DH, Beilharz TH, Marguerat S, Mata J, Watt S, Schubert F, Preiss T, Bahler J (2007) A network of multiple regulatory layers shapes gene expression in fission yeast. Mol Cell 26: 145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebreton A, Tomecki R, Dziembowski A, Seraphin B (2008) Endonucleolytic RNA cleavage by a eukaryotic exosome. Nature 456: 993–996 [DOI] [PubMed] [Google Scholar]
- Ling SH, Qamra R, Song H (2011) Structural and functional insights into eukaryotic mRNA decapping. Wiley Interdiscip Rev RNA 2: 193–208 [DOI] [PubMed] [Google Scholar]
- Lubas M, Damgaard CK, Tomecki R, Cysewski D, Jensen TH, Dziembowski A (2013) Exonuclease hDIS3L2 specifies an exosome-independent 3′-5′ degradation pathway of human cytoplasmic mRNA. EMBO J 32: 1855–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino DL, Baumgartner M, Conti E (2013) Crystal structure of an RNA-bound 11-subunit eukaryotic exosome complex. Nature 495: 70–75 [DOI] [PubMed] [Google Scholar]
- Malecki M, Jedrzejczak R, Stepien PP, Golik P (2007) In vitro reconstitution and characterization of the yeast mitochondrial degradosome complex unravels tight functional interdependence. J Mol Biol 372: 23–36 [DOI] [PubMed] [Google Scholar]
- Marshall AN, Montealegre CM, Jiménez-López C, Lorenz MC, van Hoof A (2013) Alternative splicing and subfunctionalization generates functional diversity in fungal proteomes. PLoS Genet (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos RG, Fialho AM, Giloh M, Schuster G, Arraiano CM (2012) The rnb gene of Synechocystis PCC6803 encodes a RNA hydrolase displaying RNase II and not RNase R enzymatic properties. PLoS ONE 7: e32690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama A, Arai R, Yashiroda Y, Shirai A, Kamata A, Sekido S, Kobayashi Y, Hashimoto A, Hamamoto M, Hiraoka Y, Horinouchi S, Yoshida M (2006) ORFeome cloning and global analysis of protein localization in the fission yeast Schizosaccharomyces pombe. Nat Biotechnol 24: 841–847 [DOI] [PubMed] [Google Scholar]
- Maundrell K (1990) nmt1 of fission yeast. A highly transcribed gene completely repressed by thiamine. J Biol Chem 265: 10857–10864 [PubMed] [Google Scholar]
- Meyer S, Temme C, Wahle E (2004) Messenger RNA turnover in eukaryotes: pathways and enzymes. Crit Rev Biochem Mol Biol 39: 197–216 [DOI] [PubMed] [Google Scholar]
- Mitchell P, Petfalski E, Shevchenko A, Mann M, Tollervey D (1997) The exosome: a conserved eukaryotic RNA processing complex containing multiple 3′--5′ exoribonucleases. Cell 91: 457–466 [DOI] [PubMed] [Google Scholar]
- Moazed D (2009) Small RNAs in transcriptional gene silencing and genome defence. Nature 457: 413–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol 194: 795–823 [DOI] [PubMed] [Google Scholar]
- Morozov IY, Jones MG, Razak AA, Rigden DJ, Caddick MX (2010) CUCU modification of mRNA promotes decapping and transcript degradation in Aspergillus nidulans. Mol Cell Biol 30: 460–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlrad D, Decker CJ, Parker R (1995) Turnover mechanisms of the stable yeast PGK1 mRNA. Mol Cell Biol 15: 2145–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen TE, Marzluff WF (2008) Degradation of histone mRNA requires oligouridylation followed by decapping and simultaneous degradation of the mRNA both 5′ to 3′ and 3′ to 5′. Genes Dev 22: 50–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson D, Sunnerhagen P (2011) Cellular stress induces cytoplasmic RNA granules in fission yeast. RNA 17: 120–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissan T, Rajyaguru P, She M, Song H, Parker R (2010) Decapping activators in Saccharomyces cerevisiae act by multiple mechanisms. Mol Cell 39: 773–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos DO, Parker R (2008) Analysis of cytoplasmic mRNA decay in Saccharomyces cerevisiae. Methods Enzymol 448: 409–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaut G, Shevchenko A, Rutz B, Wilm M, Mann M, Seraphin B (1999) A generic protein purification method for protein complex characterization and proteome exploration. Nat Biotechnol 17: 1030–1032 [DOI] [PubMed] [Google Scholar]
- Rissland OS, Norbury CJ (2009) Decapping is preceded by 3′ uridylation in a novel pathway of bulk mRNA turnover. Nat Struct Mol Biol 16: 616–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer D, Clark A, Klauer AA, Tsanova B, van Hoof A (2011) Functions of the cytoplasmic exosome. Adv Exp Med Biol 702: 79–90 [DOI] [PubMed] [Google Scholar]
- Schaeffer D, Reis FP, Johnson SJ, Arraiano CM, van Hoof A (2012) The CR3 motif of Rrp44p is important for interaction with the core exosome and exosome function. Nucleic Acids Res 40: 9298–9307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer D, Tsanova B, Barbas A, Reis FP, Dastidar EG, Sanchez-Rotunno M, Arraiano CM, van Hoof A (2009) The exosome contains domains with specific endoribonuclease, exoribonuclease and cytoplasmic mRNA decay activities. Nat Struct Mol Biol 16: 56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MJ, Norbury CJ (2010) Polyadenylation and beyond: emerging roles for noncanonical poly(A) polymerases. Wiley Interdiscip Rev RNA 1: 142–151 [DOI] [PubMed] [Google Scholar]
- Schneider C, Leung E, Brown J, Tollervey D (2009) The N-terminal PIN domain of the exosome subunit Rrp44 harbors endonuclease activity and tethers Rrp44 to the yeast core exosome. Nucleic Acids Res 37: 1127–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberg DR, Maquat LE (2012) Regulation of cytoplasmic mRNA decay. Nat Rev Genet 13: 246–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B, Goodman HM (2004) Uridine addition after microRNA-directed cleavage. Science 306: 997. [DOI] [PubMed] [Google Scholar]
- Sheth U, Parker R (2003) Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science 300: 805–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MG, Kiledjian M (2007) 3′ Terminal oligo U-tract-mediated stimulation of decapping. RNA 13: 2356–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staals RH, Bronkhorst AW, Schilders G, Slomovic S, Schuster G, Heck AJ, Raijmakers R, Pruijn GJ (2010) Dis3-like 1: a novel exoribonuclease associated with the human exosome. EMBO J 29: 2358–2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synowsky SA, van Wijk M, Raijmakers R, Heck AJ (2009) Comparative multiplexed mass spectrometric analyses of endogenously expressed yeast nuclear and cytoplasmic exosomes. J Mol Biol 385: 1300–1313 [DOI] [PubMed] [Google Scholar]
- Teixeira D, Parker R (2007) Analysis of P-body assembly in Saccharomyces cerevisiae. Mol Biol Cell 18: 2274–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira D, Sheth U, Valencia-Sanchez MA, Brengues M, Parker R (2005) Processing bodies require RNA for assembly and contain nontranslating mRNAs. RNA 11: 371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharun S, He W, Mayes AE, Lennertz P, Beggs JD, Parker R (2000) Yeast Sm-like proteins function in mRNA decapping and decay. Nature 404: 515–518 [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25: 4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JE, Chang HM, Piskounova E, Gregory RI (2012) Lin28-mediated control of let-7 microRNA expression by alternative TUTases Zcchc11 (TUT4) and Zcchc6 (TUT7). RNA 18: 1875–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T, Niwa H, Nemoto T, Dhut S, Eddison M, Matsusaka T, Yanagida M, Hirata D (1996) The fission yeast sts5+ gene is required for maintenance of growth polarity and functionally interacts with protein kinase C and an osmosensing MAP-kinase pathway. J Cell Sci 109(Pt 9): 2331–2342 [DOI] [PubMed] [Google Scholar]
- Tomecki R, Kristiansen MS, Lykke-Andersen S, Chlebowski A, Larsen KM, Szczesny RJ, Drazkowska K, Pastula A, Andersen JS, Stepien PP, Dziembowski A, Jensen TH (2010) The human core exosome interacts with differentially localized processive RNases: hDIS3 and hDIS3L. EMBO J 29: 2342–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S, Yamashita A, Harigaya Y, Iwata R, Yamamoto M (2010) Importance of polyadenylation in the selective elimination of meiotic mRNAs in growing S. pombe cells. EMBO J 29: 2173–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa K, Tanaka T, Ida Y, Furusawa C, Hirasawa T, Shimizu H (2011) Comprehensive phenotypic analysis of single-gene deletion and overexpression strains of Saccharomyces cerevisiae. Yeast 28: 349–361 [DOI] [PubMed] [Google Scholar]
- Zhang W, Murphy C, Sieburth LE (2010) Conserved RNaseII domain protein functions in cytoplasmic mRNA decay and suppresses Arabidopsis decapping mutant phenotypes. Proc Natl Acad Sci USA 107: 15981–15985 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.