Abstract
The chemical nature and functional significance of mitochondrial flashes associated with fluctuations in mitochondrial membrane potential is unclear. Using a ratiometric pH probe insensitive to superoxide, we show that flashes reflect matrix alkalinization transients of ∼0.4 pH units that persist in cells permeabilized in ion-free solutions and can be evoked by imposed mitochondrial depolarization. Ablation of the pro-fusion protein Optic atrophy 1 specifically abrogated pH flashes and reduced the propagation of matrix photoactivated GFP (paGFP). Ablation or invalidation of the pro-fission Dynamin-related protein 1 greatly enhanced flash propagation between contiguous mitochondria but marginally increased paGFP matrix diffusion, indicating that flashes propagate without matrix content exchange. The pH flashes were associated with synchronous depolarization and hyperpolarization events that promoted the membrane potential equilibration of juxtaposed mitochondria. We propose that flashes are energy conservation events triggered by the opening of a fusion pore between two contiguous mitochondria of different membrane potentials, propagating without matrix fusion to equilibrate the energetic state of connected mitochondria.
Keywords: bioenergetics, cell signalling, metabolism
Introduction
Mitochondria are double-membrane organelles that play a central role in cellular energy conversion, lipid metabolism, calcium signalling, and apoptosis. The generation of ATP by oxidative phosphorylation involves the generation of a proton-motive force (Δp) across the inner mitochondrial membrane (IMM) as protons are pumped by respiratory chain complexes and subsequently used to drive the activity of the ATP synthase. Δp comprises an electrical component, the mitochondrial membrane potential (ΔΨm ∼180 mV, negative inside), and a chemical component, the transmembrane pH gradient (ΔpHm ∼0.8, alkaline inside), whose generation is facilitated by the low H+-buffering capacity of the alkaline mitochondrial matrix (Poburko et al, 2011). While some electrogenic transporters are driven exclusively by ΔΨm, the transport of many ions, substrates, and metabolites depends on ΔpHm (Bernardi, 1999).
Improvements in live cell fluorescence imaging have revealed that ΔΨm fluctuates rapidly within individual mitochondria and that these electrical events can propagate along interconnected mitochondria (Duchen et al, 1998; Huser et al, 1998; Huser and Blatter, 1999; De Giorgi et al, 2000). A plethora of mechanisms were proposed to trigger the ΔΨm fluctuations: local Ca2+ elevations (Duchen et al, 1998), opening of the mitochondrial permeability transition pore (mPTP) (Huser and Blatter, 1999; De Giorgi et al, 2000; Zorov et al, 2000; Jacobson and Duchen, 2002), coupling of ΔΨm to the ATP synthase (Thiffault and Bennett, 2005), switching between active and inactive states of oxidative phosphorylation (Buckman and Reynolds, 2001), or opening of a proton-selective channel by matrix alkalinization (Hattori et al, 2005). Spontaneous ΔΨm fluctuations are also observed in permeabilized cells (Uechi et al, 2006) and in isolated mitochondria, where they are modulated by adenine nucleotides acting from the matrix side (Vergun et al, 2003; Vergun and Reynolds, 2004). In astrocytes, spontaneous ΔΨm decreases are associated with transient elevations in matrix [Na+] (Azarias et al, 2008), whereas in cardiac myocytes synchronized ΔΨm, reactive oxygen species (ROS), and NADP fluctuations were reported and attributed to the opening of a mitochondrial anion channel permeable to superoxide (Aon et al, 2003). In skeletal muscle cells and intact beating hearts, superoxide flashes coinciding with ΔΨm decreases were recorded with a circularly permutated yellow fluorescent protein (cpYFP) and proposed to be generated by stochastic openings of the mPTP that, by dissipating ΔΨm, divert electrons from the respiratory chain to generate bursts of matrix superoxide (Wang et al, 2008). Subsequent studies using cpYFP-based probes indicated that flash frequency is linked to mitochondrial respiration (Pouvreau, 2010; Wei et al, 2011) and increases during oxidative stress-induced apoptosis (Ma et al, 2011), reviewed in Fang et al (2011). The superoxide nature of the flashes is disputed, however (Muller, 2009), and because cpYFP is also pH sensitive (Nagai et al, 2001) several groups have instead proposed that the flashes are transient mitochondrial matrix pH (pHmito) elevations (Azarias and Chatton, 2011; Schwarzlander et al, 2011, 2012a), reviewed in Santo-Domingo and Demaurex (2012) and Schwarzlander et al (2012b).
Energy conservation across the IMM depends on its impermeability to protons; however, the maintenance of this permeability barrier is challenged in intact cells by the understanding that mitochondria are not isolated organelles and that they undergo cycles of fission and most importantly fusion (Twig et al, 2008). Mitochondrial fission depends on the cytoplasmic dynamin-related protein 1 (DRP1) (Smirnova et al, 2001), that is recruited on the organelle by several potential receptors like FIS1, MFF, and MID49/51 (Palmer et al, 2011). Fusion depends on the outer mitochondrial membrane (OMM) proteins Mitofusin (MFN) 1 and 2 and on the IMM Optic atrophy 1 (OPA1) (Campello and Scorrano, 2010). Mitochondrial fusion is a complex process from the membrane biology and the bioenergetic point of view: fusion of two organelles involves the generation of a fusion intermediate of four membranes; if the process of mitochondrial fusion is analogous to other organellar fusions, when the IMM fuses a fusion pore shall be generated that would link two matrixes. Such a fusion pore might connect two mitochondria of different respiratory states, with unpredictable effects on their membrane potential. Defective fusion pore assembly could even connect the matrix with the intermembrane space. In vitro experiments indicate that OPA1 induces lipid tubulation (Ban et al, 2010), and rupture of the growing IMM tubules could link the matrix to the IMS. If pore formation involves the juxtaposition of two hemichannels as for gap junctions, opening of the hemichannels on the growing IMM tubule would also connect the matrix to the IMS, equilibrating two chemically different environments with profound consequences on the bioenergetics of the organelle.
Here we set out to address, by combining genetics and physiology, how mitochondrial fusion impacts on bioenergetics. Our ratiometric probe SypHer, highly pH-sensitive but insensitive to superoxide in vitro, recorded changes in matrix pH in single mitochondria. Spontaneous alkalinization transients coincided with decreases in ΔΨm. We could unravel that these flashes represented compensatory pHmito elevations maintaining the proton-motive force during spontaneous decreases in ΔΨm. In cells lacking OPA1, the flashes were completely absent, whereas their propagation was greatly increased when mitochondria were more interconnected. A significant fraction of adjacent mitochondria exhibited opposite changes in ΔΨm during pHmito flashes that resulted in membrane potential equilibration. We propose that the pHmito flashes are energy conservation events requiring the fusion protein OPA1 and therefore triggered by the opening of fusion pores between adjacent mitochondria. The flashes propagate without matrix mixing between adjacent mitochondria, a new mode of coupling that might allow interconnected mitochondria to rapidly equilibrate their energetic state.
Results
Spontaneous alkalinization transients in single mitochondria
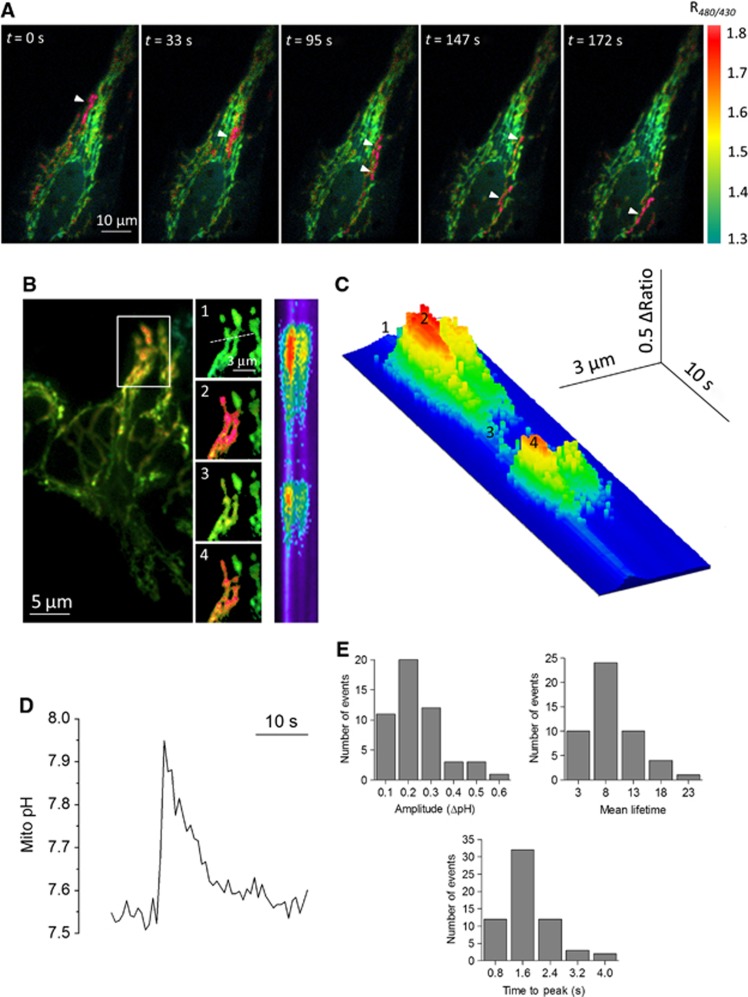
We recently generated a new pH-sensitive probe targeted to the matrix of mitochondria, mito-SypHer, and reported dynamic changes in the mitochondrial pH gradient in HeLa cells (Poburko et al, 2011). During these recordings, we frequently observed spontaneous and asynchronous increases in mito-SypHer ratio fluorescence in discrete regions of the mitochondrial network (Figure 1 and Supplementary Movie S1). The elevations occurred either in different regions of the cell (Figure 1A) or repeatedly at the same location (Figure 1B), but always remained restricted to a specific region of the mitochondrial network (Figure 1B, inset). The elevations had an abrupt onset (time to peak: 1.63±0.08 s) followed by a slower recovery towards basal levels, and a mean life time of 8.6±0.6 s (Figure 1C–E). On average, 0.54±0.04 elevations were detected per minute per cell. In situ pH calibration of the probe by titration with buffers of different pH in the presence of the K+/H+ ionophore nigericin (Supplementary Figure S1) revealed that the matrix pH increased by 0.38±0.04 pH units during a typical event, from 7.75±0.21 to 8.14±0.46 (Figure 1D and E). Transient alkalinization events of sizable magnitude thus occur in intact mitochondria.
Figure 1.
Transients pHmito elevations in single mitochondria. (A) Time sequence of F480/F430 ratio images of HeLa cells expressing mito-SypHer showing spontaneous alkalinization transients (arrows) in single mitochondria in different cellular regions. Warm colours denote high ratio values. See also Supplementary Movie S1. (B) Repetitive pH transients in a mitochondrial cluster. Numbered insets show consecutive images and the right-hand panel shows a 30 s scan along the line drawn in inset #1, the time axis running vertically from top to bottom. (C) 3D reconstruction of the line scan image. Numbers correspond to inset panels in B. (D) Calibrated pHmito transient. (E) Histograms showing the distribution of the amplitude, time to peak, and half-life time of 61 independent pHmito elevations.
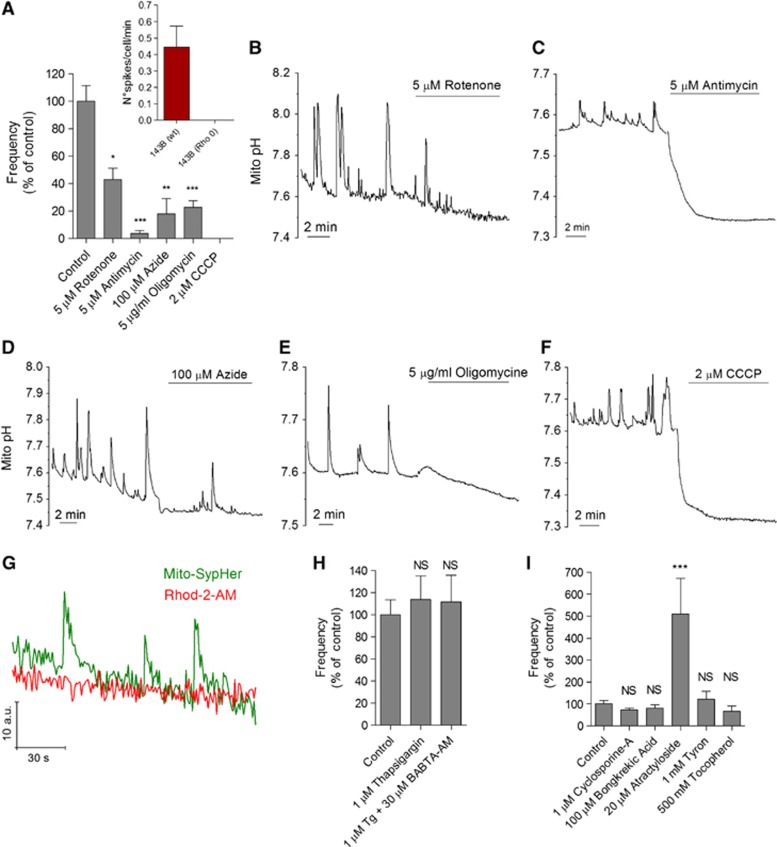
cpYFP flash activity has been shown to require an active respiratory chain (Wang et al, 2008; Schwarzlander et al, 2012a). Accordingly, inhibition of complex I, III, IV, and V with rotenone, antimycin, azide, and oligomycin, respectively, decreased the frequency of SypHer flashes (Figure 2A–D). Furthermore, Rho 0 cells, which lack mito-DNA and thus all H+-translocating complexes, lacked pHmito activity (Figure 2A, inset). The strongest inhibitors were antimycin and the protonophore carbonyl cyanide m-chlorophenyl hydrazone (CCCP) (Figure 2A), at doses increasing respiration (Supplementary Figure S2C), which both collapsed ΔpHm (Figure 2C and F). Earlier studies have linked ΔΨm fluctuations to cytosolic Ca2+ elevations (Duchen et al, 1998; Vergun and Reynolds, 2004; Guzman et al, 2010). We could not detect mitochondrial Ca2+ elevations with Rhod2 during pHmito flashes (Figure 2G) and neither intracellular Ca2+ stores depletion with thapsigargin, cytosolic Ca2+ buffering with (1,2-bis(o-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid) aceto-methyl ester (BAPTA-AM) (Figure 2H), nor genetic manipulation of the recently identified mitochondrial H+/Ca2+ exchanger protein Letm1 (Supplementary Figure S2) had any impact on flash activity. This indicates that pHmito flashes are not driven by mitochondrial Ca2+ uptake. ΔΨm fluctuations were attributed to mPTP opening by ROS (Huser and Blatter, 1999; Jacobson and Duchen, 2002; Wang et al, 2008). In our hands, mPTP inhibitors (cyclosporine A and bongkrekic acid) and ROS scavengers (Tyron and Tocopherol) did not significantly alter pHmito flash frequency (Figure 2I), indicating that flash activity is not driven by mPTP opening, although atractyloside increased flash frequency by ∼5-fold.
Figure 2.
Pharmacology of pHmito flashes. (A) Effect of respiratory chain inhibitors on the frequency of pHmito flashes in 15 min recordings of 82 (control), 10 (rotenone), 28 (antimycin), 13 (azide), 48 (oligomycin), and 11 cells (CCCP). Inset: pHmito elevations in control (n=14) and mitochondria-deficient rho 0 osteosarcoma 143b cells (n=12). (B–F) Representative recordings of HeLa cells expressing DRP1 (K38A), showing the effect of different respiratory chain inhibitors on pHmito flashes and on the basal mitochondrial pH. (G) Simultaneous mito-SypHer and Rhod-2 recordings. No [Ca2+]mito changes were observed during pHmito flashes. (H) Effect of Ca2+ depletion and Ca2+ buffering on the frequency of pHmito flashes. Cells were treated with thapsigargin (Tg) to deplete Ca2+ stores and subsequently loaded with BAPTA-AM to chelate cytosolic Ca2+ (n=26, 21, and 14 cells). (I) Effect of mPTP modulators on the frequency of pHmito flashes (n=9–44 cells, means±s.e.m.). *P<0.05, **P<0.01, and ***P<0.001. NS, not significant.
Mito-SypHer is a specific pH indicator insensitive to superoxide
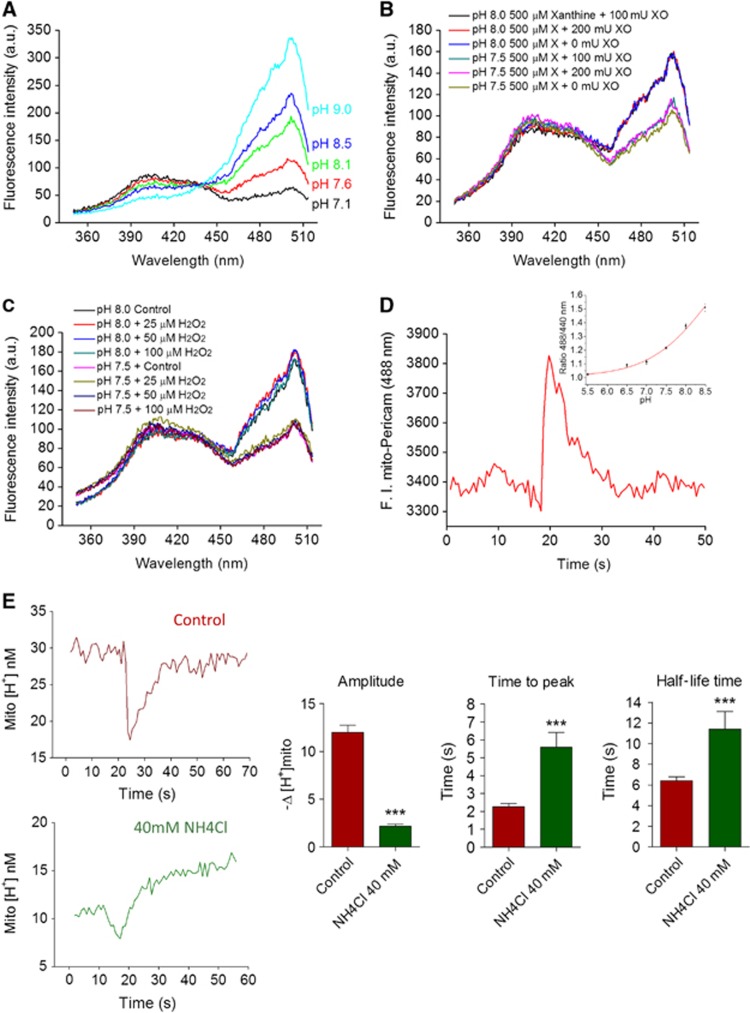
The pHmito elevations reported by the ratiometric mito-SypHer probe resemble the superoxide and pHmito flashes previously reported with cpYFP (Wang et al, 2008; Schwarzlander et al, 2012a). To clarify the chemical nature of the flashes, we evaluated the pH and superoxide sensitivity of bacterially expressed 6 × His-tag SypHer. The excitation spectra of purified SypHer was highly sensitive to changes in pH (Figure 3A) but was not affected by the addition of xanthine and xanthine oxidase (XO) at concentrations that evoked a robust superoxide dismutase (SOD)-sensitive increase in the luminescence of the superoxide probe 2-methyl-6-(p-methoxyphenyl)-3,7-dihydroimidazo[1,2-alpha]pyrazin-3-one (MCLA) (Figure 3B and Supplementary Figure S3A). Furthermore, the excitation spectra of purified SypHer were not affected by H2O2 (Figure 3C), by the NO donor S-nitroso-N-acetyl-DL-penicillamine (SNAP), by the reducing agent dithiothreitol (DTT), or by millimolar concentrations of Ca2+, PO4−, and ATP (Supplementary Figure S3B–E and Poburko et al, 2011). These in vitro data indicate that the fluorescence of mito-SypHer is insensitive to changes in redox state, ionic strength, and metabolites, and further validate the probe as a ratiometric pH indicator. Ratiometric pericam targeted to the mitochondria (RPmit) reportedly responds to superoxide at 488 nm (Pouvreau, 2010), but this indicator is not specific for superoxide as its fluorescence increases sharply above pH 7.0 (Figure 3D, inset) suggesting that the fluorescence flashes reported by RPmit in HeLa cells (Figure 3D) also reflect an increase in matrix pH. Next, we increased the proton-buffering power of mitochondria with the permeable weak base NH4Cl, a procedure expected to alter the kinetics of proton but not of superoxide changes. Since NH4Cl increases pHmito, the fluorescence data were converted to proton concentrations and expressed in a non-logarithmic scale to compare the absolute changes in [H+] (Figure 2E). Both the onset and the recovery of the [H+] transients were delayed in the presence of the weak base, and their amplitude decreased by 82% (Figure 3E, n=49–59). Thus, increasing the buffering power of mitochondria decreased the amplitude and prolonged the duration of the spontaneous transients, providing independent functional evidence that the SypHer flashes are caused by protons and not by superoxide.
Figure 3.
Mito-SypHer is a pH-sensitive probe insensitive to superoxide. (A) Excitation spectra (λem=530 nm) of purified bacterially expressed SypHer at different pH. (B) Excitation spectra of purified SypHer in the presence of xanthine and XO at pH 7.5 and pH 8.0. (C) Excitation spectra in the presence of increasing amounts of H2O2. Spectra are representative of three independent experiments in each condition. (D) Spontaneous fluorescence elevations recorded with ratiometric pericam (RPmit, λem=488 nm) in HeLa cell mitochondria. (n=52 transients from three cells). Inset: effect of pH on RPmit F488/F440 ratio fluorescence. (E) Effect of 40 mM NH4Cl on the kinetics and amplitude of the pHmito elevations recorded with SypHer. Left panels: the fluorescence recordings were converted to proton concentrations and expressed in a non-logarithmic scale to compare the absolute changes in [H+]. Right panels: averaged amplitude, time to peak, and half-life time of 56 pHmito elevations recorded in six cells before (red) and after (green) addition of 40 mM NH4Cl (means±s.e.m.). ***P<0.001.
pHmito flashes are bioenergetic events driven by decreases in ΔΨm
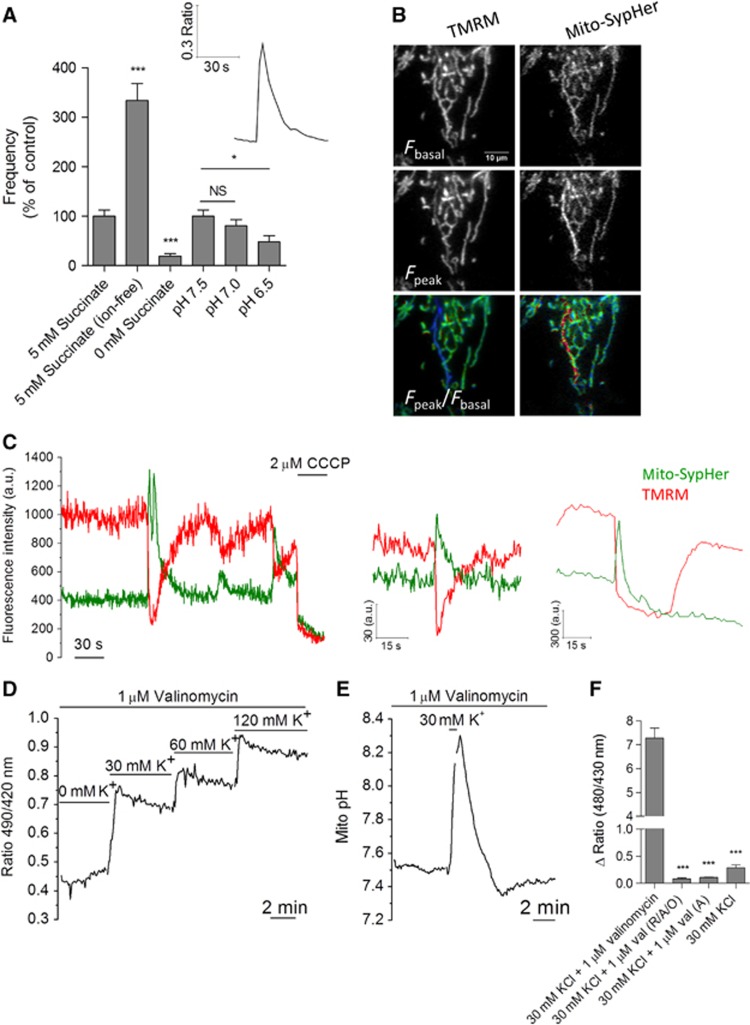
We next recorded pHmito in cells permeabilized with digitonin and perfused with succinate. pHmito flashes were readily observed in permeabilized cells (Figure 4A, inset), a configuration that, as shown previously (Poburko et al, 2011), allowed basal pHmito levels to vary rapidly and reversibly with the cytosolic pH (Supplementary Figure S4A). Succinate removal reduced flash frequency by 93%, whereas substitution of Na+, K+, Ca2+, Cl−, and PO42− with sucrose increased flash frequency without altering their kinetics or amplitude. Decreasing cytosolic pH from 7.5 to 7.0 did not significantly decrease flash frequency, but further acidification to pH 6.5 decreased flash frequency by ∼50% (Figure 4A). The pHmito flashes thus persisted in permeabilized cells, their frequency increasing in ion-free conditions and decreasing under acidic conditions and substrate removal. These data indicate that the pHmito flashes are not driven by the entry of ions into mitochondria, and that the flash activity requires respiring mitochondria and a permissive matrix or cytosolic pH but not cytosolic ions.
Figure 4.
pHmito flashes are bioenergetic events driven by decreases in ΔΨm. (A) Spontaneous elevations in mito-SypHer fluorescence in HeLa cells permeabilized in KCl-based solutions. Inset shows a typical pHmito flash, bar graph shows the frequency of flashes recorded in solutions containing (n=64) or lacking ions (Ca2+, Na+, K+, and PO4− replaced with sucrose, n=62), respiratory substrates (n=32), and of varying pH (n=64 for each condition) (means±s.e.m.). (B) Simultaneous pHmito and ΔΨm recordings in intact cells loaded with TMRM. A concomitant decrease in ΔΨm was observed in 64/64 flashes. See also Supplementary Movie S2. (C) ΔΨm typically mirrored pHmito (left panels) but occasionally exhibited delayed recovery (right panel). (D) Effect of imposed mitochondrial depolarizations on pHmito. Permeabilized cells were equilibrated with valinomycin and exposed to increasing concentration of KCl to clamp ΔΨm at different depolarized potentials. Increasing depolarization steps induced progressive matrix alkalinization (n=37 cells). (E) pHmito elevation evoked by a brief pulse of 30 mM KCl to transiently depolarize mitochondria (n=68). (F) Effect of respiratory chain inhibitors and of valinomycin on the amplitude of the pHmito elevation evoked by KCl. A, antimycin; O, oligomycin; R, rotenone; Val, valinomycin (n=50 cells for each condition; means±s.e.m.). *P<0.05 and ***P<0.001. NS, not significant.
The cpYFP flashes occur coincidentally with decreases in ΔΨm (Wang et al, 2008). We also observed a concomitant decrease in ΔΨm with every pHmito elevation during simultaneous SypHer and tetramethyl rhodamine methyl ester (TMRM) recordings (Figures 4B–C, n=64 events, Supplementary movie S2). In most cases (96%), the depolarization events were transient and mirrored the pHmito elevations (Figure 4C, left traces), but on rare occasions (4%) mitochondria remained depolarized for several seconds after the termination of the pHmito flash (Figure 4C, right traces). The upstroke of the pHmito and ΔΨm transients was faster than the temporal resolution of our imaging setup (20 Hz), and the two activities thus appeared coincidental. The mirror changes in ΔΨm and pHmito reflect opposite alterations in the electrical and chemical components of the proton-motive force, suggesting that ΔpHm increases to balance the decrease in ΔΨm (Santo-Domingo and Demaurex, 2012). To test this possibility, we clamped the ΔΨm at different potentials by equilibrating permeabilized cells with valinomycin at different K+ concentrations. As predicted, addition of 30 mM KCl evoked an immediate increase in pHmito, and subsequent additions of higher K+ concentrations further alkalinized the matrix (Figure 4D). To test whether a pHmito flash could be evoked by an artificial depolarization, we briefly added 30 mM KCl to cells equilibrated with valinomycin. This treatment faithfully reproduced pHmito flashes, the pHmito rapidly increasing upon KCl addition and slowly recovering upon KCl withdrawal (Figure 4F). KCl had no effect in the absence of valinomycin (Figure 4G) and pHmito flashes were also evoked by addition of NaCl or LiCl to permeabilized cells treated with the ionophore A23187 (Supplementary Figure S4B), ruling out K+/H+ exchange. The KCl-evoked pHmito elevations were prevented by respiratory chain inhibitors (Figure 4G), confirming that they reflected increases in proton pumping. Thus, pHmito flashes can be artificially generated by an imposed transient mitochondrial depolarization, strongly suggesting that the endogenous flash activity of intact cells reflects compensatory increases in ΔpH driven by spontaneous decreases in ΔΨm. The ΔΨm changes coincided both spatially and temporally with the matrix pH flashes (Figure 4B and Supplementary Figure S4C), suggesting that the electrical events propagate along connected mitochondria and trigger immediate pH responses in depolarized mitochondria.
Matrix pH elevations propagate faster than matrix GFP along connected mitochondria
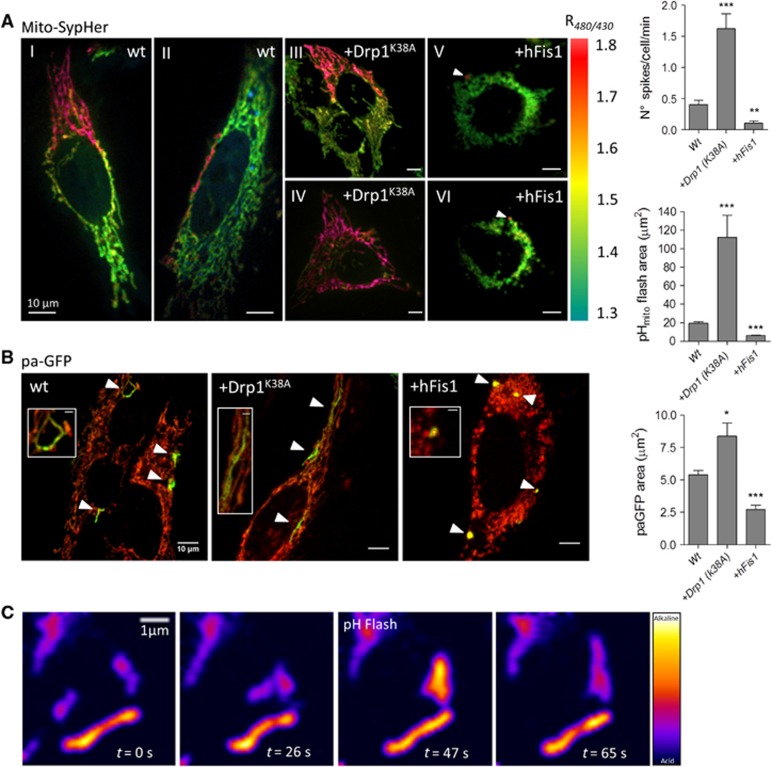
The spatial dimension of the pHmito elevations varied considerably, with some events restricted to single mitochondria and other occurring in large clusters of interconnected mitochondria (Figure 5AI). To test whether the area covered by a single pHmito elevation varied with mitochondria interconnectivity, we enforced mitochondrial shape changes by overexpressing mitochondrial-shaping proteins. Elongation was promoted by a dominant-negative DRP1 mutant (DRP1K38A) and fission by the pro-fission protein hFIS1 (Frieden et al, 2004). The spatial extension of the pHmito elevations increased dramatically in cells expressing DRP1K38A (Figure 5AIII), with global pHmito flashes observed in some cells (Figure 5AIV and Supplementary movie S3), and decreased in cells expressing hFIS1 (Figure 5AV). On average, a single pHmito flash covered 17.02±0.32 μm2 (n=81) of the fluorescent mitochondrial area in control HeLa cells, 109.08±21.12 μm2 (n=96 flashes) in cells expressing DRP1K38A, and only 6.41±0.08 μm2 (n=59) in hFIS1 expressers. Interestingly, the frequency of the pHmito elevations increased in cells with fused mitochondria and decreased in cells with fragmented mitochondria (Figure 5A), while the flash amplitude and time to peak increased upon DRP1K38A and hFIS1 expression, respectively (Supplementary Figure S5A). We next tested whether flash propagation reflected luminal continuity between neighbouring organelles by measuring the matrix diffusion of a photoactivated GFP (paGFP). Photoactivation of matrix-targeted paGFP revealed areas that were much smaller than the areas of elementary pHmito flashes (Figure 5B, the individual matrix compartments labelled with paGFP appearing in green over the red TMRM mitochondrial staining). paGFP fluorescence covered a maximal mitochondrial area 2 s after laser illumination (Supplementary Figure S5B and C), a procedure that did not alter TMRM fluorescence (Figure 5D and E), indicating that our measurements were not limited by the rates of paGFP matrix diffusion or by laser-induced toxicity. paGFP-labelled areas were ∼3-fold smaller than the pHmito flash areas in non-transfected cells and ∼13-fold smaller than flash area in DRP1K38A cells, the paGFP areas increasing by only ∼30% upon DRP1K38A expression while the pHmito flash area increased by ∼6-fold (Figure 5A and B). paGFP-labelled areas of up to 47 μm2 could be detected in DRP1K38A expressers, indicating that the small average size of paGFP regions did not reflect failure to detect paGFP fluorescence but rather probe confinement. Photoactivation of matrix-targeted paGFP in cells loaded with TMRM confirmed that the spontaneous ΔΨm drops propagated within a much wider mitochondrial area than the matrix paGFP (data not shown). These data indicate that pHmito flashes can propagate along interconnected mitochondria that have limited exchange of matrix protein content. Nevertheless, pHmito and ΔΨm flashes correlated temporally with mitochondrial fusion events in live microscopy (Figure 5C and Supplementary movie S4), indicating that flash activity is linked to mitochondrial fusion.
Figure 5.
Matrix pH elevations propagate along connected, but not fused, mitochondria. (A) Ratio mito-SypHer images taken at the peak of elementary pHmito elevations in WT HeLa cells (I, II) and in cells expressing the pro-fusion protein DRP1K38A (III, IV) or the pro-fission protein hFIS1 (V, VI). Note that in DRP1K38A cells pHmito flashes occasionally spread over the entire mitochondrial network. See also Supplementary Movie S3. Bar graphs: averaged spatial extent and frequency of pHmito flashes in 15 min continuous recordings of 11 cells for each condition (means±s.e.m.). (B) Individual matrix compartments labelled with paGFP. Representative merged TMRM/paGFP fluorescence images taken 5 s after paGFP photoactivation in HeLa cells expressing the indicated plasmid; insets show paGFP-labelled regions at higher magnification, with the irradiated region indicated by a crosshair; bar graph: averaged spatial extent of matrix paGFP propagation (n=6 independent experiments, means±s.e.m.). (C) pHmito flash occurring during transient contact between two individual mitochondria. See also Supplementary Movie S4. *P<0.05, **P<0.01, and ***P<0.001.
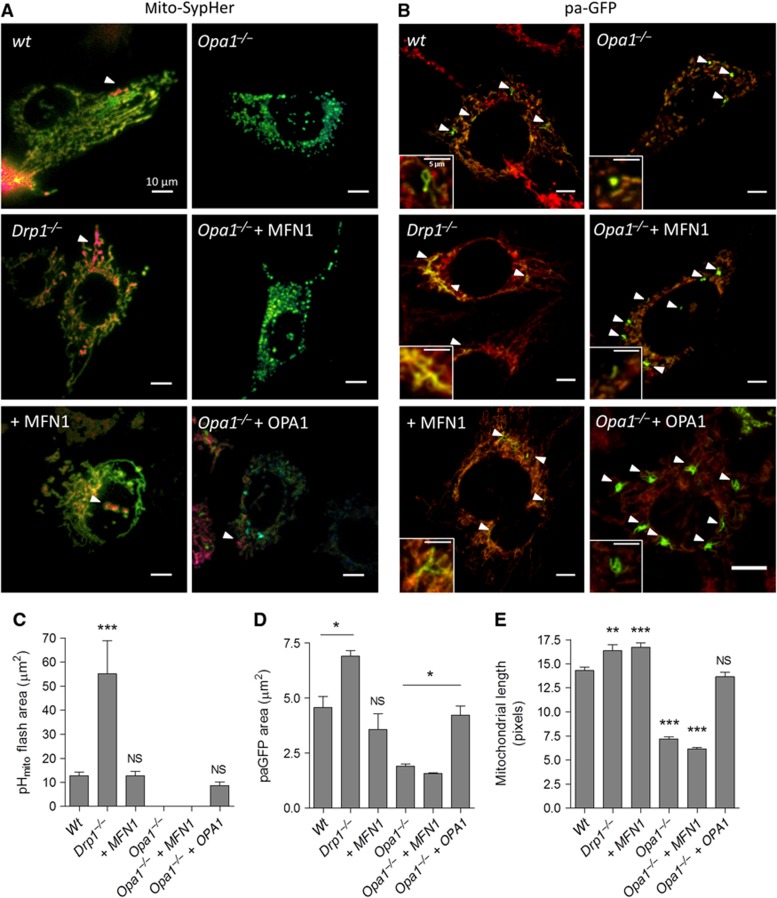
To further explore the link between mitochondrial fusion and pHmito flash propagation, we used fibroblasts (MEFs) from knockout mice that completely lack the endogenous pro-fission protein DRP1 (Ishihara et al, 2009) or the inner membrane pro-fusion protein OPA1 (Gomes et al, 2011). The size of pHmito flashes increased by ∼5-fold in Drp1−/− cells while the area of paGFP regions and the length of the smallest fluorescent objects, an independent readout of mitochondrial length, increased by only ∼30% (Figure 6). Drp1 ablation thus markedly increased the propagation of pHmito flashes without altering their frequency and amplitude (Supplementary Figure S6), consistent with the phenotype of HeLa cells expressing the dominant-negative DRP1K38A. In contrast, expression of MFN1 in WT MEFs increased the length of individual mitochondria as expected but did not increase the size of the pHmito flashes or paGFP areas (Figure 6C–E), indicating that enforced fusion of the outer membrane does not promote pHmito flash propagation. Remarkably, Opa1 ablation abrogated pHmito flash activity (Figure 6C) and ΔΨm fluctuations (not shown), and reduced as expected both the length of individual mitochondria and the size of paGFP-labelled areas by half (Figure 6D–E). Although Opa1−/− are bioenergetically competent (Gomes et al, 2011), not a single pHmito flash was detected in Opa1−/− cells even after application of atractyloside, which increased pHmito flash frequency by four-fold in control cells (Supplementary Figure S6E), or after expression of MFN1, which, as expected, Cipolat et al (2004) failed to rescue mitochondrial length and paGFP propagation (Figure 6). Importantly, re-expression of OPA1 restored pHmito flash activity, mitochondrial length, and paGFP matrix propagation to WT levels (Figure 6). These data indicate that OPA1-mediated fusion of the inner membrane, but not MFN1-mediated fusion of the outer membrane, is linked to the pHmito flash activity.
Figure 6.
pHmito flashes require OPA1 but not MFN1. (A) SypHer images taken at the peak of elementary pHmito elevations in mouse embryonic fibroblasts (MEFs) derived from control, Drp1−/−, and Opa1−/− mice transfected or not with MFN1 or OPA1. (B) Matrix compartments labelled with paGFP in cells of the indicated genotype; insets show the paGFP-labelled regions at higher magnification. Averaged spatial extent of (C) pHmito flashes and of (D) paGFP propagation in 15 min continuous recordings of 30 cells for each condition (n=6 independent experiments) (means±s.e.m.). (E) Averaged length of individual mitochondria (n=300, means±s.e.m.). *P<0.05, **P<0.01, and ***P<0.001. NS, not significant.
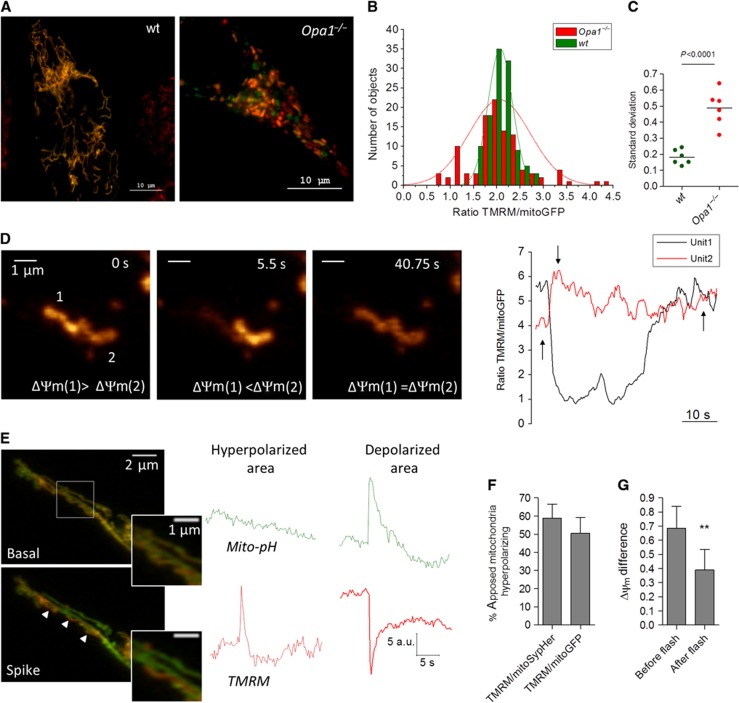
We next tested the impact of OPA1-mediated flash activity on mitochondrial bioenergetics, using ratiometric imaging of TMRM over matrix-targeted GFP to quantify ΔΨm. Opa1 ablation markedly increased the heterogeneity of ΔΨm within the mitochondrial population of individual cells (Figure 7A). The ΔΨm of 2.5-μm2-wide fluorescent objects had a Gaussian distribution (Figure 7B) whose s.d. increased by 2.5-fold upon Opa1 ablation, from 10 to 25% (Figure 7C). This indicates that OPA1-mediated fusion promotes the equilibration of mitochondrial membrane potentials. We therefore checked whether this ΔΨm equilibration was linked to the pHmito flash activity. The distribution of ΔΨm (measured with TMRM or TMRM/GFP) and of pHmito (measured with SypHer) remained unchanged within the flashing regions (Supplementary Figure S7), indicating that pHmito flashes do not promote energy equilibration within the flashing regions themselves. However, careful analysis revealed that 66% of flashes were associated with hyperpolarization events occurring in adjacent mitochondria (located <2 pixels from a flashing unit). The ΔΨm changes occurring in these adjacent mitochondria were synchronous but of opposite direction (Figure 7D and Supplementary Movie S5). The hyperpolarization events were not associated with changes in pHmito (Figure 7E) and occurred in ∼50% of mitochondria adjacent to a flashing unit (Figure 7F) but never in non-adjacent mitochondria. Importantly, the membrane potentials of the two adjacent mitochondria equilibrated after the event (Figure 7D). On average, the ΔΨm difference between adjacent mitochondria undergoing opposite changes in membrane potential decreased by ∼40% after a flash (Figure 7G). These data show that OPA1-dependent flashes equilibrate the membrane potentials of apposed mitochondria.
Figure 7.
OPA1-mediated pHmito flashes equilibrate the potentials of apposed mitochondria. (A) Representative images of wt and Opa1−/− MEFs expressing matrix GFP and loaded with TMRM. (B) Distribution of ΔΨm (expressed as TMRM/mitoGFP ratio of 2.5-μm2-wide fluorescent objects) from these two cells. (C) S.d.’s of ΔΨm Gaussian distributions in mitochondrial populations from six wt and six Opa1−/− cells. (D) Concomittant hyperpolarization (#1) and depolarization (#2) events in two adjacent mitochondria. Note that the two mitochondrial potentials equilibrated after the event. See Supplementary Movies S5a and S6b. (E) ΔΨm and pHmito recordings of adjacent mitochondria undergoing opposite changes in ΔΨm. The pHmito remained stable in the hyperpolarizing mitochondrion. (F) Fraction of adjacent mitochondria (defined as closer than 2 pixels from a flashing mitochondrion) undergoing concomitant hyperpolarization events (n=20–29 cells). (G) ΔΨm difference between adjacent mitochondria measured before and after the synchronous hyperpolarizing/depolarizing event (n=23 events, means±s.e.m.). **P<0.01.
Discussion
In this study, we provide several new insights into the mechanism and significance of spontaneous mitochondrial fluctuations. First, we clarify the chemical nature of ‘mitochondrial flashes’ by using a probe that we show to be responsive to pH but not to superoxide. Superoxide flashes coinciding with ΔΨm decreases were reported in individual mitochondria from skeletal muscle and intact beating hearts (Wang et al, 2008; Pouvreau, 2010; Fang et al, 2011; Wei et al, 2011), but the cpYFP probe used was shown to be highly sensitive to pH (Schwarzlander et al, 2011) and was subsequently used to report matrix alkalinization transients in mitochondria from Arabidopsis thaliana root cells (Schwarzlander et al, 2012a). The pH/superoxide flashes coincide with ΔΨm decreases and have similar kinetics and pharmacological profiles, suggesting that they reflect the same bioenergetic event. However, since the two activities were measured with the same cpYFP-based probe reportedly sensitive to both proton and superoxide, the chemical nature of the measured signal is uncertain. By showing that our SypHer probe is insensitive to superoxide, we demonstrate that human mitochondria do in fact transiently alkalinize during decreases in ΔΨm, validating the results obtained in astrocytes (Azarias and Chatton, 2011) and plants (Schwarzlander et al, 2012a). The SypHer flashes were altered by increased mitochondrial buffering but not by ROS scavengers and were not associated with fluorescence changes of redox-sensitive green fluorescent protein (roGFP) (data not shown), consistent with protons and not superoxide as the source of the signal. Furthermore, we show that the most potent flash inhibitors, antimycin and CCCP, which are not expected to abrogate superoxide flashes as they increase superoxide production (Muller, 2009), both collapsed ΔpHm as expected from their pharmacology. Since our probe is insensitive to superoxide, we cannot rule out that superoxide flashes occur concomitantly with pHmito flashes, and this possibility should be evaluated with new probes selective for superoxide and insensitive to pH.
Second, we have causally linked the pHmito flashes to the ΔΨm decreases by demonstrating that prototypical pHmito flashes can be evoked by artificial depolarization of mitochondria with valinomycin/K+. In isolated mitochondria equilibrated with valinomycin/K+, alterations in ΔΨm are exactly balanced by opposite alterations in ΔpH, and Δp remains constant within a wide range of voltages (Nicholls, 1974; Lambert and Brand, 2004; Nicholls, 2005). We show here that this rule holds true also in permeabilized cells. A transient mitochondrial depolarization thermodynamically favours H+ extrusion by decreasing the driving force for proton pumping by respiratory chain complexes, increasing the rate of H+ extrusion and of O2 consumption by mitochondria (Nicholls, 1974; Costa et al, 1984; Nicolli et al, 1991; Talbot et al, 2007). The demonstration that this compensatory mechanism occurs spontaneously in intact cells indicates that ΔΨm fluctuations are not an indicator of mitochondrial dysfunction as concomitant pHmito flashes preserve the proton-motive force, thus maintaining the ability of mitochondria to convert energy. A thermodynamically similar mechanism was recently proposed to account for coincident ΔΨm and pHmito fluctuations in plant mitochondria, where the pulsing activity was proposed to be triggered by the entry of calcium ions into the matrix (Schwarzlander et al, 2012a). In our hands, mitochondrial Ca2+ uptake does not appear to trigger pHmito elevations because the spontaneous activity was not altered by Ca2+ store depletion, by cytosolic Ca2+ chelation (Figure 2), or by knockdown of the mitochondrial H+/Ca2+ exchanger Letm1 (Supplementary Figure S2). By comparing the ΔΨm and pHmito changes recorded during flashes to the changes evoked by CCCP and oligomycin, we can estimate that ΔΨm was around −120 mV at rest and decreased to −50 mV during a flash, while pHmito averaged 7.8 at rest and increased by 0.4 pH unit during a flash. The IMS pH was previously measured at 6.8 in HeLa cells (Porcelli et al, 2005), and since this parameter strongly depends on proton pumping across the IMS, an acidification of 0.4 pH unit that would match the matrix alkalinization during a flash seems reasonable. We therefore estimate that ΔpHm is around 1 (−60 mV) at rest and increases to 1.8 (−110 mV) during a flash. Based on these calculations, the resting proton-motive force of −180 mV decreases by only ∼20 mV during a flash, but the relative contributions of its electrical and chemical components become inverted.
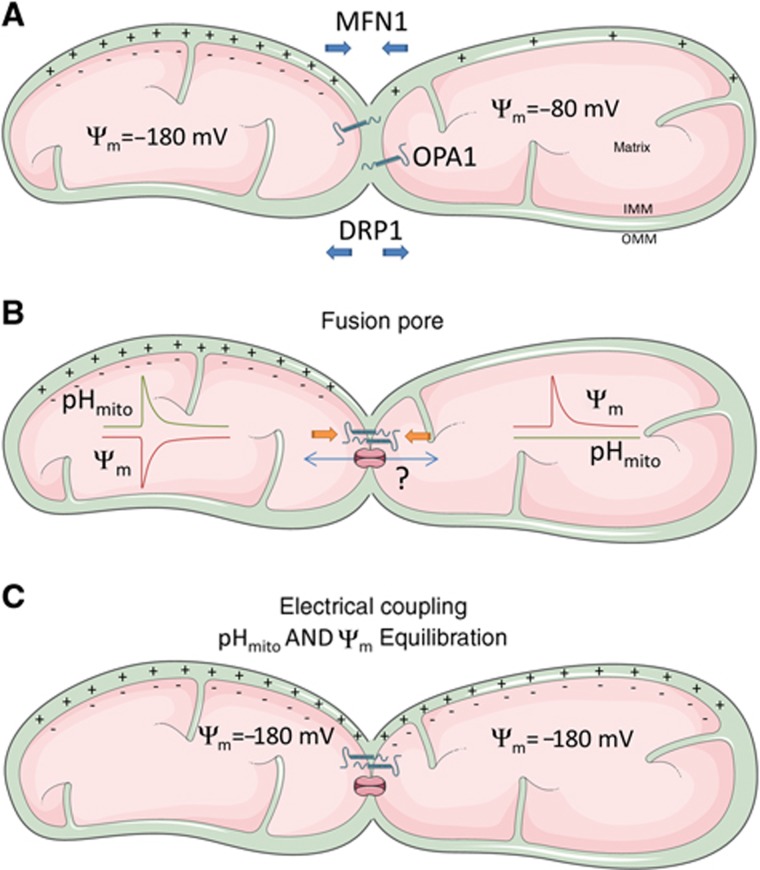
Third, we show that OPA1-mediated IMM fusion is required for the generation of the coupled pHmito/ΔΨm fluctuations. ΔΨm flickers or oscillations were previously linked to mitochondrial Ca2+ or Na+ entry (Duchen et al, 1998; De Giorgi et al, 2000; Buckman and Reynolds, 2001; Jacobson and Duchen, 2002; Vergun et al, 2003; Vergun and Reynolds, 2004; Azarias et al, 2008) or attributed to mPTP opening by mitochondria ROS (Huser and Blatter, 1999; De Giorgi et al, 2000; Jacobson and Duchen, 2002). We show that pHmito flashes persist in ion-free solutions and are not affected by mPTP inhibitors and ROS scavengers, ruling out cation entry across the mPTP as a trigger of the fluctuations. The unexpected and opposite effects of atractyloside and oligomycin might reflect the diverging effects of these inhibitors on the fusion process. Oligomycin inhibits the ATP synthase while atractyloside inhibits the ANT (Vergun and Reynolds, 2004), causing opposite changes in matrix ATP levels that might differently modulate the formation of a fusion pore. Our observation that the activity disappears in mitochondria lacking the pro-fusion protein OPA1 indicates that the fluctuations are probably linked to the fusion of the mitochondria inner membranes, but this activity differs from the ‘kiss-and-run’ mode of transient mitochondrial fusion previously reported (Liu et al, 2009), which allowed exchange of soluble matrix proteins and promoted mitochondrial mobility. Instead, we propose that the coupled pHmito/ΔΨm fluctuations reflect the transient openings of a fusion pore between contiguous mitochondria of different membrane potentials (Figure 8). Opening a fusion pore will establish electrical continuity between these mitochondria, decreasing ΔΨm in the more energized mitochondria. The ΔΨm decrease will then boost proton pumping by active respiratory chain complexes, generating a pHmito flash in the depolarizing mitochondria. Electrical coupling causes the apposed mitochondria to hyperpolarize during the flash, shutting down proton pumping and preventing pHmito flash propagation in the hyperpolarizing mitochondria. While the existence of pores electrically coupling mitochondria awaits electrophysiological confirmation, our ‘junctional coupling’ model explains why flash activity is not coupled to ion fluxes and why mitochondria can preserve their bioenergetic competence during the flashes, as connecting two matrixes will not dissipate the proton-motive force. Previous models have implicated the opening of ion channels, transporters, or large conductance pores between the matrix and the IMS/cytosol, processes that have profound impacts on mitochondria bioenergetics and ionic homeostasis. By linking flash activity to OPA1-mediated mitochondrial fusion, a highly regulated cellular process sensitive to calcium elevations and to oxidative stress (Cereghetti et al, 2008; Tang et al, 2009), our model also accounts for the reported effects of calcium and ROS on flashing activity.
Figure 8.
Proposed mechanism of mitochondrial ΔΨm/pHmito fluctuations. We propose that the ΔΨm/pHmito fluctuations are triggered by the opening of a fusion pore between two mitochondria of different potential. (A) Individual mitochondria can maintain different ΔΨm and pHm. (B) OPA1-mediated inner membrane fusion leads to the formation of a fusion pore that establishes electrical continuity between adjacent mitochondria, without allowing the diffusion of matrix proteins. The disequilibrium in membrane potentials drags electrons from the more energized mitochondria, causing a decrease in ΔΨm that boosts proton pumping by the respiratory chain, generating a pHmito flash in the depolarizing mitochondria. Electrical coupling causes the apposed mitochondria to hyperpolarize during the flash, shutting down proton pumping and preventing pHmito flash propagation in the hyperpolarizing mitochondria. (C) At the end of the flash, the two interconnected mitochondria are in electrochemical equilibrium.
Finally, our concurrent paGFP and TMRM recordings demonstrate that contiguous mitochondria can synchronize their energetic state without mixing their matrix content. Earlier studies had shown that ΔΨm flickering could propagate along interconnected mitochondria (De Giorgi et al, 2000), but whether ΔΨm propagation required matrix continuity was not known. Here, we show that inhibition of endogenous DRP1 activity either by genetic ablation or by expression of a dominant-negative mutant greatly enhances the propagation of pHmito elevations along interconnected mitochondria, but marginally increases the propagation of a photoactivated matrix protein. Mitochondrial fusion proceeds unabated in DRP1 incapacitated cells. However, although these mitochondria appear fused on the confocal microscope, their matrix compartments do not allow the free diffusion of matrix paGFP. In contrast, the pHmito fluctuations covered on average 50% of the mitochondrial area and became global in some cells, in which the matrix pH of the whole network increased within 10 ms without any clear initiation spot or visible decay in flash amplitude along labelled structures (Supplementary movie S3). This indicates that the coincident ΔΨm/pHmito fluctuations propagate by a saltatory mechanism or by a very fast regenerative mechanism. Our observation that ΔΨm and pHmito rapidly equilibrate along interconnected mitochondria confirms the hypothesis originally formulated by Zorov that ΔΨm and ΔpHm immediately spread along mitochondrial inner membranes (Amchenkova et al, 1988). We extend this concept by showing that the proton-motive force can equilibrate within milliseconds in contiguous mitochondria that do not mix their matrix protein content. One purpose of mitochondrial fusion is to allow genetic complementation of damaged mitochondrial DNA, but this function requires the mixing of matrix content. We demonstrate that another function of mitochondrial fusion proteins is to electrically couple individual mitochondria in order to synergize their metabolic activity.
In summary, we show here that mitochondria exhibit spontaneous elevations in their matrix pH triggered by bursts of depolarization that both propagate faster than matrix GFP along connected mitochondria. This indicates that mitochondria can be electrically coupled without exchanging their matrix content. We propose that the matrix alkalinization transients reflect increased pumping by the respiratory chain during ΔΨm decreases triggered by the opening of a fusion pore between neighbouring mitochondria of different membrane potentials. This new mode of mitochondrial coupling might facilitate the transmission of energy inside cells by equilibrating the proton-motive force along electrically connected, but not fused, mitochondria.
Materials and methods
Cell culture and transfection
HeLa cells and 143b cells (wt and Rho 0) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 1 and 4.5 mg/ml glucose, respectively; WT and Drp1−/− MEF cells in DMEM-Glutamax with non-essential amino acids; Opa1−/− cells in MEF medium supplemented with uridine (50 μg/ml, Sigma). All media contained 10% FCS, 1% penicillin, and 1% streptomycin. For fluorescence imaging, 105 cells were seeded on 25-mm glass cover slips, transfected 24 h later with 2 μg of DNA and 5 μl Lipofectamine 2000, and imaged 48 h later. All reagents were purchased from Invitrogen or Sigma.
Mito-SypHer purification and in vitro characterization
The N-terminal poly-His-tag mito-SypHer was generated by cloning in frame mito-SypHer into the Xho-I/ Hind-III site of the prokaryotes expression vector pBAD/HisB (Invitrogen). TOP10-competent cells (Invitrogen) were transformed and expression induced according to the manufacturer’s instructions in the presence of 0.002% arabiniose for 4 h. Cells were lysed and total protein content kept in Tris 25 mM, 150 mM NaCl. After purification in a nickel column, the samples were dialysed at 4°C for 16 h and mito-SypHer concentrated using Amicon Ultra-4 Centrifugal Filter Units (Millipore). Five millimolar β-mercaptoethanol was present throughout the isolation procedures to reduce thiol groups. Twenty microlitre (2 μM) of purified mito-SypHer was dissolved in 200 μl Tris 25 mM, 150 mM NaCl pH 7.5 (0.5 mM β-mercaptoethanol), and fluorescence spectra recorded on a LS50B spectrometer (Perkin Elmer), using 500 μM xanthine and 100 mU XO (Sigma) to generate superoxide.
Superoxide production measurements in vitro
Xanthine (X)/XO O2− production was verified by adding 0.01 mM of the luciferin analogue MCLA to a buffer containing 25 mM Tris, 150 mM NaCl, 0.3 mM ethylenediaminetetraacetic acid (EDTA), 0.3 mM β-mercaptoethanol, and 400 μM xanthine (pH 7.5). XO (100 mU) was added to initiate the reaction and the luminescence recorded every 10 s on a FLUOstar (BMG Labtech) microplate reader. To verify the O2− specificity of the signal emitted by MCLA, 50 U/ml SOD was included in control experiments.
Mitochondrial pH, ΔΨm, and Ca2+ measurements in live cells
Recordings were performed in N-2-hydroxyethylpiperazine-N′-2-ethane sulphonic acid (HEPES) buffer solution containing 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 20 mM HEPES, 10 mM glucose, pH set to 7.4 with NaOH at 37°C. The Ca2+-free solution contained 0.5 mM ethyleneglycol-bis (beta-aminoethylether)-N,N′-tetraacetic acid (EGTA) and no CaCl2. Mito-SypHer was alternately excited for 200–300 ms at 440 and 488 nm on a Nipkow spinning disk confocal microscope (Visitron Systems GmbH) equipped with a × 63 1.4 NA oil-immersion objective (Carl Zeiss AG). Images were acquired every 800 ms and ratios calculated in MetaFluor 6.3 (Universal Imaging) and analysed in Excel (Microsoft) and GraphPad Prism 5.01 (GraphPad Inc, La Jolla, USA). Mitochondrial pH was calibrated using nigericin (5 μg/ml) and monensin (5 μM) in 125 mM KCl, 20 mM NaCl, 0.5 mM MgCl2, 0.2 mM EGTA, and, Tris (pH 8.0, 9.0), HEPES (pH 7.0–7.5), or MES (pH 5.5–6.5). For each cell, a 5-point calibration curve was fitted to a variable slope sigmoid equation with 1/y weighting and constraining the top of the curve to 30 (GraphPad Prism 5.01). For simultaneous pHmito/Ca2+mito measurements, cells were incubated at room temperature for 30 min with 2 μM Rhod-2-AM, washed for 20 min, and imaged immediately. For pHmito/Ψm recordings, cells were incubated at room temperature for 20 min with 4 nM TMRM, washed, and kept at 37°C on the microscope until signal reached stability. Mito-SypHer was excited for 300 ms at 488 nm and TMRM or Rhod-2 were excited for 300 ms at 565 nm. Image pairs were acquired every 600 ms.
Measurements in permeabilized cells
Cells were imaged with a × 40, 1.3 NA objective (Zeiss Axiovert s100TV) using a cooled CCD camera (MicroMax, Roper Scientific). For pH imaging, SypHer was alternately excited for 200–300 ms at 430 and 480 nm through a 505DCXR dichroic filter and imaged with a 535DF25 band pass filter (Omega Optical). Cells were permeabilized by a short exposure to digitonin (1 min, 100 μM) in a buffer containing 120 mM KCl, 10 mM NaCl, 1 mM H2KPO4, 20 mM HEPES, 5 mM succinic acid, 1 mM ATP-Mg2+, 0.02 mM ADP-K, 1 mM MgCl2, 0.5 mM EGTA adjusted to pH 7.4 with KOH. The ion-free solution contained 10 mM HEPES, 5 mM succinic acid, 0.5 mM EGTA, and sucrose to reach 300 mOsm at pH 7.4. For manipulations of the mitochondrial membrane potential, 1 μM valinomycin was added and sucrose and KCl balanced to reach 300 mOsm at pH 7.4.
Mitochondrial length analysis
Confocal Z-stacks of cells expressing matrix-targeted red fluorescent protein (mtRFP) were acquired on a Nikon 1 AR inverted Microscope using a × 60 objective (oil; CFI Plan APO 1.4 NA) and 561 nm (50 mW) excitation. Analysis of mitochondrial length was performed with Image J tool ‘Freehand line selection’ by measuring 10 mitochondria per cell (>30 cells per condition; three experiments).
paGFP experiments
Cells transiently expressing paGFP were loaded for 25 min at 37°C with 4 nM TMRM in imaging buffer (135 mM NaCl, 5 mM KCl, 0.4 mM KH2PO4, 1 mM MgSO4*7H2O, 20 mM HEPES, 0.1% D-glucose, pH 7.4). Cells with low GFP fluorescence intensity were selected to avoid saturation of the GFP emission upon photoactivation (Patterson and Lippincott-Schwartz, 2002). GFP and TMRM were imaged concurrently on the confocal microscope with the objective described above, using 488 and 561 nm excitation and 520/35 and 624/40 emission filters, respectively. Four images were acquired (one image/second) before applying one single stimulation pulse (500 ms, 405 nm laser, 100% power) followed by live imaging at 1 frame/second for 1 min. Loss of focus and movement artifacts was minimized by using a large pinhole aperture (6.9 AU), the Perfect Focus system (Nikon), and by checking photoactivated areas in ratio images. The NIS Elements AR3.2 software was used for data acquisition and analysis of the area of paGFP detected with ‘Auto Detect Area’. Photoactivated areas were measured 5 s after photoactivation to allow paGFP equilibration across the lumen of the mitochondrial network (Twig et al, 2006).
Rapid pH and potential measurements
Time-resolved pH and potential imaging was performed on cells transiently transfected with mito-SypHer and loaded with TMRM, using the IMIC Andromeda system (Fondis Electronic) equipped with a × 60 oil objective (UPLAN × 60 oil, 1.35NA, Olympus), 488 and 561 nm lasers for excitation and FF01-446/523/600/677 (Semrock) as emission filter. Two or four binning and cropped sensor mode was used to increase frame rate. The two images were acquired with the same exposure time of 15 ms to obtain acquisition rates of 66 frames per second.
Statistical analysis
All statistical analyses were performed using Prism software (GraphPad). Significance between two sets of experiments was determined using a Student’s t-test whereas group sets were analysed using ANOVA.
Supplementary Material
Acknowledgments
We thank Prof Claes Wollheim for critical reading of the manuscript, and Mr Cyril Castelbou and Ning Li for expert technical assistance. This work was supported by the Swiss National Foundation grants 310030B-133126 (to ND) and 31003AB_133130 (to LS) and ERC-2011-StG-282280-ERMITO (to LS). JS-D was supported by a fellowship of the Spanish Ministry of Education. The Figure 8 contain illustrations made by Servier Medical Art http://www.servier.fr/servier-medical-art.
Author contributions: JS-D and MG designed and performed experiments, analysed and interpreted data, and contributed to the manuscript. DP and LS designed experiments and contributed to the manuscript. ND conceived the project, designed experiments, analysed data, and wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Amchenkova AA, Bakeeva LE, Chentsov YS, Skulachev VP, Zorov DB (1988) Coupling membranes as energy-transmitting cables. I. Filamentous mitochondria in fibroblasts and mitochondrial clusters in cardiomyocytes. J Cell Biol 107: 481–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aon MA, Cortassa S, Marban E, O'Rourke B (2003) Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem 278: 44735–44744 [DOI] [PubMed] [Google Scholar]
- Azarias G, Chatton JY (2011) Selective ion changes during spontaneous mitochondrial transients in intact astrocytes. PloS ONE 6: e28505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarias G, Van de Ville D, Unser M, Chatton JY (2008) Spontaneous NA+ transients in individual mitochondria of intact astrocytes. Glia 56: 342–353 [DOI] [PubMed] [Google Scholar]
- Ban T, Heymann JA, Song Z, Hinshaw JE, Chan DC (2010) OPA1 disease alleles causing dominant optic atrophy have defects in cardiolipin-stimulated GTP hydrolysis and membrane tubulation. Hum Mol Genet 19: 2113–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi P (1999) Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev 79: 1127–1155 [DOI] [PubMed] [Google Scholar]
- Buckman JF, Reynolds IJ (2001) Spontaneous changes in mitochondrial membrane potential in cultured neurons. J Neurosci 21: 5054–5065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campello S, Scorrano L (2010) Mitochondrial shape changes: orchestrating cell pathophysiology. EMBO Rep 11: 678–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereghetti GM, Stangherlin A, Martins de Brito O, Chang CR, Blackstone C, Bernardi P, Scorrano L (2008) Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci USA 105: 15803–15808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L (2004) OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci USA 101: 15927–15932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LE, Reynafarje B, Lehninger AL (1984) Stoichiometry of mitochondrial H+ translocation coupled to succinate oxidation at level flow. J Biol Chem 259: 4802–4811 [PubMed] [Google Scholar]
- De Giorgi F, Lartigue L, Ichas F (2000) Electrical coupling and plasticity of the mitochondrial network. Cell Calcium 28: 365–370 [DOI] [PubMed] [Google Scholar]
- Duchen MR, Leyssens A, Crompton M (1998) Transient mitochondrial depolarizations reflect focal sarcoplasmic reticular calcium release in single rat cardiomyocytes. J Cell Biol 142: 975–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H, Chen M, Ding Y, Shang W, Xu J, Zhang X, Zhang W, Li K, Xiao Y, Gao F, Shang S, Li JC, Tian XL, Wang SQ, Zhou J, Weisleder N, Ma J, Ouyang K, Chen J, Wang X et al. (2011) Imaging superoxide flash and metabolism-coupled mitochondrial permeability transition in living animals. Cell Res 21: 1295–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieden M, James D, Castelbou C, Danckaert A, Martinou JC, Demaurex N (2004) Ca(2+) homeostasis during mitochondrial fragmentation and perinuclear clustering induced by hFis1. J Biol Chem 279: 22704–22714 [DOI] [PubMed] [Google Scholar]
- Gomes LC, Di Benedetto G, Scorrano L (2011) During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol 13: 589–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman JN, Sanchez-Padilla J, Wokosin D, Kondapalli J, Ilijic E, Schumacker PT, Surmeier DJ (2010) Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature 468: 696–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T, Watanabe K, Uechi Y, Yoshioka H, Ohta Y (2005) Repetitive transient depolarizations of the inner mitochondrial membrane induced by proton pumping. Biophys J 88: 2340–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huser J, Blatter LA (1999) Fluctuations in mitochondrial membrane potential caused by repetitive gating of the permeability transition pore. Biochem J 343 (Pt 2): 311–317 [PMC free article] [PubMed] [Google Scholar]
- Huser J, Rechenmacher CE, Blatter LA (1998) Imaging the permeability pore transition in single mitochondria. Biophys J 74: 2129–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto Y, Taguchi N, Morinaga H, Maeda M, Takayanagi R, Yokota S, Mihara K (2009) Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol 11: 958–966 [DOI] [PubMed] [Google Scholar]
- Jacobson J, Duchen MR (2002) Mitochondrial oxidative stress and cell death in astrocytes--requirement for stored Ca2+ and sustained opening of the permeability transition pore. J Cell Sci 115: 1175–1188 [DOI] [PubMed] [Google Scholar]
- Lambert AJ, Brand MD (2004) Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem J 382: 511–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Weaver D, Shirihai O, Hajnoczky G (2009) Mitochondrial 'kiss-and-run': interplay between mitochondrial motility and fusion-fission dynamics. EMBO J 28: 3074–3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Fang H, Shang W, Liu L, Xu Z, Ye T, Wang X, Zheng M, Chen Q, Cheng H (2011) Superoxide flashes: early mitochondrial signals for oxidative stress-induced apoptosis. J Biol Chem 286: 27573–27581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller FL (2009) A critical evaluation of cpYFP as a probe for superoxide. Free Radic Biol Med 47: 1779–1780 [DOI] [PubMed] [Google Scholar]
- Nagai T, Sawano A, Park ES, Miyawaki A (2001) Circularly permuted green fluorescent proteins engineered to sense Ca2+. Proc Natl Acad Sci USA 98: 3197–3202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG (1974) The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur J Biochem 50: 305–315 [DOI] [PubMed] [Google Scholar]
- Nicholls DG (2005) Commentary on: 'old and new data, new issues: the mitochondrial Deltapsi' by H. Tedeschi. Biochimica Et Biophysica Acta 1710: 63–65 Discussion 66 [DOI] [PubMed] [Google Scholar]
- Nicolli A, Redetti A, Bernardi P (1991) The K+ conductance of the inner mitochondrial membrane. A study of the inducible uniport for monovalent cations. J Biol Chem 266: 9465–9470 [PubMed] [Google Scholar]
- Palmer CS, Osellame LD, Laine D, Koutsopoulos OS, Frazier AE, Ryan MT (2011) MiD49 and MiD51, new components of the mitochondrial fission machinery. EMBO Rep 12: 565–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson GH, Lippincott-Schwartz J (2002) A photoactivatable GFP for selective photolabeling of proteins and cells. Science 297: 1873–1877 [DOI] [PubMed] [Google Scholar]
- Poburko D, Santo-Domingo J, Demaurex N (2011) Dynamic regulation of the mitochondrial proton gradient during cytosolic calcium elevations. J Biol Chem 286: 11672–11684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcelli AM, Ghelli A, Zanna C, Pinton P, Rizzuto R, Rugolo M (2005) pH difference across the outer mitochondrial membrane measured with a green fluorescent protein mutant. Biochem Biophys Res Commun 326: 799–804 [DOI] [PubMed] [Google Scholar]
- Pouvreau S (2010) Superoxide flashes in mouse skeletal muscle are produced by discrete arrays of active mitochondria operating coherently. PloS ONE 5: e13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santo-Domingo J, Demaurex N (2012) Perspectives on: SGP Symposium on Mitochondrial Physiology and Medicine: the renaissance of mitochondrial pH. J Gen Physiol 139: 415–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzlander M, Logan DC, Fricker MD, Sweetlove LJ (2011) The circularly permuted yellow fluorescent protein cpYFP that has been used as a superoxide probe is highly responsive to pH but not superoxide in mitochondria: implications for the existence of superoxide 'flashes'. Biochem J 437: 381–387 [DOI] [PubMed] [Google Scholar]
- Schwarzlander M, Logan DC, Johnston IG, Jones NS, Meyer AJ, Fricker MD, Sweetlove LJ (2012a) Pulsing of membrane potential in individual mitochondria: a stress-induced mechanism to regulate respiratory bioenergetics in Arabidopsis. Plant Cell 24: 1188–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzlander M, Murphy MP, Duchen MR, Logan DC, Fricker MD, Halestrap AP, Muller FL, Rizutto R, Dick TP, Meyer AJ, Sweetlove LJ (2012b) Mitochondrial 'flashes': a radical concept repHined. Trends Cell Biol 22: 503–508 [DOI] [PubMed] [Google Scholar]
- Smirnova E, Griparic L, Shurland DL, van der Bliek AM (2001) Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 12: 2245–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot J, Barrett JN, Barrett EF, David G (2007) Stimulation-induced changes in NADH fluorescence and mitochondrial membrane potential in lizard motor nerve terminals. J Physiol 579: 783–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Le PK, Tse S, Wallace DC, Huang T (2009) Heterozygous mutation of Opa1 in Drosophila shortens lifespan mediated through increased reactive oxygen species production. PloS One 4: e4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiffault C, Bennett JP Jr. (2005) Cyclical mitochondrial deltapsiM fluctuations linked to electron transport, F0F1 ATP-synthase and mitochondrial Na+/Ca+2 exchange are reduced in Alzheimer's disease cybrids. Mitochondrion 5: 109–119 [DOI] [PubMed] [Google Scholar]
- Twig G, Graf SA, Wikstrom JD, Mohamed H, Haigh SE, Elorza A, Deutsch M, Zurgil N, Reynolds N, Shirihai OS (2006) Tagging and tracking individual networks within a complex mitochondrial web with photoactivatable GFP. Am J Physiol Cell Physiol 291: C176–C184 [DOI] [PubMed] [Google Scholar]
- Twig G, Hyde B, Shirihai OS (2008) Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochimica Et Biophysica Acta 1777: 1092–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uechi Y, Yoshioka H, Morikawa D, Ohta Y (2006) Stability of membrane potential in heart mitochondria: single mitochondrion imaging. Biochem Biophys Res Commun 344: 1094–1101 [DOI] [PubMed] [Google Scholar]
- Vergun O, Reynolds IJ (2004) Fluctuations in mitochondrial membrane potential in single isolated brain mitochondria: modulation by adenine nucleotides and Ca2+. Biophys J 87: 3585–3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergun O, Votyakova TV, Reynolds IJ (2003) Spontaneous changes in mitochondrial membrane potential in single isolated brain mitochondria. Biophys J 85: 3358–3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, Wang X, Li K, Han P, Zheng M, Yin J, Mattson MP, Kao JP, Lakatta EG, Sheu SS, Ouyang K, Chen J, Dirksen RT, Cheng H (2008) Superoxide flashes in single mitochondria. Cell 134: 279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Salahura G, Boncompagni S, Kasischke KA, Protasi F, Sheu SS, Dirksen RT (2011) Mitochondrial superoxide flashes: metabolic biomarkers of skeletal muscle activity and disease. FASEB J 25: 3068–3078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ (2000) Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med 192: 1001–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.