Abstract
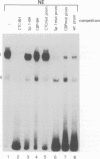
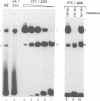
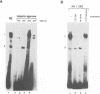
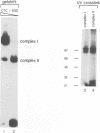
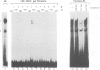
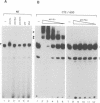
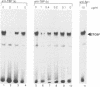
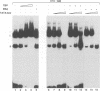
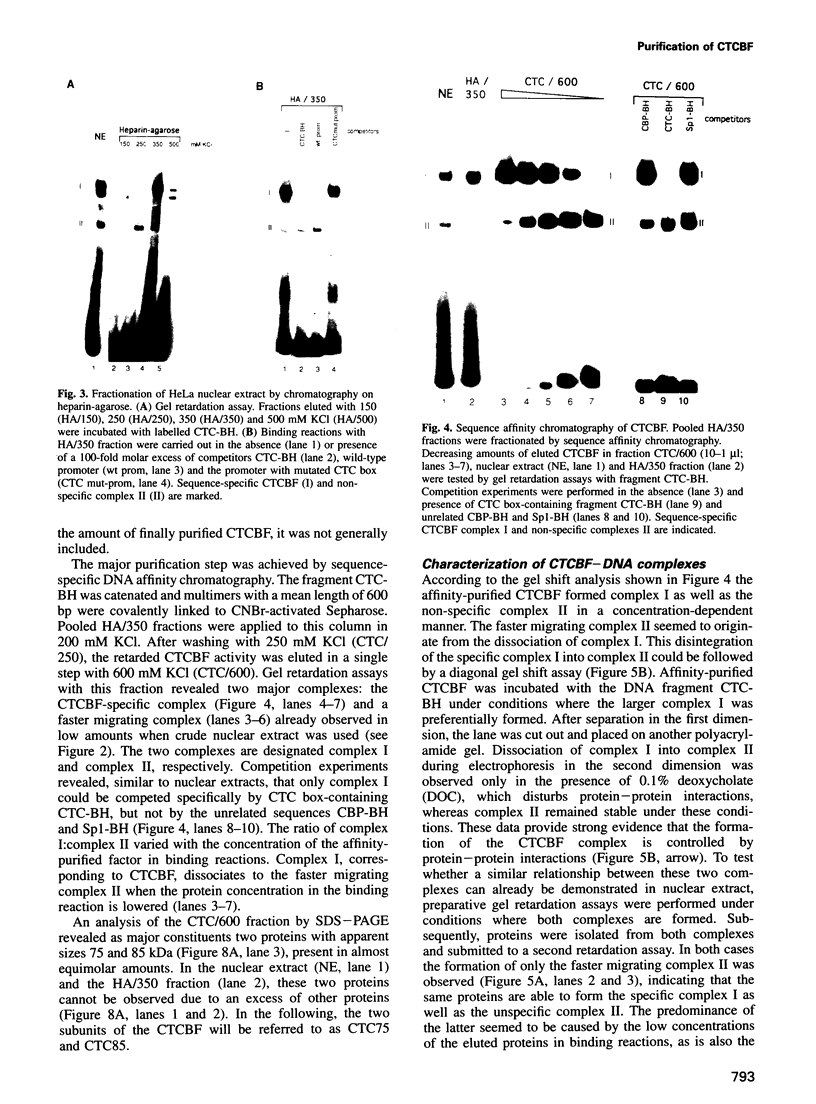
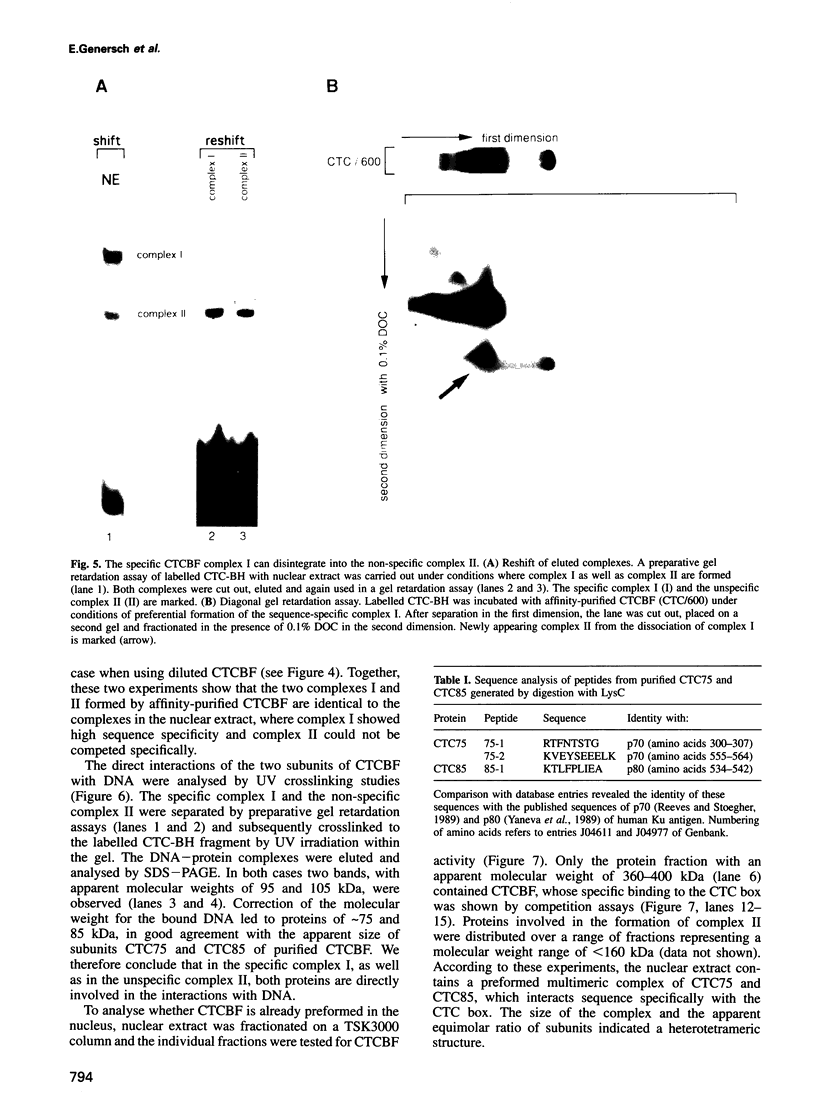
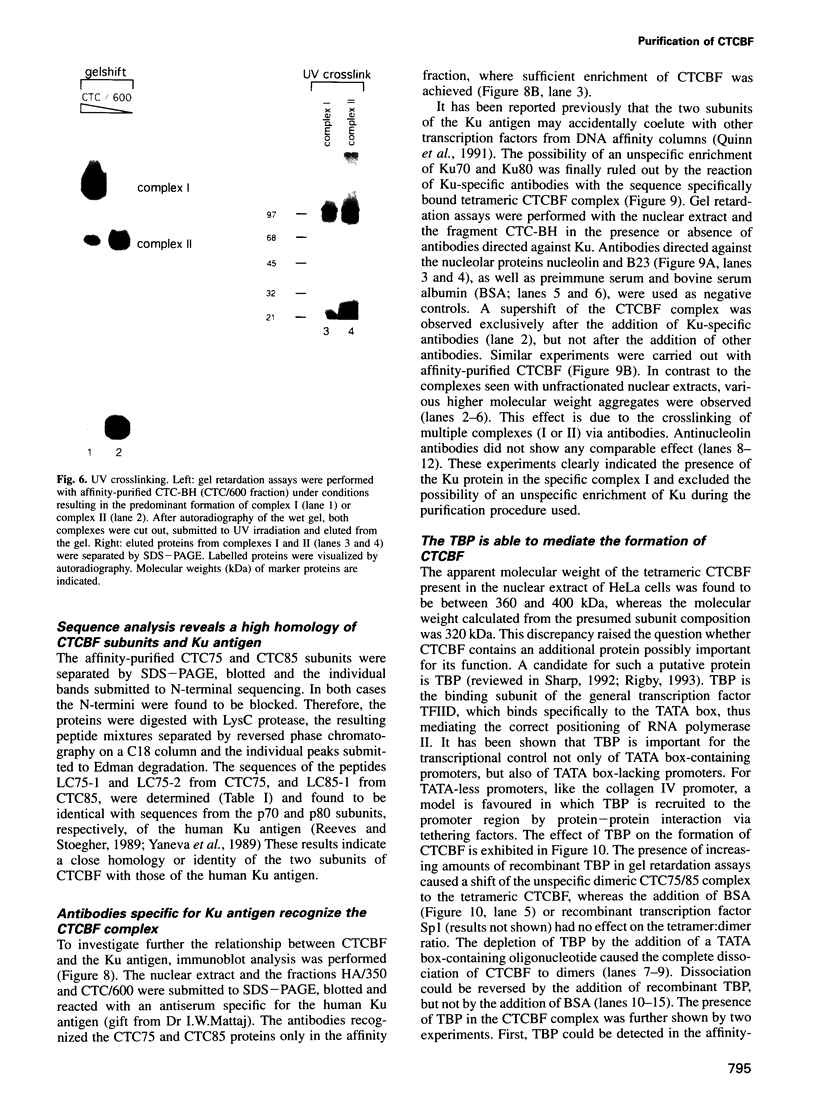
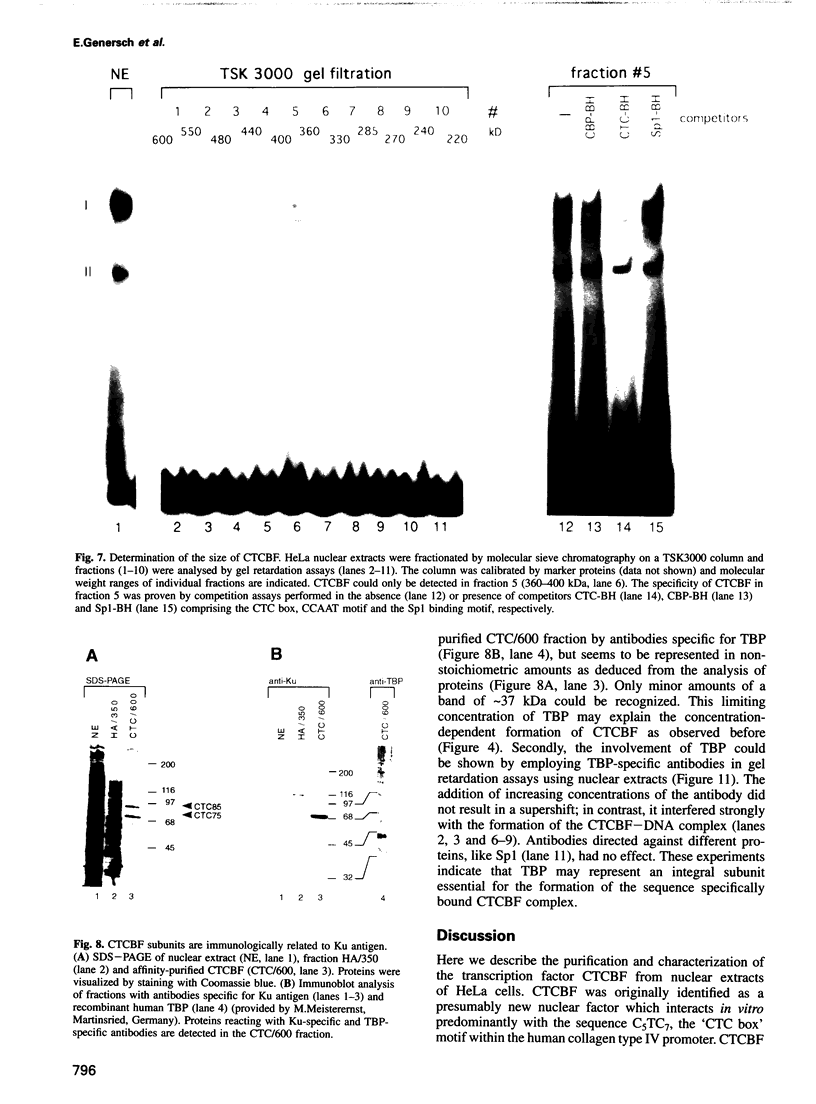
The common promoter region of the human collagen type IV genes COL4A1 and COL4A2 comprises a C5TC7 sequence ('CTC box') which is specifically recognized by the recently identified transcription factor CTC box binding factor (CTCBF) involved in the control of divergent transcription of the two genes. This factor has now been purified by affinity chromatography on heparin-agarose and CTC-Sepharose. The CTCBF contains two subunits, CTC75 and CTC85, with molecular weights of 75 and 85 kDa, respectively. Sequence analysis of LysC-derived peptides of the two subunits revealed identity or close homology to p70 and p80 subunits of the human autoantigen Ku. The sequence-specific binding CTCBF represents a presumably tetrameric complex composed of two CTC75/85 heterodimers with an apparent molecular weight of 360-400 kDa. UV crosslinking experiments, the use of Ku-specific antibodies in gel retardation assays and immunoblotting proved that both subunits are involved in sequence-specific interaction with the CTC box motif. The tetrameric complex dissociates in a concentration-dependent manner to CTC75/85 heterodimers which now bind sequence independently to DNA. Three lines of evidence indicate that TATA binding protein (TBP) is additionally involved in the formation of CTCBF: (i) TBP can be detected in purified CTCBF; (ii) the addition of recombinant TBP stimulates formation of the CTCBF-DNA complex; and (iii) antibodies directed against TBP interfere strongly with the formation of the specific protein-DNA complex. The results presented support the idea that the subunits CTC75 and CTC85 (identical or homologous to p70 and p80 of the Ku antigen) are integral parts of CTCBF, and give a first indication of the importance of TBP in the formation of CTCBF.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blier P. R., Griffith A. J., Craft J., Hardin J. A. Binding of Ku protein to DNA. Measurement of affinity for ends and demonstration of binding to nicks. J Biol Chem. 1993 Apr 5;268(10):7594–7601. [PubMed] [Google Scholar]
- Burbelo P. D., Martin G. R., Yamada Y. Alpha 1(IV) and alpha 2(IV) collagen genes are regulated by a bidirectional promoter and a shared enhancer. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9679–9682. doi: 10.1073/pnas.85.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q. P., Pitt S., Leszyk J., Baril E. F. DNA-dependent ATPase from HeLa cells is related to human Ku autoantigen. Biochemistry. 1994 Jul 19;33(28):8548–8557. doi: 10.1021/bi00194a021. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvir A., Peterson S. R., Knuth M. W., Lu H., Dynan W. S. Ku autoantigen is the regulatory component of a template-associated protein kinase that phosphorylates RNA polymerase II. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11920–11924. doi: 10.1073/pnas.89.24.11920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falzon M., Kuff E. L. The nucleotide sequence of a mouse cDNA encoding the 80 kDa subunit of the Ku (p70/p80) autoantigen. Nucleic Acids Res. 1992 Jul 25;20(14):3784–3784. doi: 10.1093/nar/20.14.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer G., Schmidt C., Opitz J., Cully Z., Kühn K., Pöschl E. Identification of a novel sequence element in the common promoter region of human collagen type IV genes, involved in the regulation of divergent transcription. Biochem J. 1993 Jun 15;292(Pt 3):687–695. doi: 10.1042/bj2920687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb T. M., Jackson S. P. The DNA-dependent protein kinase: requirement for DNA ends and association with Ku antigen. Cell. 1993 Jan 15;72(1):131–142. doi: 10.1016/0092-8674(93)90057-w. [DOI] [PubMed] [Google Scholar]
- Griffin C. A., Emanuel B. S., Hansen J. R., Cavenee W. K., Myers J. C. Human collagen genes encoding basement membrane alpha 1 (IV) and alpha 2 (IV) chains map to the distal long arm of chromosome 13. Proc Natl Acad Sci U S A. 1987 Jan;84(2):512–516. doi: 10.1073/pnas.84.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith A. J., Blier P. R., Mimori T., Hardin J. A. Ku polypeptides synthesized in vitro assemble into complexes which recognize ends of double-stranded DNA. J Biol Chem. 1992 Jan 5;267(1):331–338. [PubMed] [Google Scholar]
- Griffith A. J., Craft J., Evans J., Mimori T., Hardin J. A. Nucleotide sequence and genomic structure analyses of the p70 subunit of the human Ku autoantigen: evidence for a family of genes encoding Ku (p70)-related polypeptides. Mol Biol Rep. 1992 May;16(2):91–97. doi: 10.1007/BF00419754. [DOI] [PubMed] [Google Scholar]
- Hateboer G., Timmers H. T., Rustgi A. K., Billaud M., van 't Veer L. J., Bernards R. TATA-binding protein and the retinoblastoma gene product bind to overlapping epitopes on c-Myc and adenovirus E1A protein. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8489–8493. doi: 10.1073/pnas.90.18.8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadonaga J. T., Tjian R. Affinity purification of sequence-specific DNA binding proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5889–5893. doi: 10.1073/pnas.83.16.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaytes P., Wood L., Theriault N., Kurkinen M., Vogeli G. Head-to-head arrangement of murine type IV collagen genes. J Biol Chem. 1988 Dec 25;263(36):19274–19277. [PubMed] [Google Scholar]
- Knuth M. W., Gunderson S. I., Thompson N. E., Strasheim L. A., Burgess R. R. Purification and characterization of proximal sequence element-binding protein 1, a transcription activating protein related to Ku and TREF that binds the proximal sequence element of the human U1 promoter. J Biol Chem. 1990 Oct 15;265(29):17911–17920. [PubMed] [Google Scholar]
- Le Romancer M., Reyl-Desmars F., Cherifi Y., Pigeon C., Bottari S., Meyer O., Lewin M. J. The 86-kDa subunit of autoantigen Ku is a somatostatin receptor regulating protein phosphatase-2A activity. J Biol Chem. 1994 Jul 1;269(26):17464–17468. [PubMed] [Google Scholar]
- Lichtler A., Stover M. L., Angilly J., Kream B., Rowe D. W. Isolation and characterization of the rat alpha 1(I) collagen promoter. Regulation by 1,25-dihydroxyvitamin D. J Biol Chem. 1989 Feb 25;264(6):3072–3077. [PubMed] [Google Scholar]
- Mastrangelo I. A., Courey A. J., Wall J. S., Jackson S. P., Hough P. V. DNA looping and Sp1 multimer links: a mechanism for transcriptional synergism and enhancement. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5670–5674. doi: 10.1073/pnas.88.13.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May G., Sutton C., Gould H. Purification and characterization of Ku-2, an octamer-binding protein related to the autoantigen Ku. J Biol Chem. 1991 Feb 15;266(5):3052–3059. [PubMed] [Google Scholar]
- May T., Kern H., Müller-Taubenberger A., Nellen W. Identification of a cis-acting element controlling induction of early gene expression in Dictyostelium discoideum. Mol Cell Biol. 1989 Nov;9(11):4653–4659. doi: 10.1128/mcb.9.11.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey J. H., Nomura S., Kelly P., Mason I. J., Hogan B. L. Characterization of the mouse SPARC/osteonectin gene. Intron/exon organization and an unusual promoter region. J Biol Chem. 1988 Aug 15;263(23):11111–11116. [PubMed] [Google Scholar]
- Mimori T., Akizuki M., Yamagata H., Inada S., Yoshida S., Homma M. Characterization of a high molecular weight acidic nuclear protein recognized by autoantibodies in sera from patients with polymyositis-scleroderma overlap. J Clin Invest. 1981 Sep;68(3):611–620. doi: 10.1172/JCI110295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori T., Hardin J. A., Steitz J. A. Characterization of the DNA-binding protein antigen Ku recognized by autoantibodies from patients with rheumatic disorders. J Biol Chem. 1986 Feb 15;261(5):2274–2278. [PubMed] [Google Scholar]
- Natarajan V., Madden M. J., Salzman N. P. Proximal and distal domains that control in vitro transcription of the adenovirus IVa2 gene. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6290–6294. doi: 10.1073/pnas.81.20.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu H., Jacob S. T. Enhancer 1 binding factor (E1BF), a Ku-related protein, is a growth-regulated RNA polymerase I transcription factor: association of a repressor activity with purified E1BF from serum-deprived cells. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):9101–9105. doi: 10.1073/pnas.91.19.9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K., Burbelo P. D., Sasaki M., Yamada Y. The laminin B2 chain promoter contains unique repeat sequences and is active in transient transfection. J Biol Chem. 1988 Jun 15;263(17):8384–8389. [PubMed] [Google Scholar]
- Pöschl E., Pollner R., Kühn K. The genes for the alpha 1(IV) and alpha 2(IV) chains of human basement membrane collagen type IV are arranged head-to-head and separated by a bidirectional promoter of unique structure. EMBO J. 1988 Sep;7(9):2687–2695. doi: 10.1002/j.1460-2075.1988.tb03122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn J. P., Simpson J., Farina A. R. The Ku complex is modulated in response to viral infection and other cellular changes. Biochim Biophys Acta. 1992 Jun 15;1131(2):181–187. doi: 10.1016/0167-4781(92)90074-a. [DOI] [PubMed] [Google Scholar]
- Reeves W. H. Antibodies to the p70/p80 (Ku) antigens in systemic lupus erythematosus. Rheum Dis Clin North Am. 1992 May;18(2):391–414. [PubMed] [Google Scholar]
- Reeves W. H., Sthoeger Z. M. Molecular cloning of cDNA encoding the p70 (Ku) lupus autoantigen. J Biol Chem. 1989 Mar 25;264(9):5047–5052. [PubMed] [Google Scholar]
- Rigby P. W. Three in one and one in three: it all depends on TBP. Cell. 1993 Jan 15;72(1):7–10. doi: 10.1016/0092-8674(93)90042-o. [DOI] [PubMed] [Google Scholar]
- Roberts M. R., Miskimins W. K., Ruddle F. H. Nuclear proteins TREF1 and TREF2 bind to the transcriptional control element of the transferrin receptor gene and appear to be associated as a heterodimer. Cell Regul. 1989 Nov;1(1):151–164. doi: 10.1091/mbc.1.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossouw C. M., Vergeer W. P., du Plooy S. J., Bernard M. P., Ramirez F., de Wet W. J. DNA sequences in the first intron of the human pro-alpha 1(I) collagen gene enhance transcription. J Biol Chem. 1987 Nov 5;262(31):15151–15157. [PubMed] [Google Scholar]
- Schaufele F., Cassill J. A., West B. L., Reudelhuber T. Resolution by diagonal gel mobility shift assays of multisubunit complexes binding to a functionally important element of the rat growth hormone gene promoter. J Biol Chem. 1990 Aug 25;265(24):14592–14598. [PubMed] [Google Scholar]
- Schmidt A., Rossi P., de Crombrugghe B. Transcriptional control of the mouse alpha 2(I) collagen gene: functional deletion analysis of the promoter and evidence for cell-specific expression. Mol Cell Biol. 1986 Feb;6(2):347–354. doi: 10.1128/mcb.6.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C., Fischer G., Kadner H., Genersch E., Kühn K., Pöschl E. Differential effects of DNA-binding proteins on bidirectional transcription from the common promoter region of human collagen type IV genes COL4A1 and COL4A2. Biochim Biophys Acta. 1993 Jul 18;1174(1):1–10. doi: 10.1016/0167-4781(93)90085-r. [DOI] [PubMed] [Google Scholar]
- Sharp P. A. TATA-binding protein is a classless factor. Cell. 1992 Mar 6;68(5):819–821. doi: 10.1016/0092-8674(92)90023-6. [DOI] [PubMed] [Google Scholar]
- Soininen R., Huotari M., Hostikka S. L., Prockop D. J., Tryggvason K. The structural genes for alpha 1 and alpha 2 chains of human type IV collagen are divergently encoded on opposite DNA strands and have an overlapping promoter region. J Biol Chem. 1988 Nov 25;263(33):17217–17220. [PubMed] [Google Scholar]
- Su W., Jackson S., Tjian R., Echols H. DNA looping between sites for transcriptional activation: self-association of DNA-bound Sp1. Genes Dev. 1991 May;5(5):820–826. doi: 10.1101/gad.5.5.820. [DOI] [PubMed] [Google Scholar]
- Taccioli G. E., Gottlieb T. M., Blunt T., Priestley A., Demengeot J., Mizuta R., Lehmann A. R., Alt F. W., Jackson S. P., Jeggo P. A. Ku80: product of the XRCC5 gene and its role in DNA repair and V(D)J recombination. Science. 1994 Sep 2;265(5177):1442–1445. doi: 10.1126/science.8073286. [DOI] [PubMed] [Google Scholar]
- Timpl R., Dziadek M. Structure, development, and molecular pathology of basement membranes. Int Rev Exp Pathol. 1986;29:1–112. [PubMed] [Google Scholar]
- Timpl R. Structure and biological activity of basement membrane proteins. Eur J Biochem. 1989 Apr 1;180(3):487–502. doi: 10.1111/j.1432-1033.1989.tb14673.x. [DOI] [PubMed] [Google Scholar]
- Timpl R., Wiedemann H., van Delden V., Furthmayr H., Kühn K. A network model for the organization of type IV collagen molecules in basement membranes. Eur J Biochem. 1981 Nov;120(2):203–211. doi: 10.1111/j.1432-1033.1981.tb05690.x. [DOI] [PubMed] [Google Scholar]
- Tuteja N., Tuteja R., Ochem A., Taneja P., Huang N. W., Simoncsits A., Susic S., Rahman K., Marusic L., Chen J. Human DNA helicase II: a novel DNA unwinding enzyme identified as the Ku autoantigen. EMBO J. 1994 Oct 17;13(20):4991–5001. doi: 10.1002/j.1460-2075.1994.tb06826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Satoh M., Chou C. H., Reeves W. H. Similar DNA binding properties of free P70 (KU) subunit and P70/P80 heterodimer. FEBS Lett. 1994 Sep 5;351(2):219–224. doi: 10.1016/0014-5793(94)00863-9. [DOI] [PubMed] [Google Scholar]
- Yaneva M., Arnett F. C. Antibodies against Ku protein in sera from patients with autoimmune diseases. Clin Exp Immunol. 1989 Jun;76(3):366–372. [PMC free article] [PubMed] [Google Scholar]
- Yaneva M., Wen J., Ayala A., Cook R. cDNA-derived amino acid sequence of the 86-kDa subunit of the Ku antigen. J Biol Chem. 1989 Aug 15;264(23):13407–13411. [PubMed] [Google Scholar]
- Young M. F., Findlay D. M., Dominguez P., Burbelo P. D., McQuillan C., Kopp J. B., Robey P. G., Termine J. D. Osteonectin promoter. DNA sequence analysis and S1 endonuclease site potentially associated with transcriptional control in bone cells. J Biol Chem. 1989 Jan 5;264(1):450–456. [PubMed] [Google Scholar]
- Yurchenco P. D., Schittny J. C. Molecular architecture of basement membranes. FASEB J. 1990 Apr 1;4(6):1577–1590. doi: 10.1096/fasebj.4.6.2180767. [DOI] [PubMed] [Google Scholar]
- Zhang W. W., Yaneva M. On the mechanisms of Ku protein binding to DNA. Biochem Biophys Res Commun. 1992 Jul 15;186(1):574–579. doi: 10.1016/s0006-291x(05)80847-2. [DOI] [PubMed] [Google Scholar]