Abstract
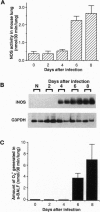
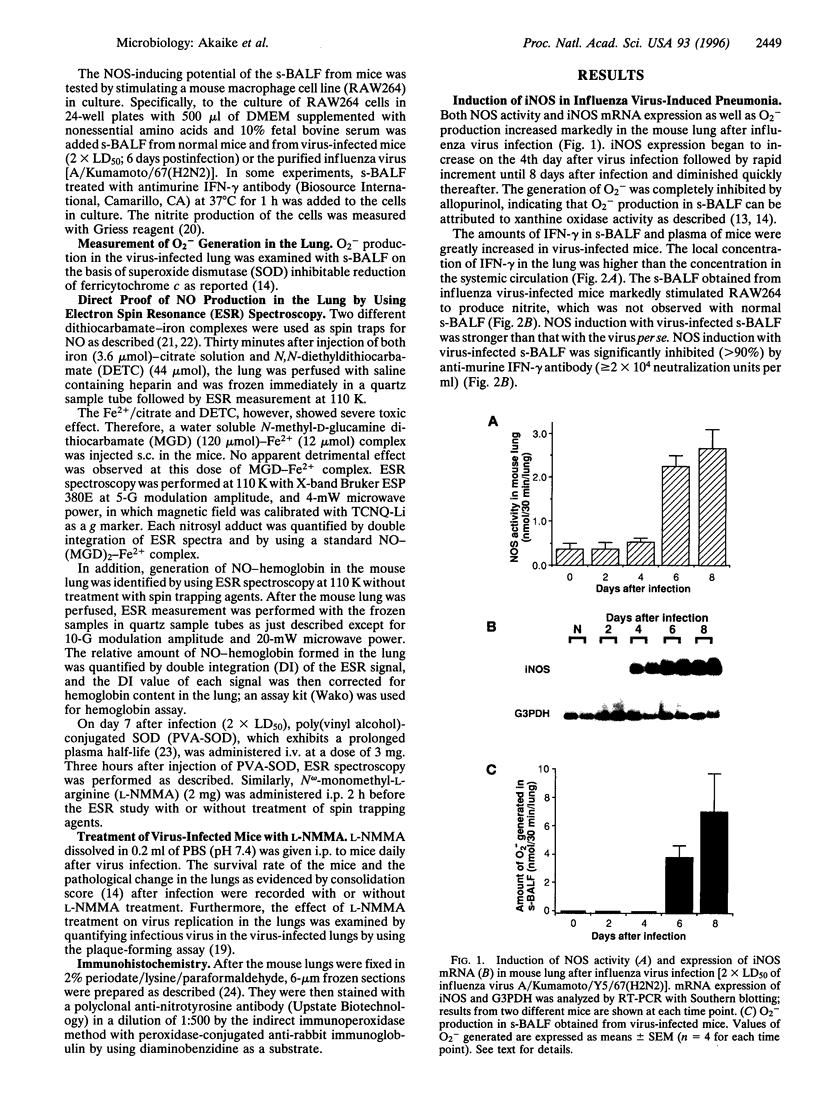
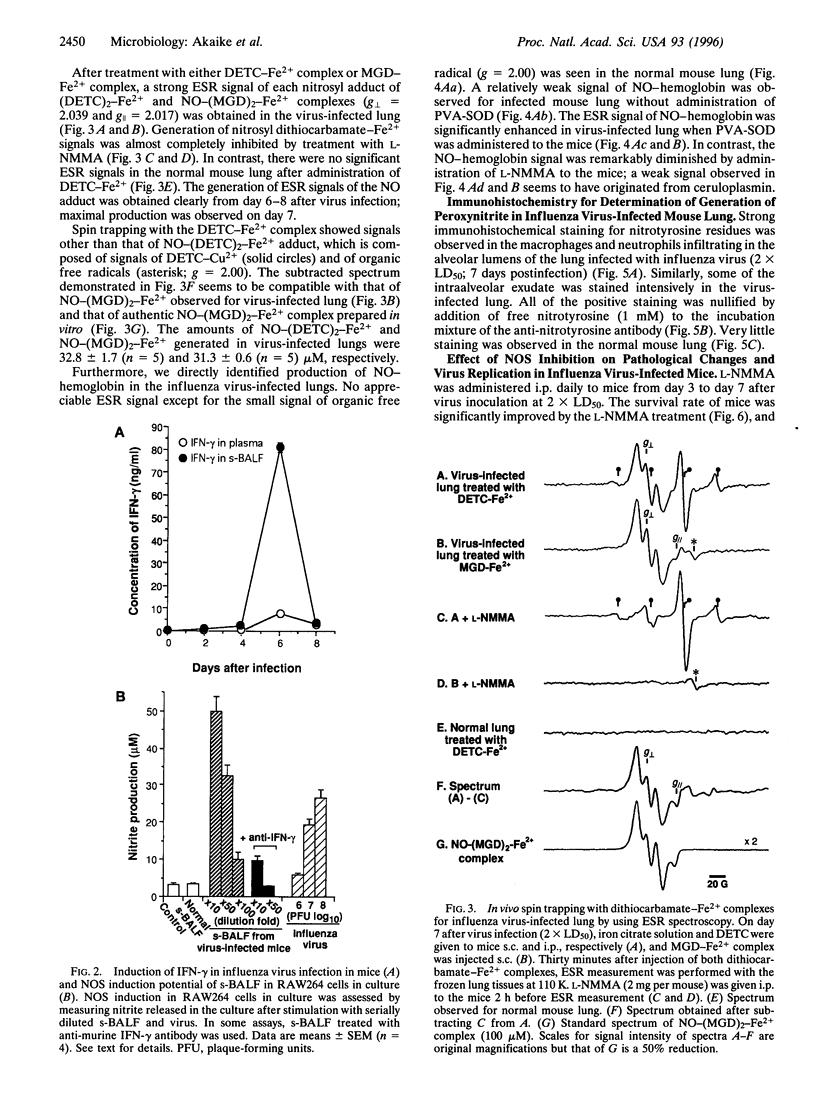
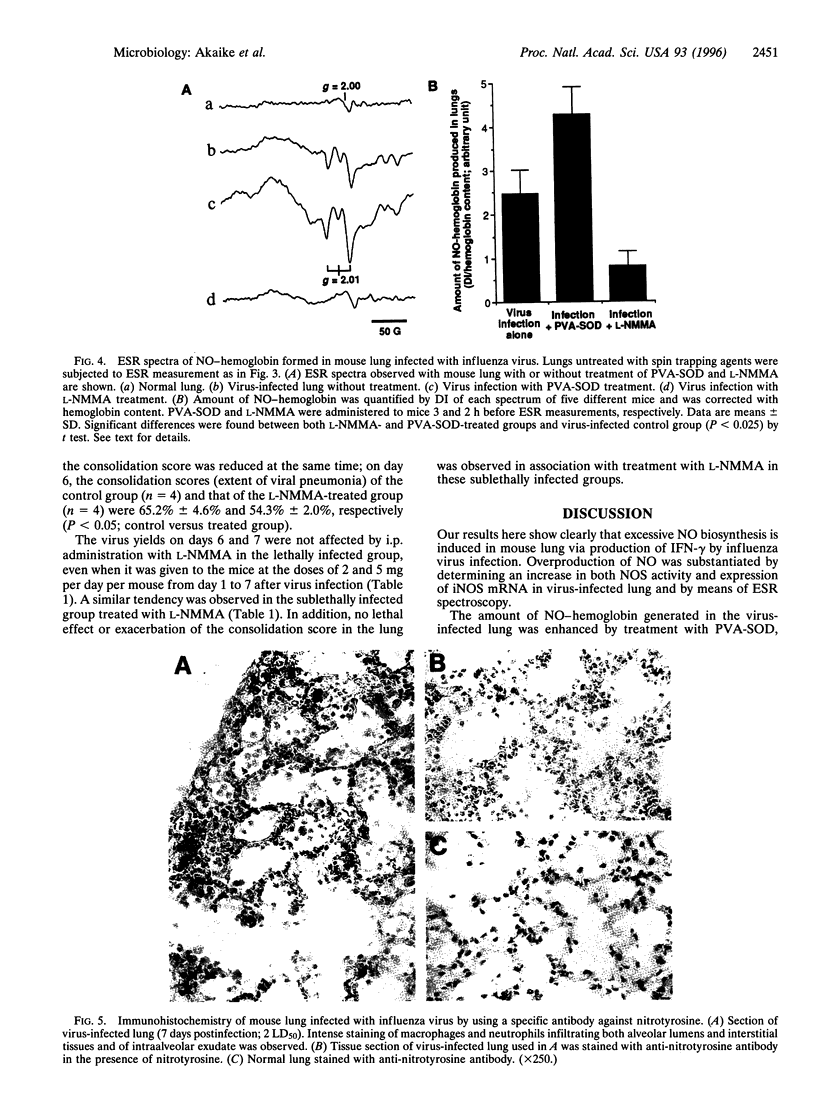
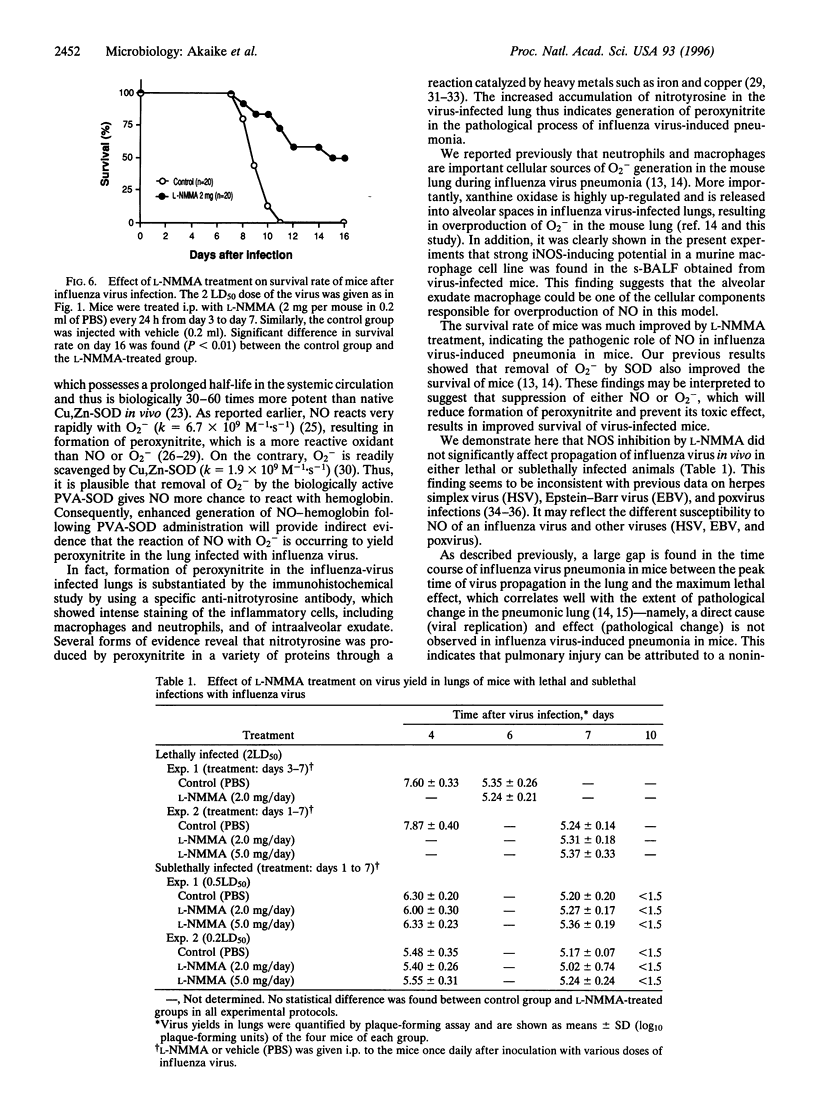
The role of nitric oxide (NO) in the pathogenesis of influenza virus-induced pneumonia in mice was investigated. Experimental influenza virus pneumonia was produced with influenza virus A/Kumamoto/Y5/67(H2N2). Both the enzyme activity of NO synthase (NOS) and mRNA expression of the inducible NOS were greatly increased in the mouse lungs; increases were mediated by interferon gamma. Excessive production of NO in the virus-infected lung was studied further by using electron spin resonance (ESR) spectroscopy. In vivo spin trapping with dithiocarbamate-iron complexes indicated that a significant amount of NO was generated in the virus-infected lung. Furthermore, an NO-hemoglobin ESR signal appeared in the virus-infected lung, and formation of NO-hemoglobin was significantly increased by treatment with superoxide dismutase and was inhibited by N(omega)-monomethyl-L-arginine (L-NMMA) administration. Immunohistochemistry with a specific anti-nitrotyrosine antibody showed intense staining of alveolar phagocytic cells such as macrophages and neutrophils and of intraalveolar exudate in the virus-infected lung. These results strongly suggest formation of peroxynitrite in the lung through the reaction of NO with O2-, which is generated by alveolar phagocytic cells and xanthine oxidase. In addition, administration of L-NMMA resulted in significant improvement in the survival rate of virus-infected mice without appreciable suppression of their antiviral defenses. On the basis of these data, we conclude that NO together with O2- which forms more reactive peroxynitrite may be the most important pathogenic factors in influenza virus-induced pneumonia in mice.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike T., Ando M., Oda T., Doi T., Ijiri S., Araki S., Maeda H. Dependence on O2- generation by xanthine oxidase of pathogenesis of influenza virus infection in mice. J Clin Invest. 1990 Mar;85(3):739–745. doi: 10.1172/JCI114499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike T., Molla A., Ando M., Araki S., Maeda H. Molecular mechanism of complex infection by bacteria and virus analyzed by a model using serratial protease and influenza virus in mice. J Virol. 1989 May;63(5):2252–2259. doi: 10.1128/jvi.63.5.2252-2259.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike T., Weihe E., Schaefer M., Fu Z. F., Zheng Y. M., Vogel W., Schmidt H., Koprowski H., Dietzschold B. Effect of neurotropic virus infection on neuronal and inducible nitric oxide synthase activity in rat brain. J Neurovirol. 1995 Mar;1(1):118–125. doi: 10.3109/13550289509111016. [DOI] [PubMed] [Google Scholar]
- Asano K., Chee C. B., Gaston B., Lilly C. M., Gerard C., Drazen J. M., Stamler J. S. Constitutive and inducible nitric oxide synthase gene expression, regulation, and activity in human lung epithelial cells. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10089–10093. doi: 10.1073/pnas.91.21.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann J. S., Ye Y. Z., Anderson P. G., Chen J., Accavitti M. A., Tarpey M. M., White C. R. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe Seyler. 1994 Feb;375(2):81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- Bukrinsky M. I., Nottet H. S., Schmidtmayerova H., Dubrovsky L., Flanagan C. R., Mullins M. E., Lipton S. A., Gendelman H. E. Regulation of nitric oxide synthase activity in human immunodeficiency virus type 1 (HIV-1)-infected monocytes: implications for HIV-associated neurological disease. J Exp Med. 1995 Feb 1;181(2):735–745. doi: 10.1084/jem.181.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle J. T., Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993 Oct 29;262(5134):689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Croen K. D. Evidence for antiviral effect of nitric oxide. Inhibition of herpes simplex virus type 1 replication. J Clin Invest. 1993 Jun;91(6):2446–2452. doi: 10.1172/JCI116479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Vanhoutte P. M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989 Jul;3(9):2007–2018. [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Huie R. E., Padmaja S. The reaction of no with superoxide. Free Radic Res Commun. 1993;18(4):195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Shimokata K., Daikoku T., Fukatsu T., Tsutsui Y., Nishiyama Y. Pathogenesis of cytomegalovirus-associated pneumonitis in ICR mice: possible involvement of superoxide radicals. Arch Virol. 1992;127(1-4):11–24. doi: 10.1007/BF01309571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischiropoulos H., Zhu L., Chen J., Tsai M., Martin J. C., Smith C. D., Beckman J. S. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys. 1992 Nov 1;298(2):431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- Karupiah G., Xie Q. W., Buller R. M., Nathan C., Duarte C., MacMicking J. D. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science. 1993 Sep 10;261(5127):1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- Klug-Roth D., Fridovich I., Rabani J. Pulse radiolytic investigations of superoxide catalyzed disproportionation. Mechanism for bovine superoxide dismutase. J Am Chem Soc. 1973 May 2;95(9):2786–2790. doi: 10.1021/ja00790a007. [DOI] [PubMed] [Google Scholar]
- Komarov A., Mattson D., Jones M. M., Singh P. K., Lai C. S. In vivo spin trapping of nitric oxide in mice. Biochem Biophys Res Commun. 1993 Sep 30;195(3):1191–1198. doi: 10.1006/bbrc.1993.2170. [DOI] [PubMed] [Google Scholar]
- Kooy N. W., Royall J. A., Ye Y. Z., Kelly D. R., Beckman J. S. Evidence for in vivo peroxynitrite production in human acute lung injury. Am J Respir Crit Care Med. 1995 Apr;151(4):1250–1254. doi: 10.1164/ajrccm/151.4.1250. [DOI] [PubMed] [Google Scholar]
- Koprowski H., Zheng Y. M., Heber-Katz E., Fraser N., Rorke L., Fu Z. F., Hanlon C., Dietzschold B. In vivo expression of inducible nitric oxide synthase in experimentally induced neurologic diseases. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):3024–3027. doi: 10.1073/pnas.90.7.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton S. A., Choi Y. B., Pan Z. H., Lei S. Z., Chen H. S., Sucher N. J., Loscalzo J., Singel D. J., Stamler J. S. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993 Aug 12;364(6438):626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- Maeda H., Akaike T. Oxygen free radicals as pathogenic molecules in viral diseases. Proc Soc Exp Biol Med. 1991 Nov;198(2):721–727. doi: 10.3181/00379727-198-43309c. [DOI] [PubMed] [Google Scholar]
- Maeda H., Akaike T., Yoshida M., Suga M. Multiple functions of nitric oxide in pathophysiology and microbiology: analysis by a new nitric oxide scavenger. J Leukoc Biol. 1994 Nov;56(5):588–592. doi: 10.1002/jlb.56.5.588. [DOI] [PubMed] [Google Scholar]
- Mannick J. B., Asano K., Izumi K., Kieff E., Stamler J. S. Nitric oxide produced by human B lymphocytes inhibits apoptosis and Epstein-Barr virus reactivation. Cell. 1994 Dec 30;79(7):1137–1146. doi: 10.1016/0092-8674(94)90005-1. [DOI] [PubMed] [Google Scholar]
- McCartney-Francis N., Allen J. B., Mizel D. E., Albina J. E., Xie Q. W., Nathan C. F., Wahl S. M. Suppression of arthritis by an inhibitor of nitric oxide synthase. J Exp Med. 1993 Aug 1;178(2):749–754. doi: 10.1084/jem.178.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993 Dec 30;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Mulligan M. S., Hevel J. M., Marletta M. A., Ward P. A. Tissue injury caused by deposition of immune complexes is L-arginine dependent. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6338–6342. doi: 10.1073/pnas.88.14.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obolenskaya MYu, Vanin A. F., Mordvintcev P. I., Mülsch A., Decker K. Epr evidence of nitric oxide production by the regenerating rat liver. Biochem Biophys Res Commun. 1994 Jul 15;202(1):571–576. doi: 10.1006/bbrc.1994.1966. [DOI] [PubMed] [Google Scholar]
- Oda T., Akaike T., Hamamoto T., Suzuki F., Hirano T., Maeda H. Oxygen radicals in influenza-induced pathogenesis and treatment with pyran polymer-conjugated SOD. Science. 1989 May 26;244(4907):974–976. doi: 10.1126/science.2543070. [DOI] [PubMed] [Google Scholar]
- Radi R., Beckman J. S., Bush K. M., Freeman B. A. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991 Mar 5;266(7):4244–4250. [PubMed] [Google Scholar]
- Shibuki K., Okada D. Endogenous nitric oxide release required for long-term synaptic depression in the cerebellum. Nature. 1991 Jan 24;349(6307):326–328. doi: 10.1038/349326a0. [DOI] [PubMed] [Google Scholar]
- Stamler J. S., Singel D. J., Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992 Dec 18;258(5090):1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Griffith O. W. Mammalian nitric oxide synthases. Adv Enzymol Relat Areas Mol Biol. 1992;65:287–346. doi: 10.1002/9780470123119.ch8. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Akaike T., Wada Y., Sato K., Ikeda K., Ueda S., Maeda H. Therapeutic effects of imidazolineoxyl N-oxide against endotoxin shock through its direct nitric oxide-scavenging activity. Biochem Biophys Res Commun. 1994 Jul 29;202(2):923–930. doi: 10.1006/bbrc.1994.2018. [DOI] [PubMed] [Google Scholar]
- Zheng Y. M., Schäfer M. K., Weihe E., Sheng H., Corisdeo S., Fu Z. F., Koprowski H., Dietzschold B. Severity of neurological signs and degree of inflammatory lesions in the brains of rats with Borna disease correlate with the induction of nitric oxide synthase. J Virol. 1993 Oct;67(10):5786–5791. doi: 10.1128/jvi.67.10.5786-5791.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]